Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Collection
2.4. Assessment of Risk of Bias
2.5. Statistical Analysis
3. Results
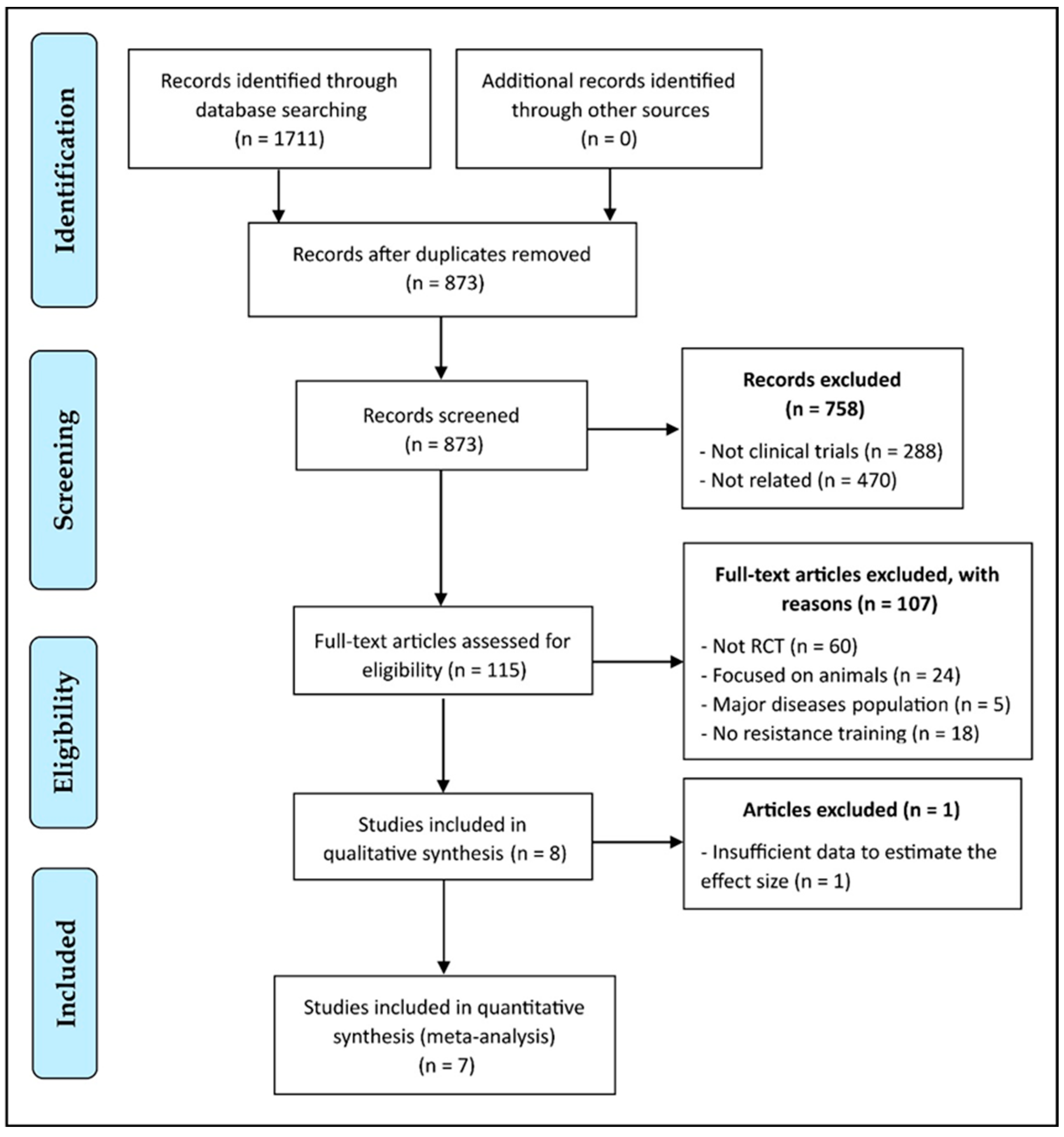
3.1. Study Selection
3.2. Characteristics of the Included RCTs
3.3. Assessment of Risk of Bias
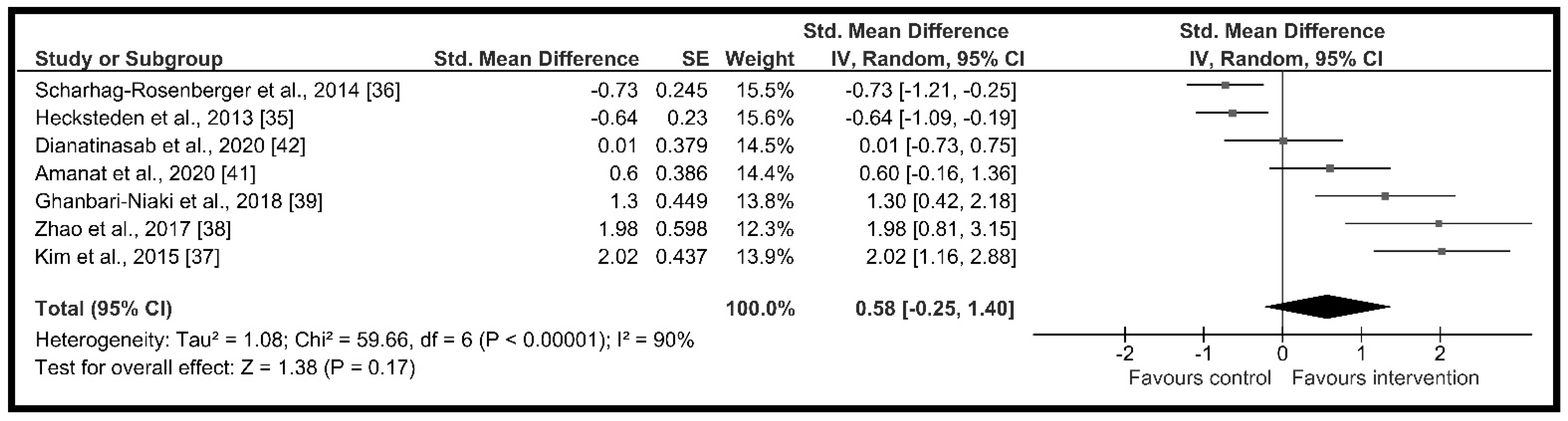
3.4. Chronic Effect of Resistance Training on Circulating Irisin
3.4.1. Meta-Analysis
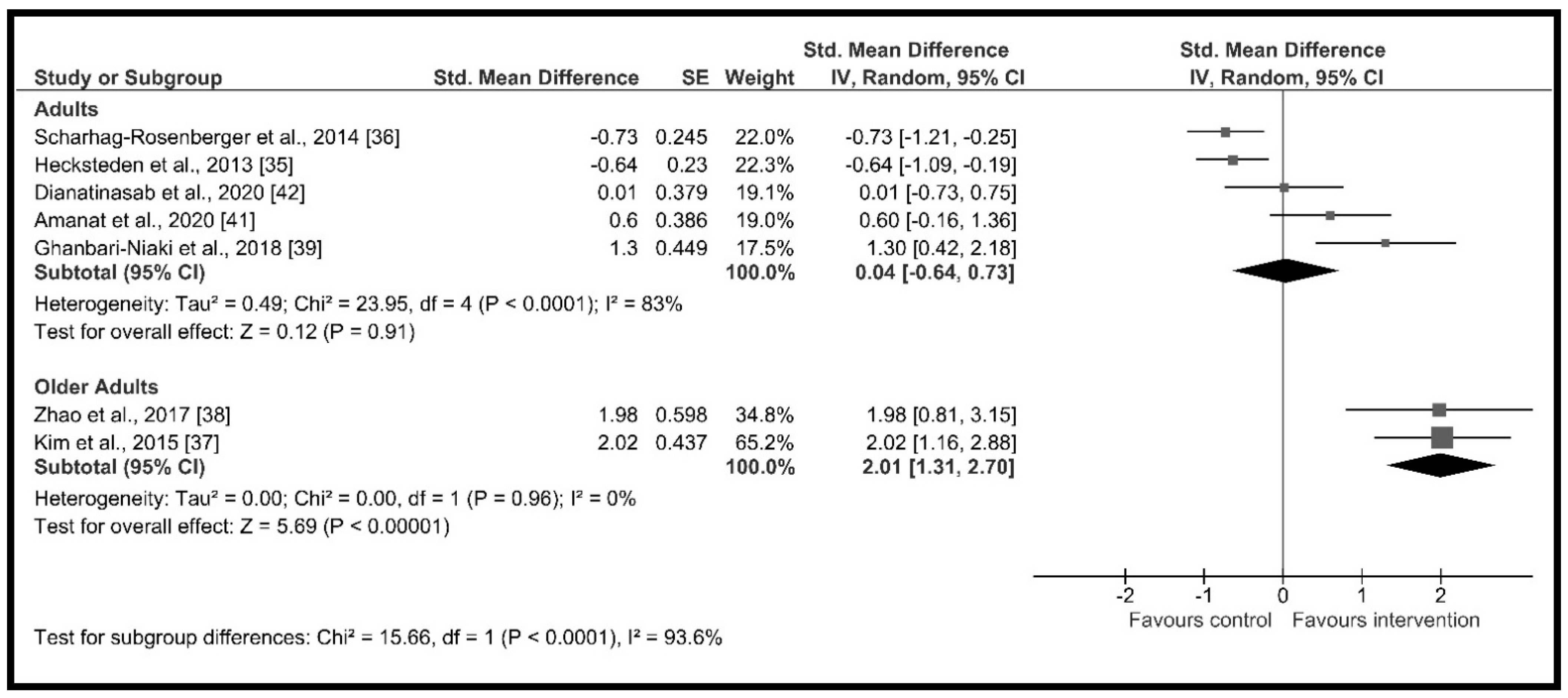
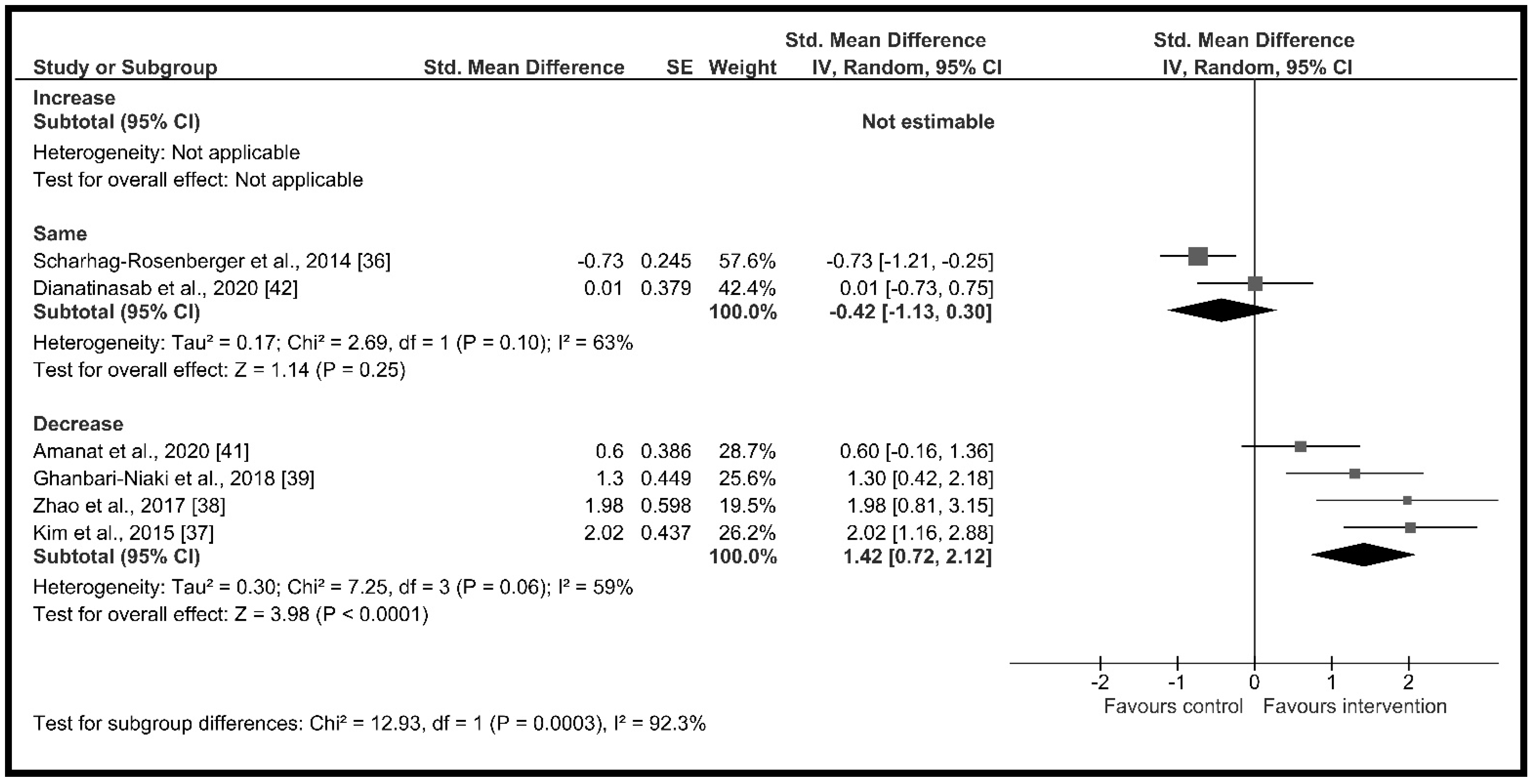
3.4.2. Effect of Moderator Variables
3.4.3. Sensitivity Analysis and Publication Bias
4. Discussion
4.1. Main Findings and Interpretation
4.2. Strengths and Limitations
4.3. Suggestions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, B.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, K.; Han, Y.; Zhu, H.; Zhou, X.; Tan, T.; Zeng, J.; Zhang, J.; Liu, Y.; Li, Y.; et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J. Cardiovasc. Pharmacol. 2018, 72, 259–269. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1α-dependent myokine that drives browning of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef]
- Gao, S.; Li, F.; Li, H.; Huang, Y.; Liu, Y.; Chen, Y. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS ONE 2016, 11, e0147480. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Colaianni, G.; Grano, M. Role of Irisin on the bone-muscle functional unit. BoneKey Rep. 2015, 4, 765. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Zhang, S.; Hou, N.; Wang, D.; Sun, X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1501–H1508. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via αV integrin receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.; Song, J. The role of irisin in Alzheimer’s disease. J. Clin. Med. 2018, 7, 407. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, D.I.; Choi, J.H.; Heo, Y.R.; Park, S.H. New role of irisin in hepatocytes: The protective effect of hepatic steatosis in vitro. Cell Signal. 2015, 27, 1831–1839. [Google Scholar] [CrossRef]
- Steiner, E.; Tata, M.; Frisén, J. A fresh look at adult neurogenesis. Nat. Med. 2019, 25, 542–543. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, J.; Yano, N.; Zhang, L.X.; Wang, H.; Zhang, S.; Qin, G.; Dubielecka, P.M.; Zhuang, S.; Liu, P.Y.; et al. Irisin promotes cardiac progenitor cell-induced myocardial repair and functional improvement in infarcted heart. J. Cell Physiol. 2019, 234, 1671–1681. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Cai, X.; Sun, Z.; Schumann, U.; Zügel, M.; Steinacker, J.M. Chronic exercise training and circulating irisin in adults: A meta-analysis. Sports Med. 2015, 45, 1577–1588. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance exercise induces a greater irisin response than endurance exercise. Metab. Exp. 2015, 64, 1042–1050. [Google Scholar] [CrossRef]
- Nygaard, H.; Slettalokken, G.; Vegge, G.; Hollan, I.; Whist, J.E.; Strand, T.; Bent, R.; Rønnestad, B.R.; Ellefsen, S. Irisin in Blood Increases Transiently after Single Sessions of Intense Endurance Exercise and Heavy Strength Training. PLoS ONE 2015, 10, E0121367. [Google Scholar] [CrossRef]
- Gentil, P.; Arruda, A.; Souza, D.; Giessing, J.; Paoli, A.; Fisher, J.; Steele, J. Is There Any Practical Application of Meta-Analytical Results in Strength Training? Front. Physiol. 2017, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, l4898. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.B. Estimating effect sizes from pretest-posttest-control group designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans—Results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Scharhag-Rosenberger, F.; Meyer, T.; Wegmann, M.; Ruppenthal, S.; Kaestner, L.; Morsch, A.; Hecksteden, A. Irisin does not mediate resistance training-induced alterations in resting metabolic rate. Med. Sci. Sports Exerc. 2014, 46, 1736–1743. [Google Scholar] [CrossRef]
- Kim, H.J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Z.; Qu, C.; Dong, Y. Effects of 12 weeks resistance training on serum irisin in older male adults. Front. Physiol. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari-Niaki, A.; Saeidi, A.; Ahmadian, M.; Gharahcholo, L.; Naghavi, N.; Fazelzadeh, M.; Mahjoub, S.; Myers, S.; Williams, A. The combination of exercise training and Zataria multiflora supplementation increase serum irisin levels in postmenopausal women. Integr. Med. Res. 2018, 7, 44–52. [Google Scholar] [CrossRef]
- Korkmaz, A.; Venojärvi, M.; Wasenius, N.; Manderoos, S.; Deruisseau, K.C.; Gidlund, E.K.; Heinonen, O.J.; Lindholm, H.; Aunola, S.; Eriksson, J.G.; et al. Plasma irisin is increased following 12 weeks of Nordic walking and associates with glucose homoeostasis in overweight/obese men with impaired glucose regulation. Eur. J. Sport Sci. 2019, 19, 258–266. [Google Scholar] [CrossRef]
- Amanat, S.; Sinaei, E.; Panji, M.; MohammadporHodki, R.; Bagheri-Hosseinabadi, Z.; Asadimehr, H.; Fararouei, M.; Dianatinasab, A. A Randomized Controlled Trial on the Effects of 12 Weeks of Aerobic, Resistance, and Combined Exercises Training on the Serum Levels of Nesfatin-1, Irisin-1 and HOMA-IR. Front. Physiol. 2020, 11, 562895. [Google Scholar] [CrossRef]
- Dianatinasab, A.; Koroni, R.; Bahramian, M.; Bagheri-Hosseinabadi, Z.; Vaismoradi, M.; Fararouei, M.; Amanat, S. The effects of aerobic, resistance, and combined exercises on the plasma irisin levels, HOMA-IR, and lipid profiles in women with metabolic syndrome: A randomized controlled trial. J. Exerc. Sci. Fit. 2020, 18, 168–176. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Ferreira, E.F.; Carneiro-Júnior, M.A.; Natalia, A.J.; Bressanc, J. Effects of exercise on the circulating concentrations of irisin in healthy adult individuals: A review. Sci. Sports 2016, 31, 251–260. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J.; So, B.; Son, J.S.; Yoon, D.; Song, W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: A pilot study. Physiol. Res. 2016, 65, 271–279. [Google Scholar] [CrossRef]
- Bang, H.S.; Seo, D.Y.; Chung, Y.M.; Oh, K.M.; Park, J.J.; Arturo, F.; Jeong, S.H.; Kim, N.; Han, J. Ursolic Acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J. Physiol. Pharmacol. 2014, 18, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef] [Green Version]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.S.; Kim, N.; Kong, I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef] [Green Version]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A. Circulating irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef] [Green Version]
- Buscemi, S.; Corleo, D.; Vasto, S.; Buscemi, C.; Massenti, M.F.; Nuzzo, D.; Lucisano, G.; Barile, A.M.; Rosafio, G.; Maniaci, V. Factors associated with circulating concentrations of irisin in the general population cohort of the ABCD study. Int. J. Obes. 2018, 42, 398–404. [Google Scholar] [CrossRef]
- Peterson, M.D.; Sen, A.; Gordon, P.M. Influence of resistance exercise on lean body mass in aging adults: A meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Bittar, S.T.; Maeda, S.S.; Marone, M.M.; Santili, C. Physical exercises with free weights and elastic bands can improve body composition parameters in postmenopausal women: WEB protocol with a randomized controlled trial. Menopause 2016, 23, 383–389. [Google Scholar] [CrossRef]
- Meng, N.H.; Li, C.I.; Liu, C.S.; Lin, C.H.; Chang, C.K.; Chang, H.W.; Yang, C.W.; Li, T.C.; Lin, C.C. Effects of concurrent aerobic and resistance exercise in frail and pre-frail older adults: A randomized trial of supervised versus home-based programs. Medicine 2020, 99, e21187. [Google Scholar] [CrossRef]
- Liang, H.; Xu, L.; Tian, X.; Wang, S.; Liu, X.; Dai, Y.; Kang, L.; Chen, L.; Jin, L.; Li, Q. The comparative efficacy of supervised- versus home-based exercise programs in patients with ankylosing spondylitis: A meta-analysis. Medicine 2020, 99, e19229. [Google Scholar] [CrossRef]
- Gentil, P.; Bottaro, M. Influence of supervision ratio on muscle adaptations to resistance training in nontrained subjects. J. Strength Cond Res. 2010, 24, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Gentil, P.; Bottaro, M. Effects of training attendance on muscle strength of young men after 11 weeks of resistance training. Asian J. Sports Med. 2013, 4, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Montes-Nieto, R.; Martínez-García, M.Á.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Differences in analytical and biological results between older and newer lots of a widely used irisin immunoassay question the validity of previous studies. Clin. Chem. Lab. Med. 2016, 54, e199–e201. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Q.; Huang, Y.; Yang, L.; Ruan, J.; Gu, W.; Zhang, X.; Zhang, Y.; Zhang, W.; Yu, Z. The effects of both age and sex on irisin levels in paired plasma and cerebrospinal fluid in healthy humans. Peptides 2019, 113, 41–51. [Google Scholar] [CrossRef]
- Albrecht, E.; Schering, L.; Buck, F.; Vlach, K.; Schober, H.C.; Drevon, C.A.; Maak, S. Irisin: Still chasing shadows. Mol. Metab. 2020, 34, 124–135. [Google Scholar] [CrossRef]
| Study, Year | Study Group | N (m/f) | Age (Years) a | BMI (kg/m2) a | D (Weeks) | F (Supervision) | Intervention | I | P | Irisin Measurement (Time Period b) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hecksteden et al., 2013 [35] | Exp | 17/23 | 48 ± 7.0 | 24.9 ± 3.4 | 26 | 3 (100%) | 8 machine-based exercises (2 s × 15 r) | 100% 20RM | No | Serum ELISA c (48 to 168 h) |
| Con | 13/26 | 50 ± 7.0 | 24.5 ± 3.1 | Not to change their previous lifestyle | ||||||
| Scharhag-Rosenberger et al., 2014 [36] | Exp | 17/20 | 47 ± 7.0 | 25.0 ± 3.4 | 24 | 3 (33%) | 8 machine-based exercises (2 s × 16/18/20 r in 6-wk cycle) | 100% 20RM | Yes (V) | Serum ELISA c (48 to 168 h) |
| Con | 12/25 | 50 ± 7.0 | 24.2 ± 3.2 | Not to change their previous lifestyle | ||||||
| Kim et al., 2015 [37] | Exp | 0/18 | 74.4 ± 2.9 | 25.3 ± 2.2 | 12 | 2 (100%) | elastic band training (2–3 s × 12–15 r) | BORG 12–13 | Yes (V/I) | Serum ELISA c (48 h) |
| Con | 0/14 | 76.0 ± 5.7 | 23.8 ± 3.0 | Not to change their lifestyle and perform stretching exercises once a week for one hour | ||||||
| Zhao et al., 2017 [38] | Exp | 10/0 | 62.3 ± 3.5 | NS | 12 | 3 (100%) | 6 machine-based exercises (2–4 s × 5–12 r) + 5 CORE exercises | 70–85% 1RM | Yes (I) | Serum ELISA d (NS) |
| Con | 7/0 | 61.9 ± 3.1 | NS | No exercise intervention | ||||||
| Ghanbari-Niaki et al., 2018 [39] | Exp | 0/12 | 58.0 ± 4.7 | 26.6 ± 3.1 | 8 | 3 (100%) | 12 exercise circuit training (2 s × 30″) | 55% 1RM | No | Serum ELISA e (48 h) |
| Con | 0/12 | 56.5 ± 4.2 | 27.9 ± 2.2 | Not to change their usual care | ||||||
| Korkmaz et al., 2019 [40] | Exp | 36/0 | 54 ± 6.1 | 30.3 ± 3.2 | 12 | 3 (100%) | 8 machine-based exercises + 6 bodyweight exercises | 85% 1RM | Yes (I) | Plasma ELISA c (NS) |
| Con | 40/0 | 54 ± 7.2 | 28.6 ± 3.0 | Not to change their lifestyle | ||||||
| Amanat et al., 2020 [41] | Exp | 0/14 | 54.5 ± 6.9 | 29.0 ± 2.9 | 12 | 3 (100%) | 10 machine-based exercises (2 s × 8–10 r) | 75–80% 1RM | Yes (V/I) | Serum ELISA c (24 h) |
| Con | 0/14 | 54.5 ± 6.9 | 29.1 ± 4.6 | Not to change their habitual physical activity | ||||||
| Dianatinasab et al., 2020 [42] | Exp | 0/13 | 53.5 ± 6.5 | 29.5 ± 2.9 | 8 | 3 (100%) | 10 machine-based exercises (2 s × 8–10 r) | 75–80% 1RM | Yes (V/I) | Serum ELISA c (24 h) |
| Con | 0/15 | 53.5 ± 6.5 | 30.4 ± 3.8 | Not to change their habitual physical activity | ||||||
| Study, Year | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Judgment |
|---|---|---|---|---|---|---|
| Hecksteden et al., 2013 [35] | LR | LR | LR | LR | LR | LR |
| Scharhag-Rosenberger et al., 2014 [36] | LR | LR | LR | SC | LR | SC |
| Kim et al., 2015 [37] | LR | LR | LR | SC | LR | SC |
| Zhao et al., 2017 [38] | LR | LR | LR | SC | LR | SC |
| Ghanbari-Niaki et al., 2018 [39] | LR | LR | LR | SC | LR | SC |
| Korkmaz et al., 2019 [40] | LR | LR | LR | SC | LR | SC |
| Amanat et al., 2020 [41] | LR | LR | LR | LR | LR | LR |
| Dianatinasab et al., 2020 [42] | LR | LR | LR | LR | LR | LR |
| Variable | Subgroup | Groups | n | Effect Size with 95% Confidence Interval | Test for Subgroup Effect a | Test for Subgroup Differences b |
|---|---|---|---|---|---|---|
| Age | Adults | 5 | 230 | 0.04 [−0.64, 0.73] | p = 0.91, I2 = 83.0% | p < 0.001 I2 = 93.6% |
| Older adults | 2 | 49 | 2.01 [1.31, 2.70] | p < 0.001, I2 = 0.0% | ||
| Gender | Male | 1 | 17 | 1.98 [0.81, 3.15] | p < 0.001 I2 = NA | p = 0.17 I2 = 47.3% |
| Female | 4 | 112 | 0.96 [0.11, 1.82] | p = 0.03, I2 = 78.0% | ||
| Change in body weight (kg) | Increase | 0 | 0 | Not estimable | Not applicable | p = 0.36 I2 = 0.0% |
| Same | 3 | 131 | 0.40 [−1.13, 1.93] | p = 0.61, I2 = 93.0% | ||
| Decrease | 3 | 69 | 1.20 [0.44, 1.95] | p = 0.002, I2 = 51.0% | ||
| Change in BMI (kg/m2) | Increase | 0 | 0 | Not estimable | Not applicable | p = 0.93 I2 = 0.0% |
| Same | 2 | 60 | 1.00 [−0.97, 2.97] | p = 0.32, I2 = 92.0% | ||
| Decrease | 2 | 52 | 0.91 [0.23, 1.59] | p = 0.009, I2 = 28.0% | ||
| Change in body fat (%) | Increase | 0 | 0 | Not estimable | Not applicable | p < 0.001 I2 = 92.3% |
| Same | 2 | 99 | −0.42 [−1.13, 0.30] | p = 0.25, I2 = 63.0% | ||
| Decrease | 4 | 101 | 1.42 [0.72, 2.12] | p < 0.001, I2 = 59.0% | ||
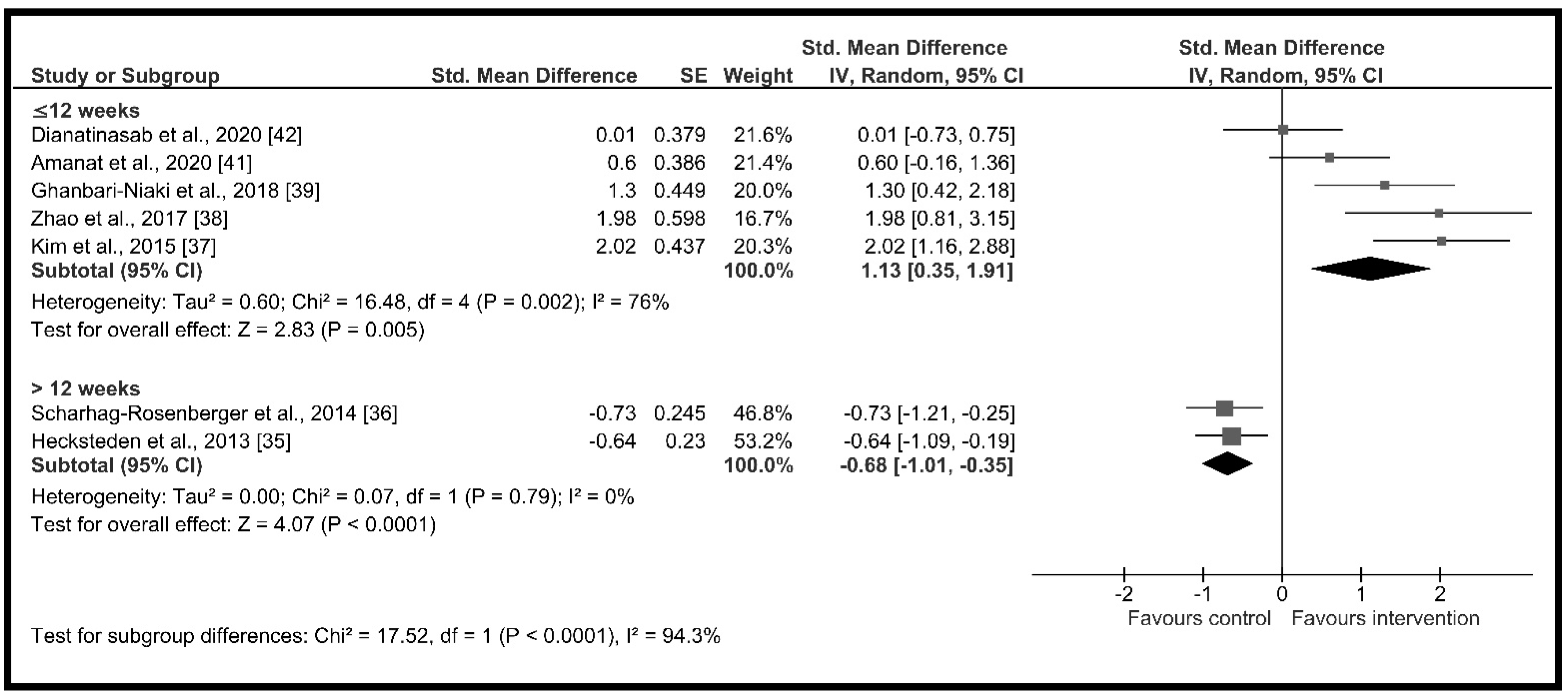
| Intervention duration (weeks) | ≤12 weeks | 5 | 129 | 1.13 [0.35, 1.91] | p = 0.005, I2 = 76.0% | p < 0.001 I2 = 94.3% |
| >12 weeks | 2 | 150 | −0.68 [−1.01, −0.35] | p < 0.001, I2 = 0.0% | ||
| Training intensity | ≤60% | 3 | 174 | −0.11 [−1.07, 0.86] | p = 0.83, I2 = 88.0% | p = 0.09 I2 = 65.9% |
| 61 to 85% | 4 | 105 | 1.10 [0.11, 2.09] | p = 0.03, I2 = 81.0% | ||
| >85% | 0 | 0 | Not estimable | Not applicable | ||
| Progression in intensity | No | 3 | 174 | −0.11 [−1.07, 0.86] | p = 0.83, I2 = 88.0% | p = 0.09 I2 = 65.9% |
| Yes | 4 | 105 | 1.10 [0.11, 2.09] | p = 0.03, I2 = 81.0% | ||
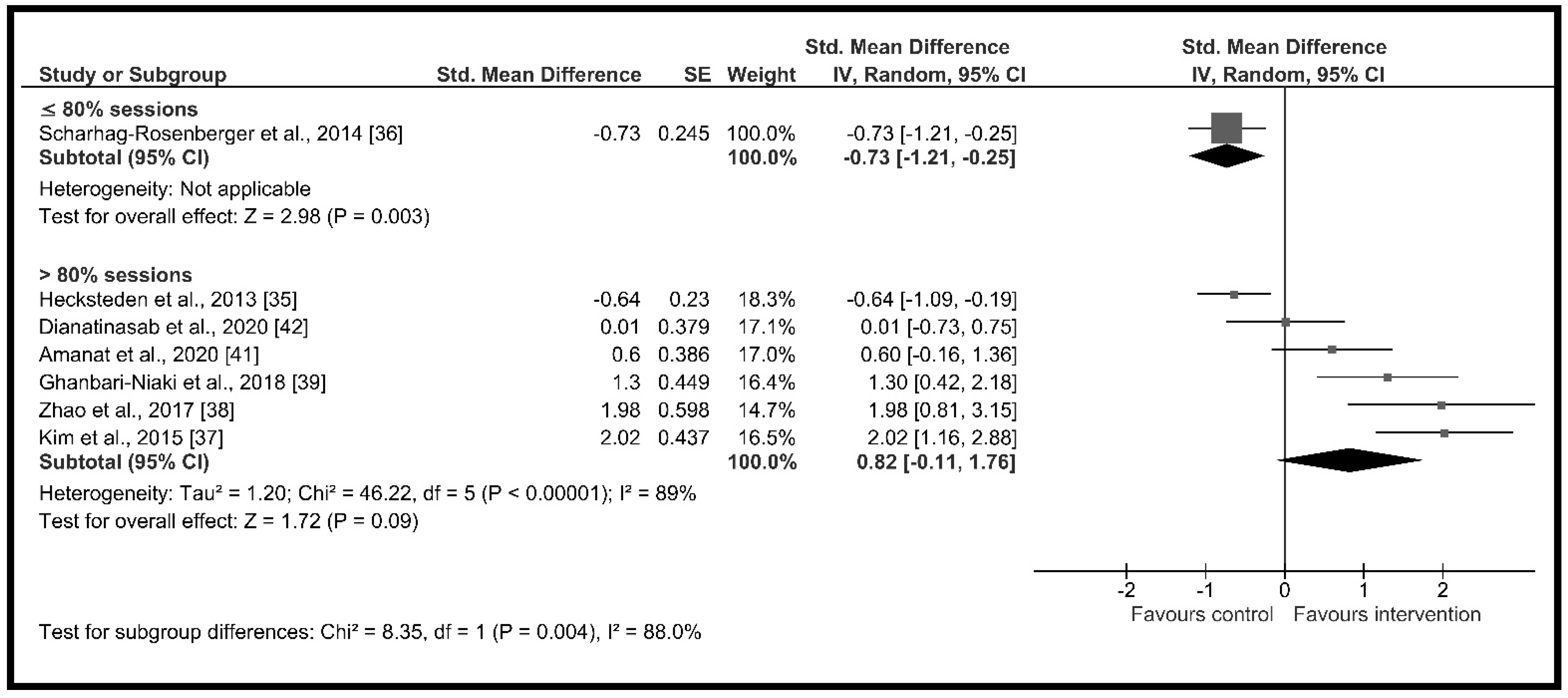
| Supervised sessions (%) | ≤80% | 1 | 71 | −0.73 [−1.21, −0.25] | p = 0.003, I2 = NA | p = 0.004 I2 = 88.0% |
| >80% | 6 | 208 | 0.82 [−0.11, 1.76] | p = 0.09, I2 = 89.0% | ||
| Risk of bias | Low risk | 3 | 135 | −0.06 [−0.81, 0.69] | p = 0.88, I2 = 76.0% | p = 0.19 I2 = 42% |
| Some concerns | 4 | 144 | 1.10 [−0.46, 2.67] | p = 0.17, I2 = 93.0% | ||
| Journal rank | Q1 | 5 | 227 | 0.57 [−0.49, 1.63] | p = 0.29, I2 = 92.0% | p = 0.09 I2 = 58.5% |
| Q2 | 1 | 24 | 1.30 [0.42, 2.18] | p = 0.004, I2 = NA | ||
| Q3 | 0 | 0 | Not estimable | Not applicable | ||
| Q4 | 1 | 28 | 0.01 [−0.73, 0.75] | p = 0.98, I2 = NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosio, P.L.; Crespo-Posadas, M.; Velarde-Sotres, Á.; Pelaez, M. Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 2476. https://doi.org/10.3390/ijerph18052476
Cosio PL, Crespo-Posadas M, Velarde-Sotres Á, Pelaez M. Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2021; 18(5):2476. https://doi.org/10.3390/ijerph18052476
Chicago/Turabian StyleCosio, Pedro L., Manuel Crespo-Posadas, Álvaro Velarde-Sotres, and Mireia Pelaez. 2021. "Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Environmental Research and Public Health 18, no. 5: 2476. https://doi.org/10.3390/ijerph18052476
APA StyleCosio, P. L., Crespo-Posadas, M., Velarde-Sotres, Á., & Pelaez, M. (2021). Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health, 18(5), 2476. https://doi.org/10.3390/ijerph18052476








