Saprochaete clavata Infection in Immunosuppressed Patients: Systematic Review of Cases and Report of the First Oral Manifestation, Focusing on Differential Diagnosis
Abstract
1. Introduction
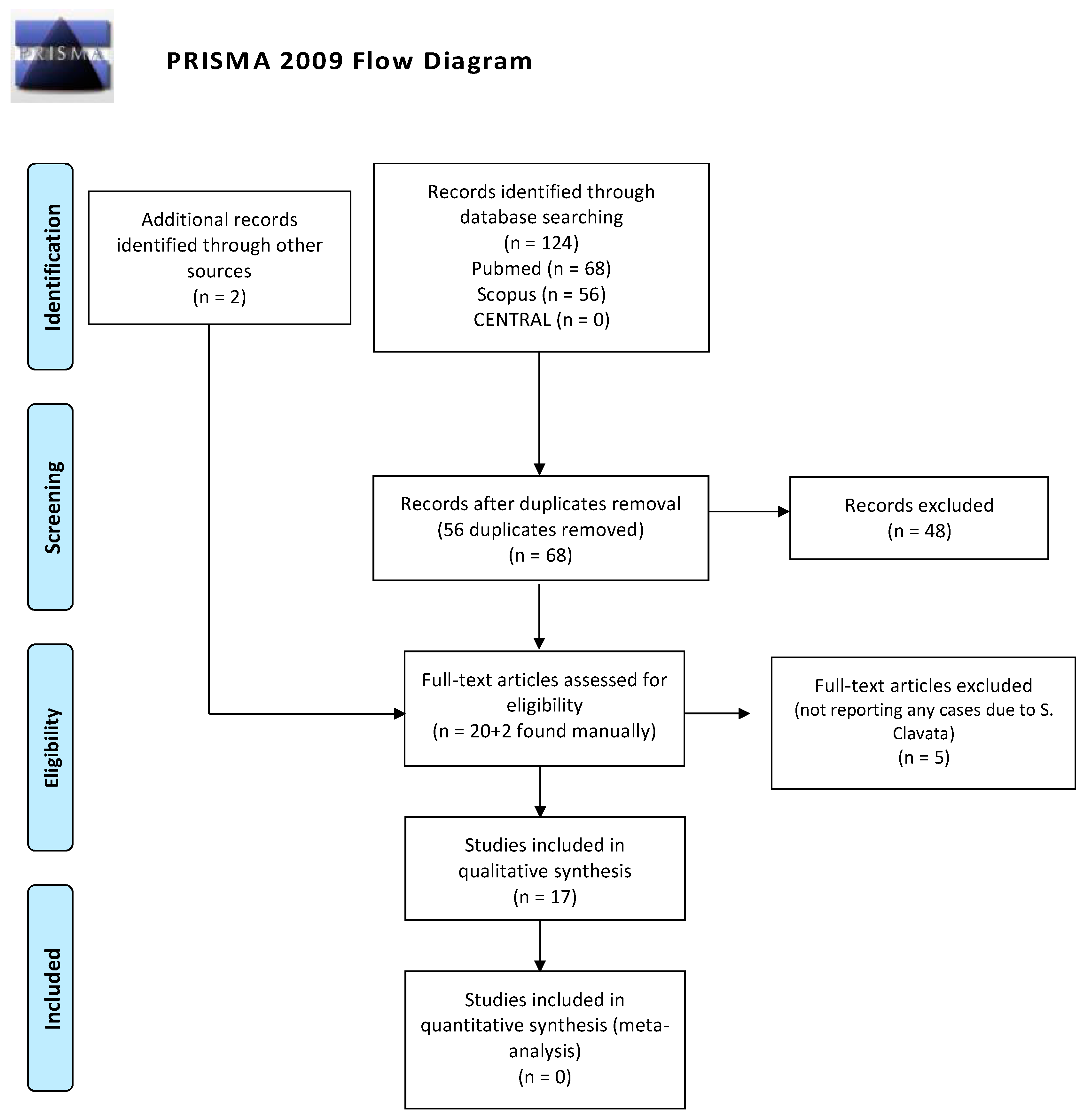
2. Methods
3. Results
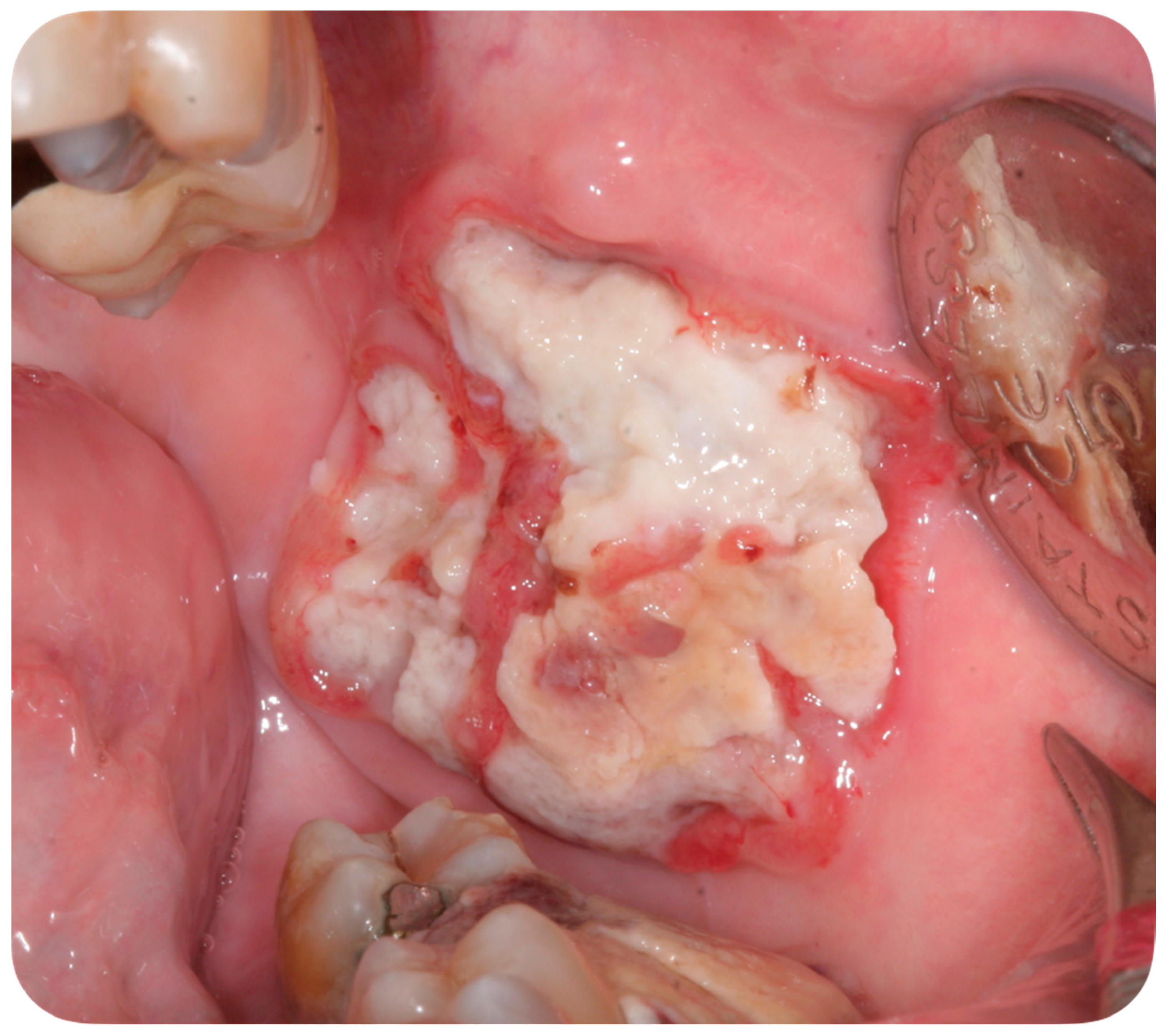
4. Case Report
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A new broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Bicanic, T.; Brown, G.D.; Hoving, J.C.; Meintjes, G.; Nielsen, K. AIDS-related mycoses: Current progress in the field and future priorities. Trends Microbiol. 2017, 26, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Pagano, L.; Martino, B.; D’Antonio, D.; Fanci, R.; Specchia, G.; Melillo, L.; Buelli, M.; Pizzarelli, G.; Venditti, M.; et al. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematologic Malignancies: A Retrospective Multicenter Study from Italy and Review of the Literature. J. Clin. Microbiol. 2005, 43, 1818–1828. [Google Scholar] [CrossRef]
- Del Principe, M.; Sarmati, L.; Cefalo, M.; Fontana, C.; De Santis, G.; Buccisano, F.; Maurillo, L.; De Bellis, E.; Postorino, M.; Sconocchia, G.; et al. A cluster of Geotrichum clavatum (Saprochaete clavata) infection in haematological patients: A first Italian report and review of literature. Mycoses 2016, 59, 594–601. [Google Scholar] [CrossRef]
- Deepa, A.; Nair, B.; Sivakumar, T.; Joseph, A. Uncommon opportunistic fungal infections of oral cavity: A review. J. Oral Maxillofac. Pathol. 2014, 18, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Pottier, I.; Gente, S.; Vernoux, J.; Guéguen, M. Safety assessment of dairy microorganisms: Geotrichum candidum. Int. J. Food Microbiol. 2008, 126, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.; Lee, S. Emerging moulds: Epidemiological trends and antifungal resistance. Mycoses 2011, 54, e666–e678. [Google Scholar] [CrossRef]
- Gadea, I.; Cuenca, E.M.; Prieto, E. Genotyping and antifungal susceptibility profile of Dipodascus capitatus isolates causing disseminated infection in seven hematological patients of a tertiary hospital. J. Clin. Microbiol. 2004, 42, 1832–1836. [Google Scholar] [CrossRef]
- Vaux, S.; Criscuolo, A.; Desnos Ollivier, M.; Diancourt, L.; Tarnaud, C.; Vandenbogaert, M.; Brisse, S.; Coignard, B.; Dromer, F. Geotrichum Investigation Group. Multicenter outbreak of infections by Saprochaete clavata, an unrecognized opportunistic fungal pathogen. Mbio 2014, 5, 02309–02314. [Google Scholar] [CrossRef]
- Stanzani, M.; Cricca, M.; Sassi, C.; Sutto, E.; De Cicco, G.; Bonifazi, F.; Bertuzzi, C.; Bacci, F.; Paolini, S.; Cavo, M.; et al. Saprochaete clavata infections in patients undergoing treatment for haematological malignancies: A report of a monocentric outbreak and review of the literature. Mycoses 2019, 62, 1100–1107. [Google Scholar] [CrossRef]
- El Zein, S.; Hindy, J.; Kanj, S. Invasive Saprochaete Infections: An Emerging Threat to Immunocompromised Patients. Path-Ogens 2020, 9, 922. [Google Scholar] [CrossRef]
- Favre, S.; Rougeron, A.; Levoir, L.; Pérard, B.; Milpied, N.; Accoceberry, I.; Gabriel, F.; Vigouro, S. Saprochaete clavata invasive infection in a patient with severe aplastic anemia: Efficacy of voriconazole and liposomal amphotericin B with adjuvant granulocyte transfusions before neutrophil recovery following allogeneic bone marrow transplantation. Med. Mycol. Case Rep. 2016, 11, 21–23. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Guinea, J.; Puig Asensio, M.; Zaragoza, Ó.; Almirante, B.; Cuenca-Estrella, M.; Aguado, J.M.; CANDIPOP Project, GEIH-GEMICOMED (SEIMC) and REIPI. Fungemia due to rare opportunistic yeasts: Data from a population-based surveil-lance in Spain. Med. Mycol. 2017, 55, 125–136. [Google Scholar] [CrossRef]
- Camus, V.; Thibault, M.; David, M.; Gargala, G.; Compagnon, P.; Lamoureux, F.; Girault, C.; Michot, J.; Stamatoullas, A.; Lanic, H.; et al. Invasive Geotrichum clavatum fungal infection in an acute myeloid leukaemia patient: A case report and review. Mycopathologia 2014, 177, 319–324. [Google Scholar] [CrossRef]
- Lacroix, C.; Brethon, B.; Boissel, N.; Desnos, M.; Leblanc, T.; Raffoux, E. Geotrichum clavatum an emerging pathogen responsible for invasive infection in two neutropenic leukemia patients. J. Chemother. 2007, 19, 81. [Google Scholar]
- Picard, M.; Cassaing, S.; Letocart, P.; Verdeil, X.; Protin, C.; Chauvin, P.; Iriart, X.; Cavalié, L.; Valentin, A.; Marchou, B.; et al. Concomitant cases of disseminated Geotrichum clavatum infections in patients with acute myeloid leukemia. Leuk. Lymphoma 2014, 55, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Júnior, J.; Sztajnbok, J.; da Silva, A.; Vieira, V.; Galastri, A.; Bissoli, L.; Litvinov, N.; del Negro, G.M.B.; Motta, A.L.; Rossi, F.; et al. Rapid identification of moulds and arthroconidial yeasts from positive blood cultures by MALDI-TOF mass spectrometry. Med. Mycol. 2016, 54, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Esposto, M.; Prigitano, A.; Lo Cascio, G.; Ossi, C.; Grancini, A.; Cavanna, C.; Lallitto, F.; Tejada, M.; Bandettini, R.; Mularoni, A.; et al. Yeast-like filamentous fungi: Molecular identification and in vitro susceptibility study. Med. Mycol. 2018, 57, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, W.; Jiang, Y.; Kuang, L. Invasive fungal infection caused by Geotrichum clavatum in an acute leukemia child: First documented case from mainland China. JPN J. Infect. Dis. 2018, 72, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Salguero Fernández, I.; Nájera Botello, L.; Orden Martinez, B.; Roustan Gullón, G. Disseminated fungemia by Saprochaete clavata. Enferm. Infecc. Microbiol. Clin. 2018, 37, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Leoni, M.; Riccardi, N.; Rotulo, G.A.; Godano, E.; Faraci, M.; Bandettini, R.; Esposto, M.C.; Castagnola, E. Magnusiomyces clavatus infection in a child after allogeneic hematotopoetic stem cell transplantation: Diagnostic and therapeutic implications. Med. Mycol. Case Rep. 2018, 23, 65–67. [Google Scholar] [CrossRef]
- Buchta, V.; Bolehovská, R.; Hovorková, E.; Cornely, O.; Seidel, D.; Žák, P. Saprochaete clavata Invasive Infections—A New Threat to Hematological-Oncological Patients. Front Microbiol. 2019, 10, 2196. [Google Scholar] [CrossRef] [PubMed]
- Pavone, P.; Oliva, A.; Raponi, G.; Pugliese, F.; Martelli, S.; Celli, P.; Saccoa, F.; Vullo, V.; Mastroianni, C.M.; Russo, G. Disseminated fungal infection due to Saprochaete clavata in a kidney transplant recipient. J. Mycol. Med. 2019, 3, 278–281. [Google Scholar] [CrossRef]
- Wee, L.; Ling, H.; Chong, C.; Soe, M.; Koh, M. A rare case of purpuric rash caused by Saprochaete clavata in a pediatric patient with acute leukemia. Pediatr. Dermatol. 2019, 6, 990–991. [Google Scholar] [CrossRef] [PubMed]
- Lo Cascio, G.; Vincenzi, M.; Soldani, F.; De Carolis, E.; Maccacaro, L.; Sorrentino, A.; Nadali, G.; Cesaro, S.; Sommavilla, M.; Niero, V. Outbreak of Saprochaete clavata Sepsis in Hematology Patients: Combined Use of MALDI-TOF and Sequencing Strategy to Identify and Correlate the Episodes. Front. Microbiol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Menu, E.; Criscuolo, A.; Desnos-Ollivier, M.; Cassagne, C.; D’Incan, E.; Furst, S.; Ranque, S.; Berger, P.; Dromer, F. Saprochaete clavata Outbreak Infecting Cancer Center through Dishwasher. Emerg. Infect. Dis. 2020, 26, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 7, E246–E247. [Google Scholar] [CrossRef] [PubMed]
- Durán, G.; Seidel, D.; Vehreschild, M.; Hamprecht, A.; Kindo, A.; Raci, Z.; Demeter, J.; de Hoog, S.; Aurbach, U.; Ziegler, M.; et al. Invasive infections due to Saprochaete and Geotrichum species: Report of 23 cases from the FungiScope Registry. Mycoses 2017, 60, 273–279. [Google Scholar] [CrossRef]
- Camera, A.; Andretta, C.; Villa, M.; Volpicelli, M.; Picardi, M.; Rossi, M.; Rinaldi, C.R.; della Cioppa, P.; Ciancia, R.; Selleri, C.; et al. Intestinal toxicity during induction chemotherapy with cytarabine-based regimens in adult acute myeloid leukemia. Hematol. J. 2003, 4, 346–350. [Google Scholar] [CrossRef]
- Martino, R.; Salavert, M.; Parody, R.; Tomás, J.; Vázquez, L.; Jarque, I.; Prieto, E.; Sastre, J.L.; Gadea, I.; Pemán, J.; et al. Blastoschizomyces capitatus infection in patients with leukemia: Report of 26 cases. J. Clin. Infect. Dis. 2004, 38, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Schuermans, C.; van Bergen, M.; Coorevits, L.; Verhaegen, J.; Lagrou, K.; Surmont, I.; Jeurissen, A. Breakthrough Saprochaete capitata infections in patients receiving echinocandins: Case report and review of the literature. Med. Mycol. 2011, 49, 414–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arendrup, M.; Boekhout, T.; Akova, M.; Meis, J.; Cornely, O. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar] [CrossRef]
- Muriel, P.; Sophie, C.; Philippe, L.; Xavier, V.; Caroline, P.; Pamela, C. Concomitant cases of disseminated Geotrichum clavatum infections. Leuk. Lymphoma 2014, 55, 1029–2403. [Google Scholar]
- Naples, J.; Martin, A.; Sobelman, D.; Schoem, S. Unusual Fungal Lesion Presenting as a Neoplastic Pediatric Tongue Mass. Pediatrics 2017, 139, e20161345. [Google Scholar] [CrossRef]
- Jefferies, S.D.; Ord, R.A. An unusual presentation of oral syphilis. Br. J. Oral Maxillofac. Surg. 1985, 23, 376–380. [Google Scholar] [CrossRef]
- Forrestel, A.K.; Kovarik, C.L.; Katz, K.A. Sexually acquired syphilis: Laboratory diagnosis, management, and prevention. J. Am. Acad. Dermatol. 2020, 82, 17–28. [Google Scholar] [CrossRef]
- Sharma, S.; Bajpai, J.; Pathak, P.K.; Pradhan, A.; Singh, P.; Kant, S. Oral tuberculosis-Current concepts. J. Family Med. Prim. Care 2019, 8, 1308–1312. [Google Scholar] [PubMed]
- Kaplan, I.; Anavi, K.; Anavi, Y.; Calderon, S.; Schwartz-Arad, D.; Teicher, S.; Hirshberg, A. The clinical spectrum of Actinomyces-associated lesions of the oral mucosa and jaw-bones: Correlation with histomorphometric analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 738–746. [Google Scholar] [CrossRef]
- Alamillos-Granados, F.J.; Dean-Ferrer, A.; García-López, A.; López-Rubio, F. Actinomycotic ulcer of the oral mucosa: An unusual presentation of oral actinomycosis. Br. J. Oral Maxillofac. Surg. 2000, 38, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Balakrishna, J.P.; Pittaluga, S.; Jaffe, E.S. Diagnosis of Hodgkin lymphoma in the modern era. Br. J. Haematol. 2019, 184, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.D.; Ferreira, C.B.; Leite, G.B.; de Menezes Pontes, J.R.; Antunes, H.S. Oral manifestations of lymphoma: A systematic review. Ecancermedicalscience 2016, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Mashhadiabbas, F.; Ahsaie, M.G. Diagnostic Biomarkers in Oral Verrucous Carcinoma: A Systematic Review. Pathol. Oncol. Res. 2017, 23, 19–32. [Google Scholar] [CrossRef]
- Sonalika, W.G.; Anand, T. Oral verrucous carcinoma: A retrospective analysis for clinicopathologic features. J. Cancer Res. Ther. 2016, 12, 142–145. [Google Scholar] [CrossRef]
- Almouhawis, H.A.; Leao, J.C.; Fedele, S.; Porter, S.R. Wegener’s granulomatosis: A review of clinical features and an update in diagnosis and treatment. J. Oral Pathol. Med. 2013, 42, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Wu, Y.Q.; Ruan, W.H. A rare case of pediatric Crohn’s disease and alveolar bone loss: A report and review. Transl. Pediatr. 2020, 9, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Laube, R.; Liu, K.; Schifter, M.; Yang, J.L.; Suen, M.K.; Leong, R.W. Oral and upper gastrointestinal Crohn’s disease. J. Gastroenterol. Hepatol. 2018, 33, 355–364. [Google Scholar] [CrossRef]
- Badhey, A.K.; Kadakia, S.; Carrau, R.L.; Iacob, C.; Khorsandi, A. Sarcoidosis of the head and neck. Head Neck Pathol. 2015, 9, 260–268. [Google Scholar] [CrossRef]
- Dhawan, S.R.; Saini, A.G.; Singhi, P.D. Management Strategies of Melkersson-Rosenthal Syndrome: A Review. Int. J. Gen. Med. 2020, 13, 61–65. [Google Scholar] [CrossRef]
- Hartwick, R.; Batsakis, J. Sinus aspergillosis and allergic fungal sinusitis. Ann. Otol. Rhinol. Laryngol. 1991, 100, 427–430. [Google Scholar] [CrossRef]
- Dreizen, S.; Keating, M.; Beran, M. Orofacial fungal infections. Nine pathogens that may invade during chemotherapy. Postgrad. Med. 1992, 91, 349–364. [Google Scholar] [CrossRef]
- Benson-Mitchell, R.; Tolley, N.; Croft, C.; Gallimore, A. Aspergillosis of the larynx. J. Laryngol. Otol. 1994, 108, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Martínez, E.; Ruiz-Gaitán, A.; Pemán-García, J. Update on the diagnosis of invasive fungal infection. Rev. Esp. Quimioter. 2017, 30, 16–21. [Google Scholar]
- Latgé, J. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Denning, D. Invasive aspergillosis. Clin. Infect. Dis. 1998, 26, 781–803. [Google Scholar] [CrossRef]
- Dreizen, S. Oral complications of cancer therapies. Description and incidence of oral complications. NCI Monogr. 1990, 9, 11–15. [Google Scholar]
- Cano, M.; Hajjeh, R. The epidemiology of histoplasmosis: A review. Sem. Resp. Inf. 2001, 16, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Johnson, P.; Sarosi, G. Fungal pneumonias. The endemic mycoses. Clin. Chest Med. 1999, 20, 507–519. [Google Scholar] [CrossRef]
- Folk, G.A.; Nelson, B.L. Oral Histoplasmosis. Head Neck Pathol. 2017, 11, 513–516. [Google Scholar] [CrossRef]
- Fitzpatrick, S.; Cohen, D.; Clark, A. Ulcerated Lesions of the Oral Mucosa: Clinical and Histologic Review. Head Neck Pathol. 2019, 13, 91–102. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, E.; Morán-Estefanía, M.; López-Avila, A.; Piris, J.; Fernández-Blasco, G.; García, J.I.; Armijo, M. Clinical expression of secondary syphilis in a patient with HIV infection. J. Dermatol. 1994, 21, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ali, M.; Adegbite, N.; Vaidhyanath, R.; Avery, C. Actinomycosis of tongue: Rare presentation mimicking malignancy with literature review and imaging features. Radiol. Case Rep. 2019, 14, 190–194. [Google Scholar] [CrossRef]
- Valour, F.; Sénéchal, A.; Dupieux, C.; Karsenty, J.; Lustig, S.; Breton, P.; Gleizal, A.; Boussel, L.; Laurent, F.; Braun, E.; et al. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Resist. 2014, 14, 183–189. [Google Scholar]
- Verma, A.; Stock, W.; Lait, M.; Ferrer, K.; Quinn, J.; Platanias, L. Actinomycosis presenting as an oral ulcer in a neutropenic patient. South Med. J. 2002, 95, 1105. [Google Scholar] [CrossRef]
- Marcoval, J.; Penín, R.M. Histopathological Features of Orofacial Granulomatosis. Am. J. Dermatopathol. 2016, 38, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Van Assche, G.; Lindsay, J.O.; Colombel, J.F.; Danese, S.; D’Hoore, A.; Gassull, M.; Gomollón, F.; Hommes, D.W.; Michetti, P.; et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Current management. J. Crohn’s Colitis 2010, 4, 28–62. [Google Scholar] [CrossRef] [PubMed]


| Study | N. of Cases | Country | Gender | Age | Underlying Disease | Clinical Form | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Lacroix et al., 2007 [15] | 2 | France | M | 14 | AML | Disseminated | VCZ, Amph | Survived |
| M | 59 | AML | Disseminated | PCZ, Amph, 5-F | Survived | |||
| Picard et al., 2014 [16] | 3 | France | F | 46 | AML | Disseminated | VCZ, Amph | Died |
| M | 70 | AML | Disseminated, Pulmonary | Caspo | Died | |||
| F | 63 | AML | Disseminated | VCZ, Amph | Died | |||
| Vaux et al., 2014 [9] | 30 | France | M (15), F (15) | 63 (mean) | AML (21), ALL (6), Other (3) | Disseminated (26), Pulmonary (12), Diarrhea (18) | Ns | Died (24), Survived (6) |
| Camus et al., 2014 [14] | 1 | France | M | 32 | AML | Disseminated, Peritonitis, Hepatic Lesions | VCZ | Survived |
| Del Principe et al., 2016 [4] | 3 | Italy | F | 36 | AML | Pulmonary, Cholecystitis, Hepatosplenic abscesses | VCZ, Amph | Survived |
| F | 50 | Lymphoma | Disseminated, Pulmonary, Splenic infiltrated | VCZ, Amph | Died | |||
| M | 21 | AML | Splenic Abscesses | VCZ, Amph | Survived | |||
| Favre et al., 2016 [12] | 1 | France | M | 27 | AA | Disseminated | VCZ, Amph | Survived |
| De Almeida et al., 2016 [17] | 1 | Brazil | F | 6 | Other | Ns | VCZ, Amph | Died |
| 18 | Italy | M (11) F (6) Ns (1) | Ns | AML (8), Lymphoma (3), AA (2), Other (3), Ns (2) | Disseminated (18) | Ns | ||
| Esposto et al., 2018 [18] | Ns | |||||||
| Liu et al., 2018 [19] | 1 | China | M | 10 | ALL | Disseminated, Pulmonary | VCZ, Mica, Amph | Survived |
| Salguero-Fernandez et al., 2018 [20] | 1 | Spain | M | 47 | Lymphoma | Disseminated, Skin | Amph, 5-F | Died |
| Leoni et al., 2018 [21] | 1 | Italy | M | 6 | Other | Disseminated, Pulmonary, Skin, Renal | VCZ, Amph | Survived |
| Buchta et al., 2019 [22] | 11 | Czechia | M | 45 | AML | Disseminated | VCZ, Amph | Died |
| Czechia | F | 61 | AML | Disseminated | Amph | Died | ||
| Czechia | F | 63 | AML | Disseminated | VCZ, Amph | Survived | ||
| Czechia | F | 58 | AML | Disseminated, Pulmonary | VCZ, Amph | Died | ||
| Czechia | F | 50 | AML | Disseminated, Pulmonary | Amph | Died | ||
| Czechia | F | 66 | Lymphoma | Disseminated | VCZ, Mica | Died | ||
| Turkey * | F | 37 | AML | Disseminated | VCZ | Survived | ||
| Israel * | F | 17 | AML | Disseminated, Liver, Spleen, CNS | VCZ, Amph, 5-F | Survived | ||
| Spain * | M | 48 | Lymphoma | Disseminated, CNS, Liver, Pulmonary, Spleen | VCZ, Amph, 5-F | Died | ||
| Germany * | M | 55 | AML | Disseminated | VCZ, Amph | Survived | ||
| Serbia * | M | 19 | ALL | Disseminated, Pulmonary | Caspo | Died | ||
| Pavone et al., 2019 [23] | 1 | Italy | F | 54 | PCKD | Disseminated, Peritonitis | VCZ, Amph | Died |
| Wee et al., 2019 [24] | 1 | Singapore | M | 13 | ALL | Disseminated, Kidney, Liver, Skin | VCZ, Amph | Survived |
| Stanzani et al., 2019 [10] | 4 | Italy | M | 66 | Lymphoma | Pulmonary | VCZ | Survived |
| F | 48 | AML | Disseminated, Pulmonary, Liver, Spleen, Kidney | VCZ, Amph | Survived | |||
| M | 34 | Lymphoma | Disseminated | VCZ | Survived | |||
| M | 64 | AML | Disseminated, Pulmonary, Spleen, CNS | VCZ, Amph | Died | |||
| Lo Cascio et al., 2020 [25] | 7 | Italy | M (6) F (1) | 41.1 (mean) | AML (5), ALL (1), Lymphoma (1) | Disseminated (7), Diarrhea (3) | Amph (3), Echi (2), AZ (2) | Died (3) Survived (4) |
| Menu et al., 2020 [26] | 9 | France | M (6) F (3) | 57.8 (mean) | AML (4), Lymphoma (2), ALL (1), Other (2) | Disseminated (8), Pulmonary (3), Diarrhea (3) Digestive Symptoms (2) | VCZ (4), PCZ (3), Echi (2) | Died (5) Survived (4) |
| This study | 1 | Italy | M | 56 | AML | Oral Lesions | VCZ | Survived |
| Age | |
|---|---|
| Years | 51.8 (mean) |
| Gender | |
| Male | 57 |
| Female | 38 |
| Non-Specified | 1 |
| Country | Tot 96 |
| France | 46 |
| Italy | 35 |
| Czech | 6 |
| Other Countries (Spain, Germany, Brazil, Turkey, Israel, Serbia, China, Singapore) | 9 |
| Outcome | Total Number (mortality rate *) |
| Death | 47 (60.2%) |
| Survival | 31 (38.8%) |
| Clinical Entity | Etiology and Pathogenesis | Clinical Manifestation | Diagnosis | Reason for Exclusion during the Diagnostic Flow-Chart | ||
|---|---|---|---|---|---|---|
| Usual Features | Unusual Oral Presentation | Site | ||||
| Opportunistic Infections | ||||||
| Candidiasis [34] | C. Albicans and other C. spp. | Pseudomembranous Candidiasis: white, soft plaques, removable, sometimes burning sensation and altered taste. Acute erythematous candidiasis: Erythema, usually painful | Rapidly growing exophytic lesions | Buccal mucosa Palate Dorsal tongue | Microbiological culture | Usually do not comprise rapidly growing asymptomatic ulcerated swellings |
| Aspergillosis | Aspergillus spp. | Oral manifestations do not arise in immunocompetent hosts | Invasive form: swelling, ulceration, necrosis, usually painful | Paranasal sinuses Oropharynx Palate Dorsal Tongue | Microbiological culture | (a) Uncommon location of the lesions (b) Absence of symptoms (c) Microbiological culture |
| Histoplasmosis | H. Capsulatum | Histoplasmosis of the head and neck is rarely seen in immunocompetent patients | Rapidly growing asymptomatic oral ulceration with firm margins, single or multiple, nonhealing. | Tongue, Palate, Buccal mucosa | Microbiological culture and pathology | (a) Oral Histoplasmosis usually does not appear as a friable swelling (b) Medical history: no travels in US (c) Microbiological culture and pathology |
| Syphilis [35,36] | T. Pallidum | Primary syphilis indurated ulcer, asymptomatic. Secondary syphilis: red rash characterized by maculopapular areas, oral ulcers covered by membrane, or condyloma lata | Vascular proliferation, multiple ulcerated areas, hemorrhage | Lips, but any other site can be involved | Direct detection of T. pallidum and serologic testing (treponemal and non-treponemal tests, i.e., TPHA) | (a) Negative TPHA (b) Absence of characteristic systemic symptoms (secondary syphilis) (c) Pathology (absence of proliferative endarteritis and infiltration of plasma cells) |
| Tuberculosis [37] | Mycobacterium Tuberculosis | Oral lesions are uncommon and occur due to infected sputum or hematogenous spread | Chronic and indurated ulcer, non-healing extraction sockets, osteomyelitis, and mandibular swellings. | Tongue and palate but any mucosal surface can be involved. Bone of maxilla or mandible | Molecular tests (nucleic acid amplification test—Xpert MTB/RIF), Pathology, microbiological tests (mycobacterial growth indicator tube—MGIT) | (a) Pathology (absence of typical caseous tuberculous granulomas) (b) Clinical presentation: Tuberculosis oral lesions usually have an “infiltrating” feature more than exophytic |
| Actinomycosis [38,39] | Actinomyces spp. | Fibrosis, swellings, cutaneous draining sinus tracts | Ulcerations of the tongue and osteomyelitis. One reported case of involvement of floor of the mouth and buccal mucosa | Mandible and surrounding tissues. Salivary glands | Microbiological culture and pathology | Microbiological culture and pathology (absence of granulomatous inflammatory response) |
| Malignant Diseases | ||||||
| Lymphomas [40,41,42] | Heterogeneous malignant disease of the lymphatic system | Hodgkin’s lymphomas: very rare in the oral cavity. Non-Hodgkin’s lymphomas: rapidly growing asymptomatic ulcerated swellings, bone resorption, or bone loss | Pathologic fracture. Pain, numbness of the lip. | Tonsils, Salivary glands, maxilla, base of the tongue, Soft palate | Histopathological examination (presence of Reed-Stemberg cells for Hodgkin’s lymphomas), immunophenotyping, flow cytometry | Pathology and immunohistochemistry according to the lymphoma type |
| Verrucous Carcinoma [43,44] | Exophytic variant of oral squamous cell carcinoma | Plaque-like or exophytic mass typically white, with a warty, ulcerated, or papillary surface. Slow continuous growth rate. Usually painless | Bone involvement (especially if invasive transformation occurs) | Buccal mucosa, Gingiva | Pathology (well-differentiated epithelial cells mass extending into the connective tissue generally with a pushing appearance) | (a) Pathology (b) Rapid onset (c) Multiple lesions |
| Orofacial Granulomatosis | ||||||
| Wegener Granulomatosis [45] | Rare immune-based inflammatory necrotizing vasculitis of unknown cause | Strawberry gingivitis, sinusitis, oral ulceration | Facial paralysis, Labial mucosal nodules, Necrosis and perforation of the nasal septum or palate, Swelling and desquamation of the lips, Salivary gland enlargement, Arthralgia of the TMJ Tongue involvement | Gingiva but any mucosal surface can be involved. Rarely major salivary glands | Pathology Clinical diagnostic criteria Presence of proteinase-3 antineutrophil cytoplasm antibodies (PR3-ANCA) myeloperoxidase antineutrophil cytoplasm antibodies (MPO-ANCA) | (a) Pathology (absence of granulomatous lesions, with necrotizing vasculitis) (b) Absence of clinical diagnostic criteria (Oral ulcerations or nasal discharge, nodules on chest radiograph, abnormal urinary sediment, granulomatous inflammation upon biopsy) |
| Crohn’s Disease [46,47] | Chronic inflammatory disease of the gastrointestinal tract | Ulcers, diffuse or nodular swellings, cobblestone appearance of the mucosa, macules and plaques involving the gingiva | Angular cheilitis Alveolar bone loss | Lips Gingiva Buccal mucosa | Endoscopy Pathology | (a) Pathology (absence of classic non-caseating granulomas) (b) Absence of peculiar clinical features |
| Sarcoidosis [48] | Multisystem granulomatous disease of unknown cause that can affect any organ | Oral manifestations are uncommon and associated with salivary gland and lymph node involvement | Submucosal mass, ulcerations, nodular swellings, an area of granularity, or an isolated papule. Bone involvement | Salivary glands Buccal mucosa but any mucosal surface can be involved | Symptoms Radiology Elevated serum angiotensin-converting enzyme (ACE) levelsPathology | (a) Pathology (absence of classic non-caseating granulomas) (b) Absence of peculiar clinical features |
| Melkersson-Rosenthal Syndrome [49] | Rare disorder of unknown cause | Recurring facial paralysis, swelling of the lips, and a fissured tongue | Edema, ulcers, papules, swellings, cobblestone mucosal alterations, or focal areas of submucosal enlargement | Lips Tongue | Pathology Clinical diagnostic criteria | (a) Pathology (absence of classic non-caseating granulomas) (b) Absence of clinical diagnostic criteria (labial swelling, facial paralysis, and fissured tongue) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajolo, C.; Rupe, C.; Schiavelli, A.; Gioco, G.; Metafuni, E.; Contaldo, M.; Sica, S. Saprochaete clavata Infection in Immunosuppressed Patients: Systematic Review of Cases and Report of the First Oral Manifestation, Focusing on Differential Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 2385. https://doi.org/10.3390/ijerph18052385
Lajolo C, Rupe C, Schiavelli A, Gioco G, Metafuni E, Contaldo M, Sica S. Saprochaete clavata Infection in Immunosuppressed Patients: Systematic Review of Cases and Report of the First Oral Manifestation, Focusing on Differential Diagnosis. International Journal of Environmental Research and Public Health. 2021; 18(5):2385. https://doi.org/10.3390/ijerph18052385
Chicago/Turabian StyleLajolo, Carlo, Cosimo Rupe, Anna Schiavelli, Gioele Gioco, Elisabetta Metafuni, Maria Contaldo, and Simona Sica. 2021. "Saprochaete clavata Infection in Immunosuppressed Patients: Systematic Review of Cases and Report of the First Oral Manifestation, Focusing on Differential Diagnosis" International Journal of Environmental Research and Public Health 18, no. 5: 2385. https://doi.org/10.3390/ijerph18052385
APA StyleLajolo, C., Rupe, C., Schiavelli, A., Gioco, G., Metafuni, E., Contaldo, M., & Sica, S. (2021). Saprochaete clavata Infection in Immunosuppressed Patients: Systematic Review of Cases and Report of the First Oral Manifestation, Focusing on Differential Diagnosis. International Journal of Environmental Research and Public Health, 18(5), 2385. https://doi.org/10.3390/ijerph18052385







