BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blotting Analysis, Immunocytochemistry, and Microscopy
2.3. RNA Extraction and Quantitative RT-PCR (qRT-PCR)
2.4. Detection of Reactive Oxygen Species (ROS) and ATP Content
2.5. Determination of Mitochondrial Membrane Potential (MMP)
2.6. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC1α) Luciferase Assay
2.7. Preparation of BST204 (Fermented Ginseng Extract)
2.8. Statistical Analysis
3. Results and Discussion
3.1. BST204 Induces Myotube Hypertrophy Through Akt Activation
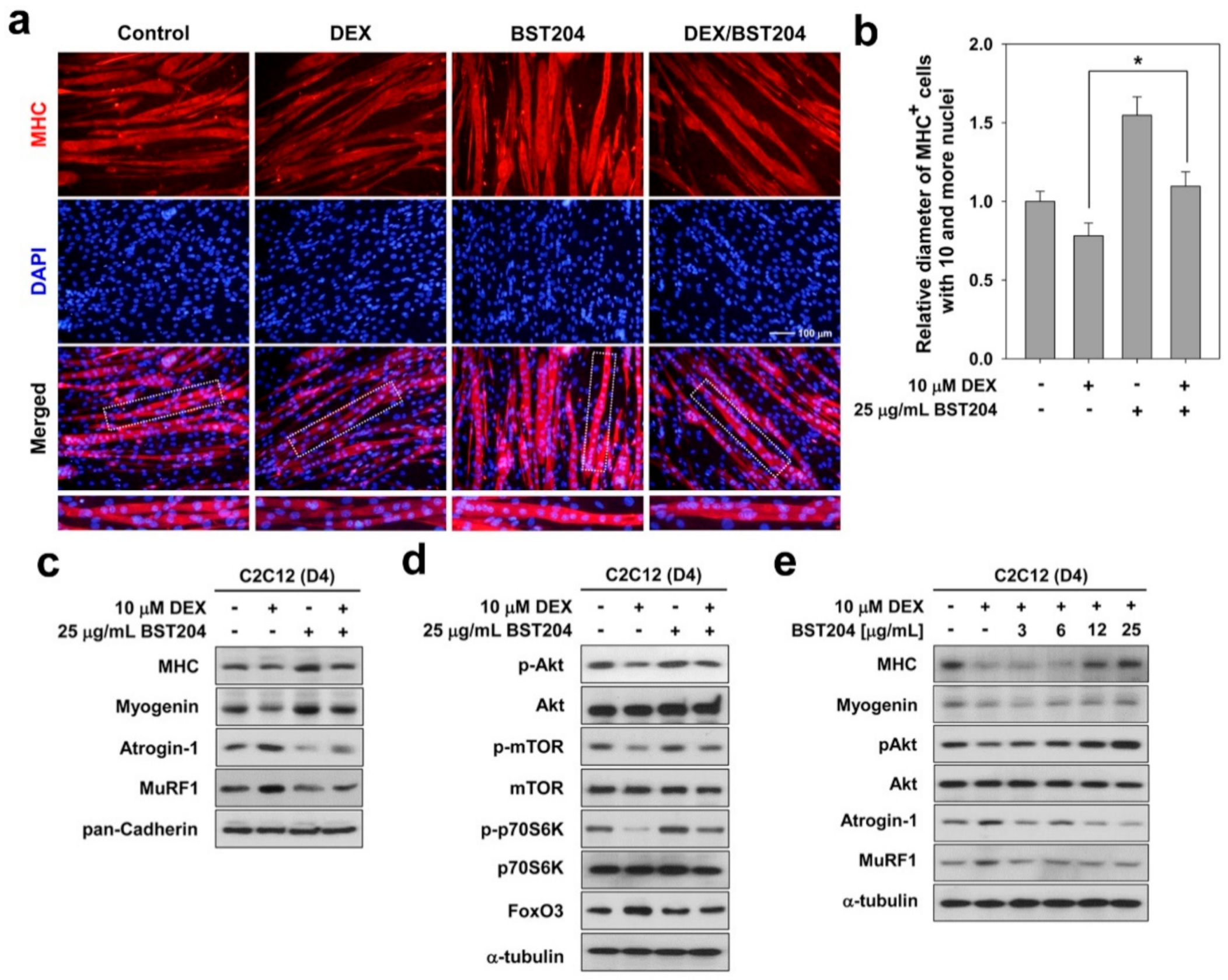
3.2. BST204 Ameliorates DEX-Induced Myotube Atrophy Through Akt/mTOR Signaling
3.3. BST204 Improves Mitochondrial Function in DEX-Treated Myotubes
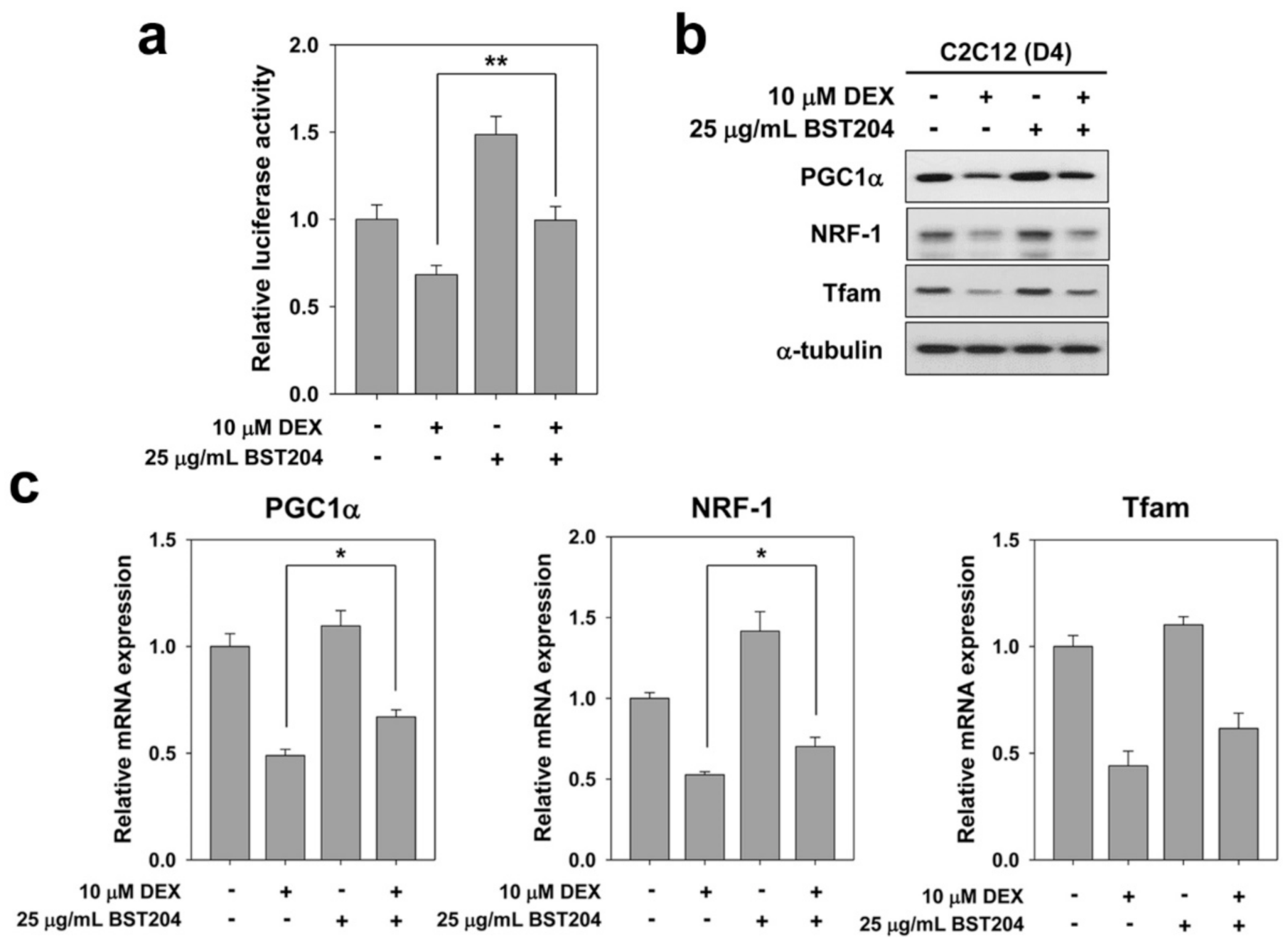
3.4. BST204 Improves the Activity and Expression of PGC1α in DEX-Treated Myotubes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakuma, K.; Yamaguchi, A. Sarcopenia and cachexia: The adaptations of negative regulators of skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2012, 3, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef]
- Glass, D.J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care. 2010, 13, 225–229. [Google Scholar] [CrossRef]
- Petroski, M.D.; Deshaies, R.J. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell. Biol. 2005, 6, 9–20. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Fujio, Y.; Mitsuuchi, Y.; Testa, J.R.; Walsh, K. Activation of Akt2 inhibits anoikis and apoptosis induced by myogenic differentiation. Cell Death Differ. 2001, 8, 1207–1212. [Google Scholar] [CrossRef]
- Gonzalez, I.; Tripathi, G.; Carter, E.J.; Cobb, L.J.; Salih, D.A.; Lovett, F.A.; Holding, C.; Pell, J.M. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol. Cell. Biol. 2004, 24, 3607–3622. [Google Scholar] [CrossRef]
- Vandromme, M.; Rochat, A.; Meier, R.; Carnac, G.; Besser, D.; Hemmings, B.A.; Fernandez, A.; Lamb, N.J. Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. J. Biol. Chem. 2001, 276, 8173–8179. [Google Scholar] [CrossRef]
- Lawlor, M.A.; Rotwein, P. Insulin-like growth factor-mediated muscle cell survival: Central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol. Cell. Biol. 2000, 20, 8983–8995. [Google Scholar] [CrossRef]
- Lawlor, M.A.; Rotwein, P. Coordinate control of muscle cell survival by distinct insulin-like growth factor activated signaling pathways. J. Cell Biol. 2000, 151, 1131–1140. [Google Scholar] [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, J.H.; Kim, N.W.; Kim, Y.J.; Chang, S.H.; Ko, N.Y.; Her, E.; Yoo, Y.H.; Kim, J.W.; Lee, B.Y.; et al. Inhibitory effects of a fermented ginseng extract, BST204, on the expression of inducible nitric oxide synthase and nitric oxide production in lipopolysaccharide-activated murine macrophages. J. Pharm. Pharm. 2005, 57, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Lee, J.H.; Kim, N.W.; Her, E.; Chang, S.H.; Ko, N.Y.; Yoo, Y.H.; Kim, J.W.; Seo, D.W.; Han, J.W.; et al. Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages. Int. Immunopharmacol. 2005, 5, 929–936. [Google Scholar] [CrossRef]
- Park, H.J.; Shim, H.S.; Kim, J.Y.; Kim, J.Y.; Park, S.K.; Shim, I. Ginseng purified dry extract, BST204, improved cancer chemotherapy-related fatigue and toxicity in mice. Evid. Based Complement. Altern. Med. 2015, 2015, 197459. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.U.; Lee, J.R.; Kim, B.G.; Han, J.W.; Leem, Y.E.; Lee, H.J.; Ho, S.M.; Hahn, M.J.; Kang, J.S. Cdo interacts with APPL1 and activates Akt in myoblast differentiation. Mol. Biol. Cell. 2010, 21, 2399–2411. [Google Scholar] [CrossRef]
- Bae, G.U.; Kim, B.G.; Lee, H.J.; Oh, J.E.; Lee, S.J.; Zhang, W.; Krauss, R.S.; Kang, J.S. Cdo binds Abl to promote p38alpha/beta mitogen-activated protein kinase activity and myogenic differentiation. Mol. Cell Biol. 2009, 29, 4130–4143. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, H.J.; Vuong, T.A.; Choi, K.S.; Choi, D.; Koo, S.H.; Cho, S.C.; Cho, H.; Kang, J.S. Prmt7 deficiency causes reduced skeletal muscle oxidative metabolism and age-related obesity. Diabetes 2016, 65, 1868–1882. [Google Scholar] [CrossRef]
- Shanker, G.; Aschner, J.L.; Syversen, T.; Aschner, M. Free radical formation in cerebral cortical astrocytes in culture induced by methylmercury. Brain Res. Mol. Brain Res. 2004, 128, 48–57. [Google Scholar] [CrossRef]
- Crouch, S.P.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Befroy, D.E.; Petersen, K.F.; Dufour, S.; Mason, G.F.; de Graaf, R.A.; Rothman, D.L.; Shulman, G.I. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 2007, 56, 1376–1381. [Google Scholar] [CrossRef]
- Handschin, C.; Rhee, J.; Lin, J.; Tarr, P.T.; Spiegelman, B.M. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 7111–7116. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yoo, G.; Kim, H.S.; Kim, J.Y.; Kim, S.O.; Yoo, Y.H.; Sung, S.H. Implication of the stereoisomers of ginsenoside derivatives in the antiproliferative effect of HSC-T6 cells. J. Agric. Food Chem. 2012, 60, 11759–11764. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, X.L.; Zhu, Y.H.; Li, X.M.; Xu, Q.; Lin, H.D.; Wang, M.W. Tetramethylpyrazine protects palmitate-induced oxidative damage and mitochondrial dysfunction in C2C12 myotubes. Life Sci. 2011, 88, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial fission and remodelling contributes to muscle atrophy. Embo. J. 2010, 29, 1774–1785. [Google Scholar] [CrossRef]
- Arany, Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.P.; Scarpulla, R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004, 18, 357–368. [Google Scholar] [CrossRef]
- Li, P.A.; Hou, X.; Hao, S. Mitochondrial Biogenesis in Neurodegeneration. J. Neurosci. Res. 2017, 95, 2025–2029. [Google Scholar] [CrossRef]
- Artem, P.; Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of mitochondrial biogenesis as a way for active longevity; Interaction between the Nrf2 and PGC-1a signaling pathways. Front. Genet. 2019, 10, 435. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.; Kim, H.; Im, M.; Park, S.K.; Han, H.J.; An, S.; Kang, J.-S.; Lee, S.-J.; Bae, G.-U. BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function. Int. J. Environ. Res. Public Health 2021, 18, 2367. https://doi.org/10.3390/ijerph18052367
Kim R, Kim H, Im M, Park SK, Han HJ, An S, Kang J-S, Lee S-J, Bae G-U. BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function. International Journal of Environmental Research and Public Health. 2021; 18(5):2367. https://doi.org/10.3390/ijerph18052367
Chicago/Turabian StyleKim, Ryuni, Hyebeen Kim, Minju Im, Sun Kyu Park, Hae Jung Han, Subin An, Jong-Sun Kang, Sang-Jin Lee, and Gyu-Un Bae. 2021. "BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function" International Journal of Environmental Research and Public Health 18, no. 5: 2367. https://doi.org/10.3390/ijerph18052367
APA StyleKim, R., Kim, H., Im, M., Park, S. K., Han, H. J., An, S., Kang, J.-S., Lee, S.-J., & Bae, G.-U. (2021). BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function. International Journal of Environmental Research and Public Health, 18(5), 2367. https://doi.org/10.3390/ijerph18052367





