Drug Injection-Related and Sexual Behavior Changes in Drug Injecting Networks after the Transmission Reduction Intervention Project (TRIP): A Social Network-Based Study in Athens, Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Behavioral Data
- the number of different people they injected drugs with (Q2),
- how often they injected drugs (Q3),
- the proportion of the time that they had shared or given to someone injection equipment (syringe, a cooker, filter or rinse water) or they had backloaded (piggy-back) to share injection drugs (Q4a–Q4e),
- the number of people they had sex with (Q5 and Q7) and
- the number of people they had sex with and always used a condom (Q6 and Q8).
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
3.1. Socio-Demographic Characteristics of Participants
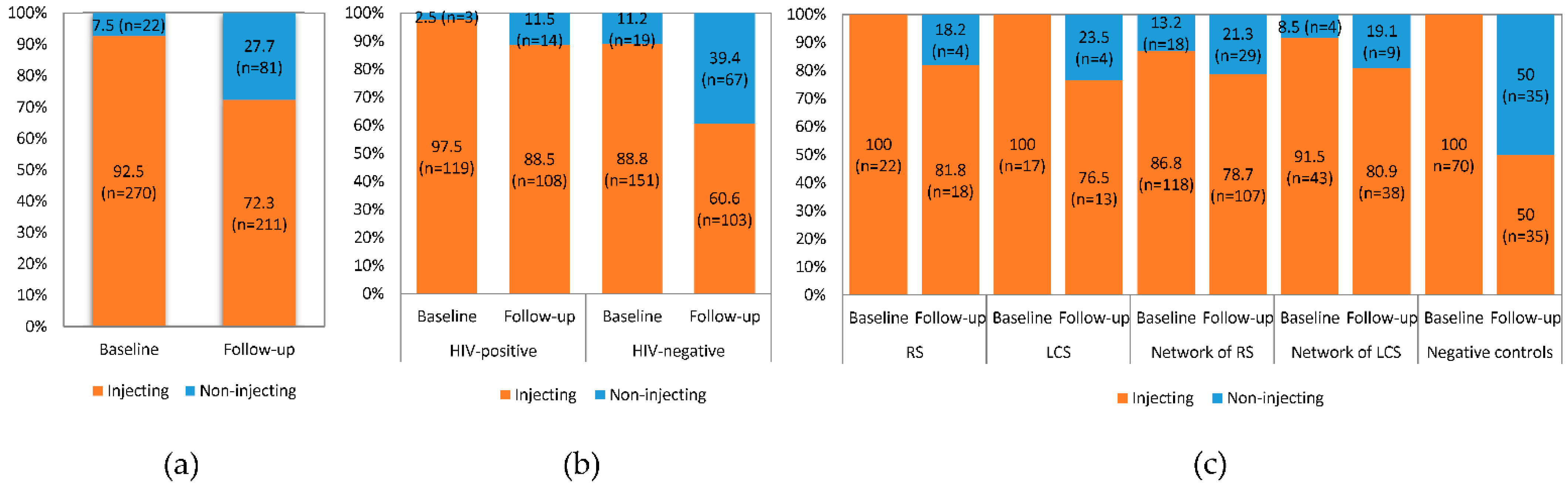
3.2. Drug Injection Status
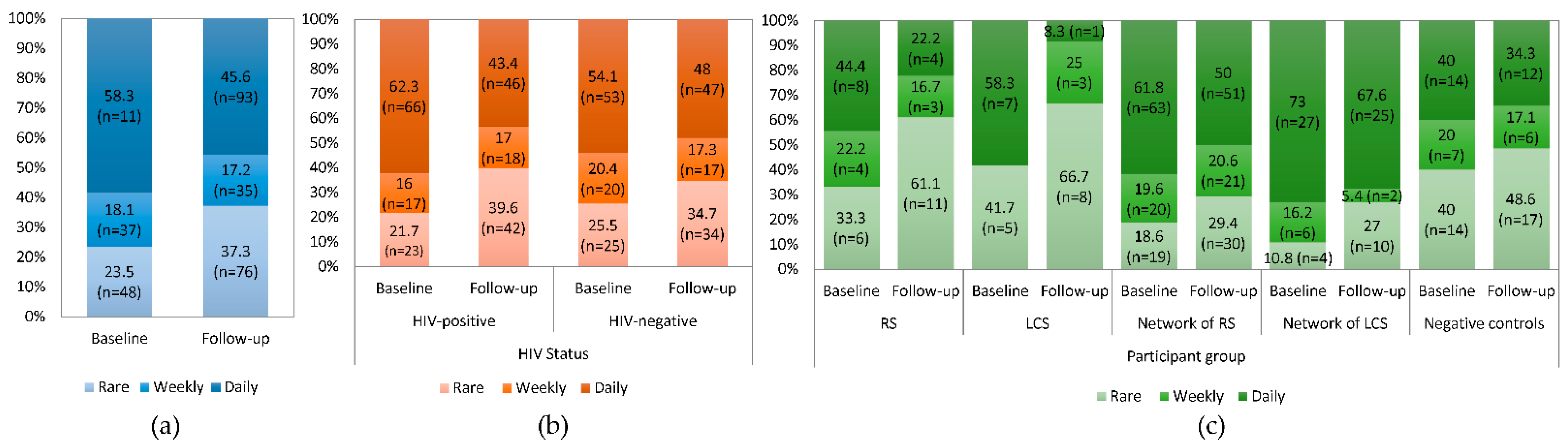
3.3. Drug Injection-Related Behaviors
3.4. Sexual Behaviors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization HIV/AIDS: Key facts. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 14 October 2020).
- Ghosn, J.; Taiwo, B.; Seedat, S.; Autran, B.; Katlama, C. HIV. Lancet 2018, 392, 685–697. [Google Scholar]
- Alchin, D.R. HIV vaccine development: An exploratory review of the trials and tribulations. Immunol. Res. 2014, 60, 35–37. [Google Scholar]
- Giannou, F.K.; Tsiara, C.G.; Nikolopoulos, G.K.; Talias, M.; Benetou, V.; Kantzanou, M.; Bonovas, S.; Hatzakis, A. Condom effectiveness in reducing heterosexual HIV transmission: A systematic review and meta-analysis of studies on HIV serodiscordant couples. Expert Rev. Pharm. Outcomes Res. 2016, 16, 489–499. [Google Scholar]
- Bailey, R.C.; Moses, S.; Parker, C.B.; Agot, K.; Maclean, I.; Krieger, J.N.; Williams, C.F.; Campbell, R.T.; Ndinya-Achola, J.O. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet 2007, 369, 643–656. [Google Scholar]
- Nikolopoulos, G.K.; Christaki, E.; Paraskevis, D.; Bonovas, S. Pre-Exposure Prophylaxis for HIV: Evidence and Perspectives. Curr. Pharm. Des. 2017, 23, 2579–2591. [Google Scholar]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapía, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar]
- Molina, J.-M.; Capitant, C.; Spire, B.; Pialoux, G.; Cotte, L.; Charreau, I.; Tremblay, C.; Le Gall, J.-M.; Cua, E.; Pasquet, A.; et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N. Engl. J. Med. 2015, 373, 2237–2246. [Google Scholar]
- Cohen, M.S.; Gay, C.L.; Busch, M.P.; Hecht, F.M. The Detection of Acute HIV Infection. J. Infect. Dis. 2010, 202, S270–S277. [Google Scholar]
- Wodak, A.; Cooney, A. Effectiveness of sterile needle and syringe programmes. Int. J. Drug Policy 2005, 16, 31–44. [Google Scholar]
- Mukandavire, C.; Low, A.; Mburu, G.; Trickey, A.; May, M.T.; Davies, C.F.; French, C.E.; Looker, K.J.; Rhodes, T.; Platt, L.; et al. Impact of opioid substitution therapy on the HIV prevention benefit of antiretroviral therapy for people who inject drugs. AIDS 2017, 31, 1181–1190. [Google Scholar]
- Kurth, A.E.; Celum, C.; Baeten, J.M.; Vermund, S.H.; Wasserheit, J.N. Combination HIV Prevention: Significance, Challenges, and Opportunities. Curr. HIV/AIDS Rep. 2011, 8, 62–72. [Google Scholar]
- UNAIDS Combination HIV Prevention: Tailoring and Coordinating Biomedical, Behavioural and Structural Strategies to Reduce New HIV Infections. Available online: http://www.unaids.org/en/resources/documents/2010/20101006_JC2007_Combination_Prevention_paper (accessed on 8 December 2020).
- Nikolopoulos, G.K.; Sypsa, V.; Bonovas, S.; Paraskevis, D.; Malliori-Minerva, M.; Hatzakis, A.; Friedman, S.R. Big Events in Greece and HIV Infection Among People Who Inject Drugs. Subst. Use Misuse 2015, 50, 825–838. [Google Scholar]
- Nikolopoulos, G.K.; Fotiou, A.; Kanavou, E.; Richardson, C.; Detsis, M.; Pharris, A.; Suk, J.E.; Semenza, J.C.; Costa-Storti, C.; Paraskevis, D.; et al. National Income Inequality and Declining GDP Growth Rates Are Associated with Increases in HIV Diagnoses among People Who Inject Drugs in Europe: A Panel Data Analysis. PLoS ONE 2015, 10, e0122367. [Google Scholar]
- Paraskevis, D.; Nikolopoulos, G.; Tsiara, C.; Paraskeva, D.; Antoniadou, A.; Lazanas, M.; Gargalianos, P.; Psychogiou, M.; Malliori, M.; Kremastinou, J.; et al. HIV-1 outbreak among injecting drug users in Greece, 2011: A preliminary report. EuroSurveillance 2011, 16, 19962. [Google Scholar]
- Paraskevis, D.; Nikolopoulos, G.; Fotiou, A.; Tsiara, C.; Paraskeva, D.; Sypsa, V.; Lazanas, M.; Gargalianos, P.; Psichogiou, M.; Skoutelis, A.; et al. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: A longitudinal study. PLoS ONE 2013, 8, e78941. [Google Scholar]
- Malliori, M.; Golna, C.; Souliotis, K.; Hatzakis, A. Managing opioid dependence treatment and controlling for HIV incidence among injecting drug users in Greece: A case study of optimism in the face of adversity. Addiction 2013, 108, 1174–1175. [Google Scholar]
- Nikolopoulos, G.; Pavlitina, E.; Muth, S.; Schneider, J.; Psichogiou, M.; Williams, L.; Paraskevis, D.; Sypsa, V.; Magiorkinis, G.; Smyrnov, P.; et al. A network intervention that locates and intervenes with recently HIV-infected persons: The Transmission Reduction Intervention Project (TRIP). Sci. Rep. 2016, 6, 38100. [Google Scholar]
- CDC—Centers for Disease Control and Prevention. Recommendations for Partner Services Programs for HIV Infection, Syphilis, Gonorrhea, and Chlamydial Infection; CDC—Centers for Disease Control and Prevention: Atlanta, GA, USA, 2008; Volume 57, pp. 1–63.
- Psichogiou, M.; Giallouros, G.; Pantavou, K.; Pavlitina, E.; Papadopoulou, M.; Williams, L.D.; Hadjikou, A.; Kakalou, E.; Skoutelis, A.; Protopapas, K.; et al. Identifying, linking, and treating people who inject drugs and were recently infected with HIV in the context of a network-based intervention. AIDS Care Psychol. Socio-Medical Asp. AIDS/HIV 2019, 31, 1376–1383. [Google Scholar]
- Bowker, A.H. A Test for Symmetry in Contingency Tables. J. Am. Stat. Assoc. 1948, 43, 572–574. [Google Scholar]
- Horyniak, D.; Dietze, P.; Degenhardt, L.; Higgs, P.; McIlwraith, F.; Alati, R.; Bruno, R.; Lenton, S.; Burns, L. The relationship between age and risky injecting behaviours among a sample of Australian people who inject drugs. Drug Alcohol Depend. 2013, 132, 541–546. [Google Scholar]
- Tassiopoulos, K.; Bernstein, J.; Bernstein, E. Age and sharing of needle injection equipment in a cohort of Massachusetts injection drug users: An observational study. Addict. Sci. Clin. Pract. 2013, 8, 20. [Google Scholar]
- French, M.T.; Roebuck, M.C.; Alexandre, P.K. Illicit Drug Use, Employment, and Labor Force Participation. South Econ. J. 2001, 68, 349. [Google Scholar]
- Richardson, L.; Wood, E.; Li, K.; Kerr, T. Factors associated with employment among a cohort of injection drug users. Drug Alcohol Rev. 2010, 29, 293–300. [Google Scholar]
- Karlsson, N.; Santacatterina, M.; Käll, K.; Hägerstrand, M.; Wallin, S.; Berglund, T.; Ekström, A.M. Risk behaviour determinants among people who inject drugs in Stockholm, Sweden over a 10-year period, from 2002 to 2012. Harm. Reduct. J. 2017, 14, 57. [Google Scholar]
- Evans, J.L.; Hahn, J.A.; Page-Shafer, K.; Lum, P.J.; Stein, E.S.; Davidson, P.J.; Moss, A.R. Gender Differences in Sexual and Injection Risk Behavior Among Active Young Injection Drug Users in San Francisco (the UFO Study). J. Urban. Health Bull. N. Y. Acad. Med. 2003, 80, 137–146. [Google Scholar]
- Bryant, J.; Treloar, C. The gendered context of initiation to injecting drug use: Evidence for women as active initiates. Drug Alcohol Rev. 2007, 26, 287–293. [Google Scholar]
- Frajzyngier, V.; Neaigus, A.; Gyarmathy, V.A.; Miller, M.; Friedman, S.R. Gender differences in injection risk behaviors at the first injection episode. Drug Alcohol Depend. 2007, 89, 145–152. [Google Scholar]
- Montgomery, S.B.; Hyde, J.; De Rosa, C.J.; Rohrbach, L.A.; Ennett, S.; Harvey, S.M.; Clatts, M.; Iverson, E.; Kipke, M.D. Gender differences in HIV risk behaviors among young injectors and their social network members. Am. J. Drug Alcohol Abuse 2002, 28, 453–475. [Google Scholar]
- Latkin, C.; Mandell, W.; Knowlton, A.; Doherty, M.; Vlahov, D.; Suh, T.; Celentano, D. Gender differences in injection-related behaviors among injection drug users in Baltimore, Maryland. AIDS Educ. Prev. 1998, 10, 257–263. [Google Scholar]
- Strehlau, V.; Torchalla, I.; Patterson, M.; Moniruzzaman, A.; Laing, A.; Addorisio, S.; Frankish, J.; Krausz, M.; Somers, J. Recruitment and retention of homeless individuals with mental illness in a housing first intervention study. Contemp. Clin. Trials Commun. 2017, 7, 48–56. [Google Scholar]
- Marin, A.; Wellman, B. Social network analysis: An introduction. In The SAGE Handbook of Social Network Analysis; Scott, J., Carrington, P.J., Eds.; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2011; pp. 11–25. [Google Scholar]
- Coates, T.J.; Richter, L.; Caceres, C. Behavioural strategies to reduce HIV transmission: How to make them work better. Lancet 2008, 372, 669–684. [Google Scholar]
- Semaan, S.; Des Jarlais, D.C.; Malow, R. Behavior Change and Health-Related Interventions for Heterosexual Risk Reduction Among Drug Users. Subst. Use Misuse 2006, 41, 1349–1378. [Google Scholar]
| Sociodemographic Characteristics | Participant Groups [n (%)] | Total | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| RS | LCS | Network of RS | Network of LCS | Negative Controls | ||||
| Overall | 22 (7.5) | 17 (5.8) | 136 (46.6) | 47 (16.1) | 70 (24.0) | 292 | _ | |
| Gender | Male | 17 (77.3) | 14 (82.4) | 106 (77.9) | 37 (78.7) | 57 (81.4) | 231 (79.1) | 0.974 |
| Female | 5 (22.7) | 3 (17.6) | 30 (22.1) | 10 (21.3) | 13 (18.6) | 61 (20.9) | ||
| Age | median (IQR) | 39.5 (31–44) | 36 (32–40) | 35 (30–39) | 34 (31–37) | 36 (32–45) | 35 (31–41) | 0.161 |
| Nationality | Greek | 20 (90.9) | 15 (88.2) | 123 (90.4) | 42 (89.4) | 69 (98.6) | 269 (92.1) | 0.246 |
| Non-Greek | 2 (9.1) | 2 (11.8) | 13 (9.6) | 5 (10.6) | 1 (1.4) | 23 (7.9) | ||
| Education | Up-to high school | 19 (86.4) | 15 (88.2) | 115 (84.6) | 39 (83.0) | 62 (88.6) | 250 (85.6) | 0.911 |
| Post high School | 3 (13.6) | 2 (11.8) | 21 (15.4) | 8 (17.0) | 8 (11.4) | 42 (14.4) | ||
| Employment 1 | Employed | 3 (13.6) | 2 (11.8) | 23 (16.9) | 12 (25.5) | 20 (28.6) | 60 (20.5) | 0.197 |
| Unemployed | 19 (86.4) | 15 (88.2) | 113 (83.1) | 35 (74.5) | 50 (71.4) | 232 (79.5) | ||
| Homelessness 1 | Homeless | 2 (9.1) | 2 (11.8) | 24 (17.8) | 9 (19.2) | 2 (2.9) | 39 (13.4) | 0.031 |
| Non-homeless | 20 (90.9) | 15 (88.2) | 111 (82.2) | 38 (80.8) | 68 (97.1) | 252 (86.6) | ||
| HIV status | Positive | 22 (100) | 17 (100) | 57 (41.9) | 26 (55.3) | 0 (0) | 122 (41.8) | <0.001 |
| Negative | 0 (0) | 0 (0) | 79 (58.1) | 21 (44.7) | 70 (100) | 170 (58.2) | ||
| Factors | Drug Injection-Related Behaviors in Follow-up | ||||
|---|---|---|---|---|---|
| Q4a | Q4c | ||||
| Univariable | Multivariable | Univariable | Multivariable | ||
| Baseline response | 3.767 | 3.237 | 2.929 | 2.530 | |
| (1.919–7.392) | (1.595–6.569) | (1.554–5.521) | (1.304–4.909) | ||
| Gender (Females vs. males) | 2.151 | - | 1.561 | - | |
| (0.996–4.646) | (0.761–3.199) | ||||
| Age | 0.923 | 0.936 | 0.946 | 0.961 | |
| (0.878–0.971) | (0.887–0.988) | (0.908–0.985) | (0.919–1.004) | ||
| Education (Post vs. up to high School) | 0.553 | - | 1.190 | - | |
| (0.182–1.687) | (0.522–2.715) | ||||
| Employment (Unemployed vs. employed) | 1.150 | - | 1.919 | - | |
| (0.439–3.011) | (0.776–4.747) | ||||
| Homelessness 1 (Homeless vs. non-homeless) | 1.046 | - | 1.048 | - | |
| (0.439–2.490) | (0.492–2.232) | ||||
| HIV status (Positive vs. negative) | 1.167 | - | 1.021 | - | |
| (0.612–2.226) | (0.583–1.788) | ||||
| Participant group (Reference group: Negative controls) | RS | 1.143 | 0.881 | 0.577 | 0.483 |
| (0.286–4.570) | (0.200–3.878) | (0.168–2.930) | (0.133–1.749) | ||
| LCS | 1.200 | 1.073 | 0.750 | 0.883 | |
| (0.259–5.559) | (0.211–5.464) | (0.189–2.974) | (0.206–3.794) | ||
| Network of RS | 0.920 | 0.657 | 0.699 | 0.615 | |
| (0.352–2.402) | (0.234–1.845) | (0.317–1.538) | (0.267–1.417) | ||
| Network of LCS | 2.333 | 1.648 | 1.853 | 1.334 | |
| (0.810–6.725) | (0.532–5.101) | (0.731–4.700) | (0.499–1.417) | ||
| SociodemographicCharacteristics | Women, Men Always Used Condom with | Men, Women Always Used Condom with | |||||
|---|---|---|---|---|---|---|---|
| [Median (%); (IQR)/Mean ± SD] | [Median (%); (IQR)/Mean ± SD]] | ||||||
| Baseline | Follow-up | p Value | Baseline | Follow-up | p Value | ||
| Overall | 50 (0–100)/ | 100 (0–100)/ | 0.019 | 25 (0–97)/ | 50 (0–100)/ | 0.939 | |
| 48 ± 46 | 59 ± 47 | 0.023 | 42 ± 45 | 49 ± 45 | 0.417 | ||
| HIV status | Positive | 73 (0–100)/ | 100 (46–100)/ | 0.026 | 95 (0–100)/ | 83 (0–100)/ | 0.462 |
| 55 ± 47 | 75 ± 43 | 0.035 | 62 ± 46 | 58 ± 45 | 0.727 | ||
| Negative | 45 (0–100)/ | 67 (0–100)/ | 0.188 | 0 (0–55)/ | 23 (0–100)/ | 0.472 | |
| 45 ± 46 | 52 ± 47 | 0.195 | 28 ± 38 | 41 ± 45 | 0.209 | ||
| Participant group | RS | 97 (0–100)/ | 100 (100–100)/ | 0.048 | 0 (0–0)/ | 25 (0–75)/ | 0.162 |
| 57 ± 53 | 100 ± 0 | 0.074 | 0 ± 0 | 38 ± 48 | 0.215 | ||
| LCS | 0 (0–100)/ | 0 (0–100)/ | 1.000 | 75 (50–100)/ | 50 (0–100)/ | 0.317 | |
| 40 ± 55 | 40 ± 55 | 1.000 | 75 ± 35 | 50 ± 71 | 0.500 | ||
| Network of RS | 0 (0–100)/ | 88 (0–100)/ | 0.007 | 21 (0–96)/ | 33 (0–100)/ | 0.825 | |
| 36 ± 44 | 58 ± 47 | 0.006 | 43 ± 46 | 47 ± 49 | 0.703 | ||
| Network of LCS | 100 (0–100)/ | 100 (0–100)/ | 0.774 | 97 (0–100)/ | 70 (0–98)/ | 0.181 | |
| 63 ± 45 | 65 ± 46) | 0.885 | 63 ± 48 | 56 ± 45 | 0.694 | ||
| Negative controls | 67 (0–100)/ | 73 (0–100)/ | 0.491 | 25 (0–60)/ | 50 (0–100)/ | 0.671 | |
| 57 ± 45 | 52 ± 48) | 0.292 | 34 ± 38 | 50 ± 41 | 0.344 | ||
| Factors | Sexual Behavior | ||||
|---|---|---|---|---|---|
| Women, Who Men Always Used Condom with | Men, Who Women Always Used Condom with | ||||
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | ||
| Baseline response | 0.153 | 0.16 | 0.13 | 0.13 | |
| (0.108–0.197) | (0.114–0.207) | (0.032–0.228) | (0.022–0.239) | ||
| Age | −0.088 | - | −0.684 | −0.402 | |
| (−0.428–0.252) | (−1.301–−0.066) | (−1.095–0.292) | |||
| Education (Post vs. up-to high School) | 0.018 | 0.013 | −0.025 | - | |
| (0.007–0.030 | (−0.007–0.033) | (−0.094–0.044) | |||
| Employment (Unemployed vs. employed) | 0.099 | - | −0.018 | - | |
| (−0.011–0.210) | (−0.289–0.254) | ||||
| Homelessness (Homeless vs. non-homeless) | 0.012 | 0.006 | 0.015 | 0.014 | |
| (0.001–0.023 | (−0.013–0.025) | (0.009–0.021) | (0.004–0.024) | ||
| HIV Positive (Positive vs. negative) | 0.061 | - | 0.069 | - | |
| (0.040–0.082) | (−0.035–0.173) | ||||
| Participant group (Reference group: Negative controls) | RS | 0.472 | 0.514 | −0.075 | −0.023 |
| (0.330–0.614) | (0.406–0.622) | (−0.549–0.398) | (−0.479–0.432) | ||
| LCS | −0.128 | −0.056 | 0.05 | −0.113 | |
| (−0.582–0.326) | (−0.470–0.295) | (−0.689–0.788) | (−0.651–0.425) | ||
| Network of RS | 0.062 | 0.133 | 0.021 | −0.053 | |
| (−0.120–0.244) | (−0.030–0.295) | (−0.284–0.325) | (−0.335–0.230) | ||
| Network of LCS | 0.109 | 0.102 | 0.053 | −0.161 | |
| (−0.130–0.347) | (−0.123–0.326) | (−0.308–0.414) | (−0.548–0.227) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giallouros, G.; Pantavou, K.; Pampaka, D.; Pavlitina, E.; Piovani, D.; Bonovas, S.; Nikolopoulos, G.K. Drug Injection-Related and Sexual Behavior Changes in Drug Injecting Networks after the Transmission Reduction Intervention Project (TRIP): A Social Network-Based Study in Athens, Greece. Int. J. Environ. Res. Public Health 2021, 18, 2388. https://doi.org/10.3390/ijerph18052388
Giallouros G, Pantavou K, Pampaka D, Pavlitina E, Piovani D, Bonovas S, Nikolopoulos GK. Drug Injection-Related and Sexual Behavior Changes in Drug Injecting Networks after the Transmission Reduction Intervention Project (TRIP): A Social Network-Based Study in Athens, Greece. International Journal of Environmental Research and Public Health. 2021; 18(5):2388. https://doi.org/10.3390/ijerph18052388
Chicago/Turabian StyleGiallouros, George, Katerina Pantavou, Despina Pampaka, Eirini Pavlitina, Daniele Piovani, Stefanos Bonovas, and Georgios K. Nikolopoulos. 2021. "Drug Injection-Related and Sexual Behavior Changes in Drug Injecting Networks after the Transmission Reduction Intervention Project (TRIP): A Social Network-Based Study in Athens, Greece" International Journal of Environmental Research and Public Health 18, no. 5: 2388. https://doi.org/10.3390/ijerph18052388
APA StyleGiallouros, G., Pantavou, K., Pampaka, D., Pavlitina, E., Piovani, D., Bonovas, S., & Nikolopoulos, G. K. (2021). Drug Injection-Related and Sexual Behavior Changes in Drug Injecting Networks after the Transmission Reduction Intervention Project (TRIP): A Social Network-Based Study in Athens, Greece. International Journal of Environmental Research and Public Health, 18(5), 2388. https://doi.org/10.3390/ijerph18052388








