Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields
Abstract
1. Introduction
2. Materials and Methods
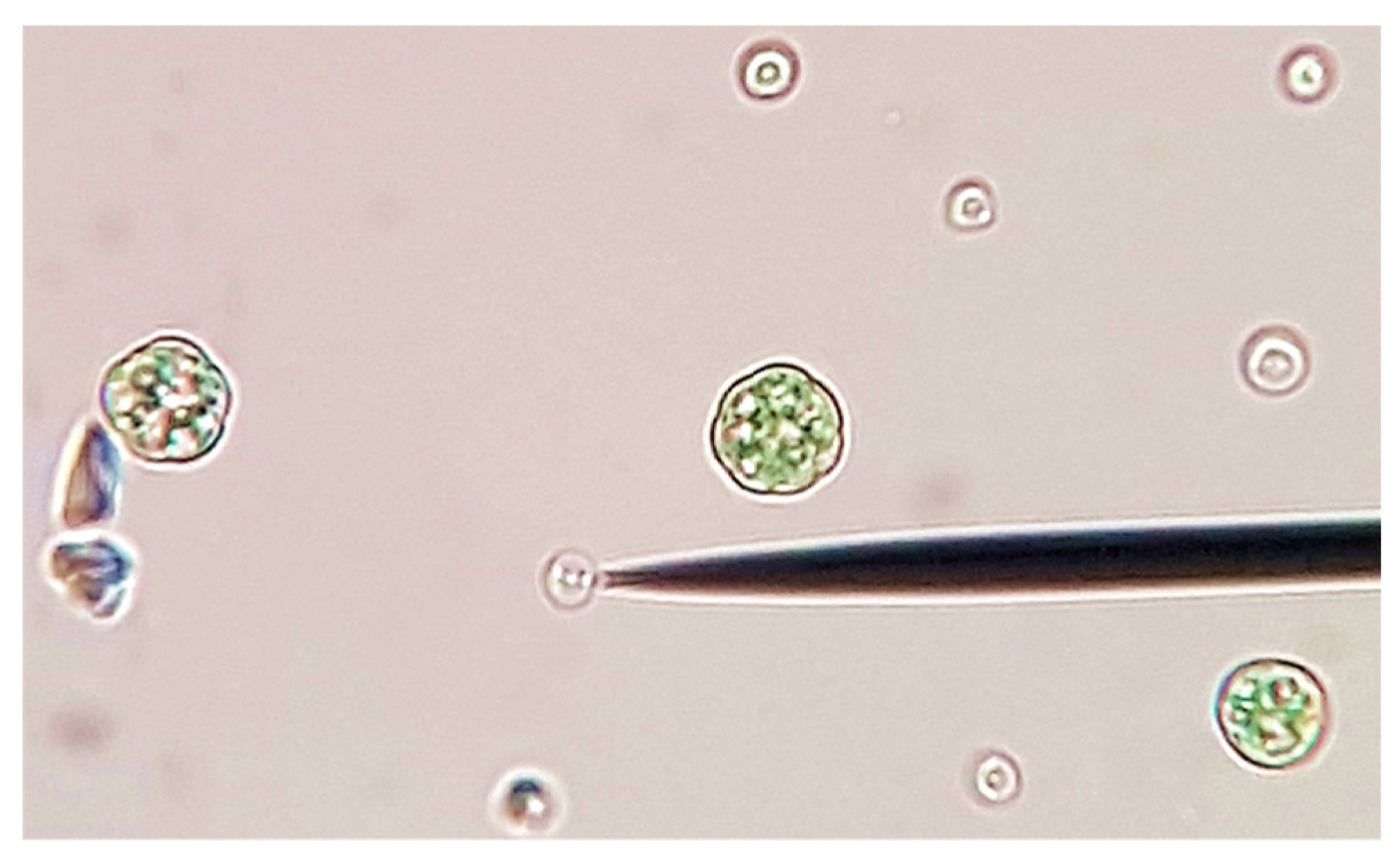
2.1. Algal Culture and Growth Media
2.2. Experimental Setup
2.2.1. Ammonium and Phosphate
2.2.2. Biomass Concentration and Phycocyanin Content
2.2.3. Lipid Content
2.2.4. C and N Content and Evaluation of δ13C and δ15N
2.2.5. Statistical Analysis
3. Results and Discussion
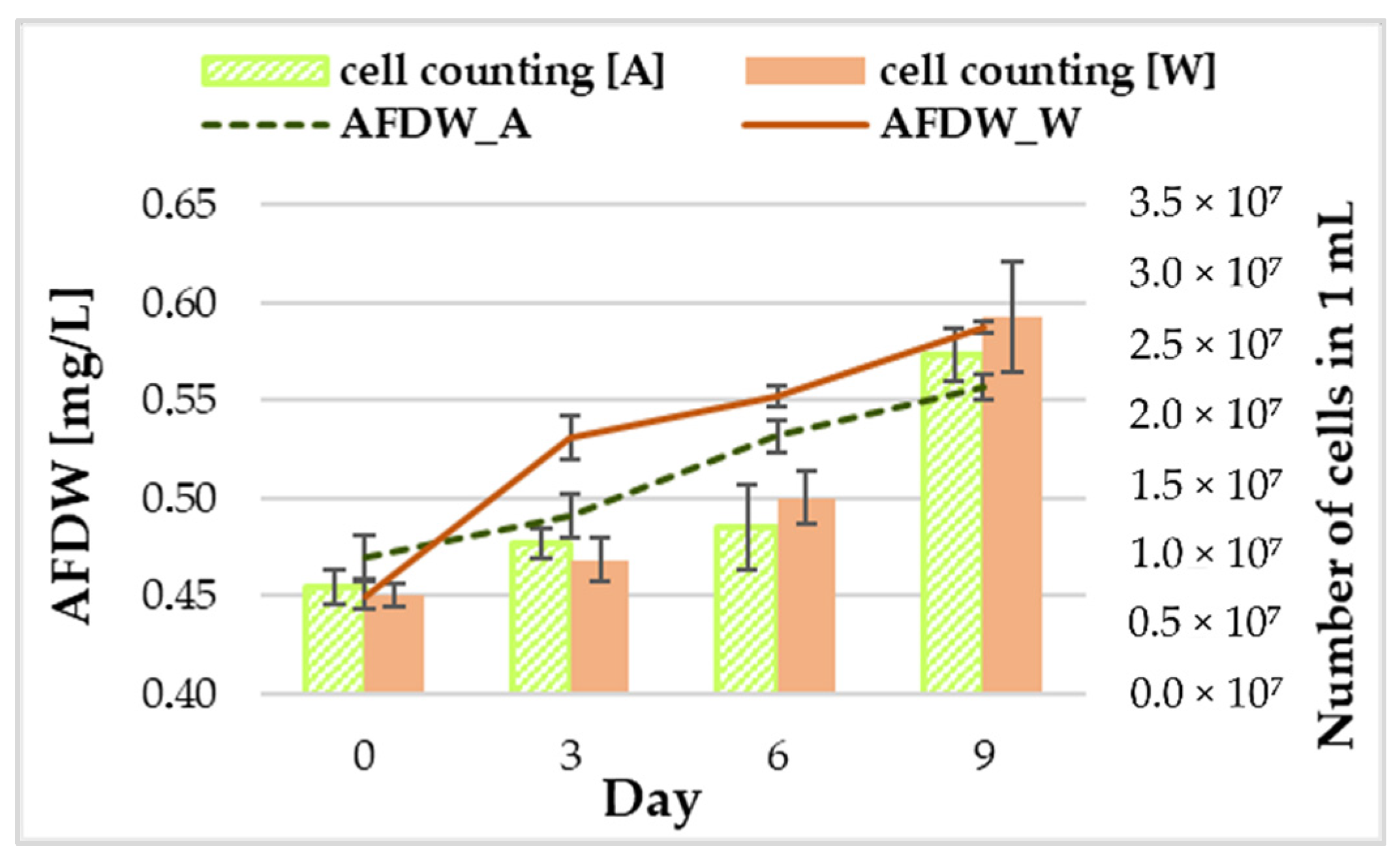
3.1. Biomass Growth and Phycocyanin Content
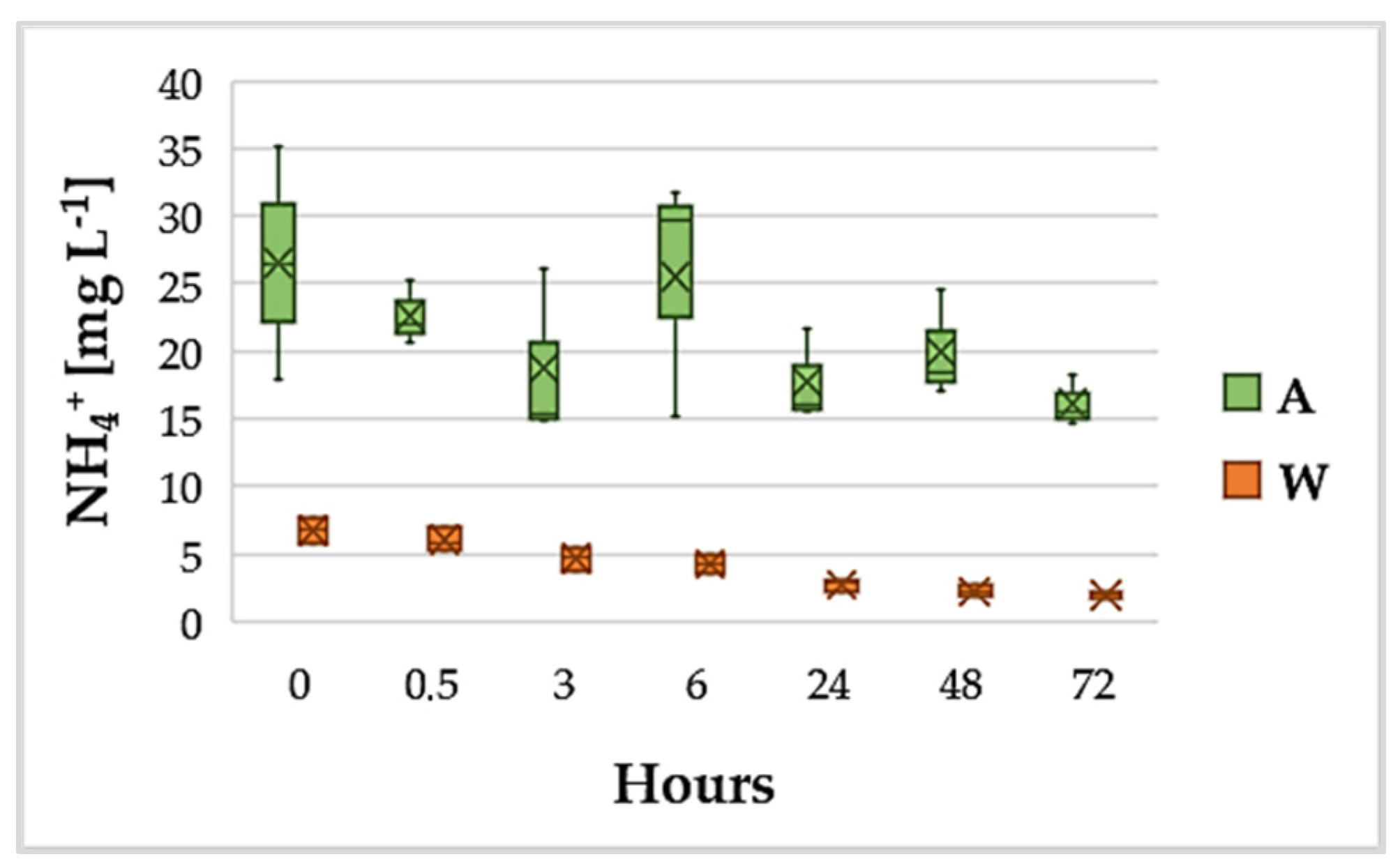
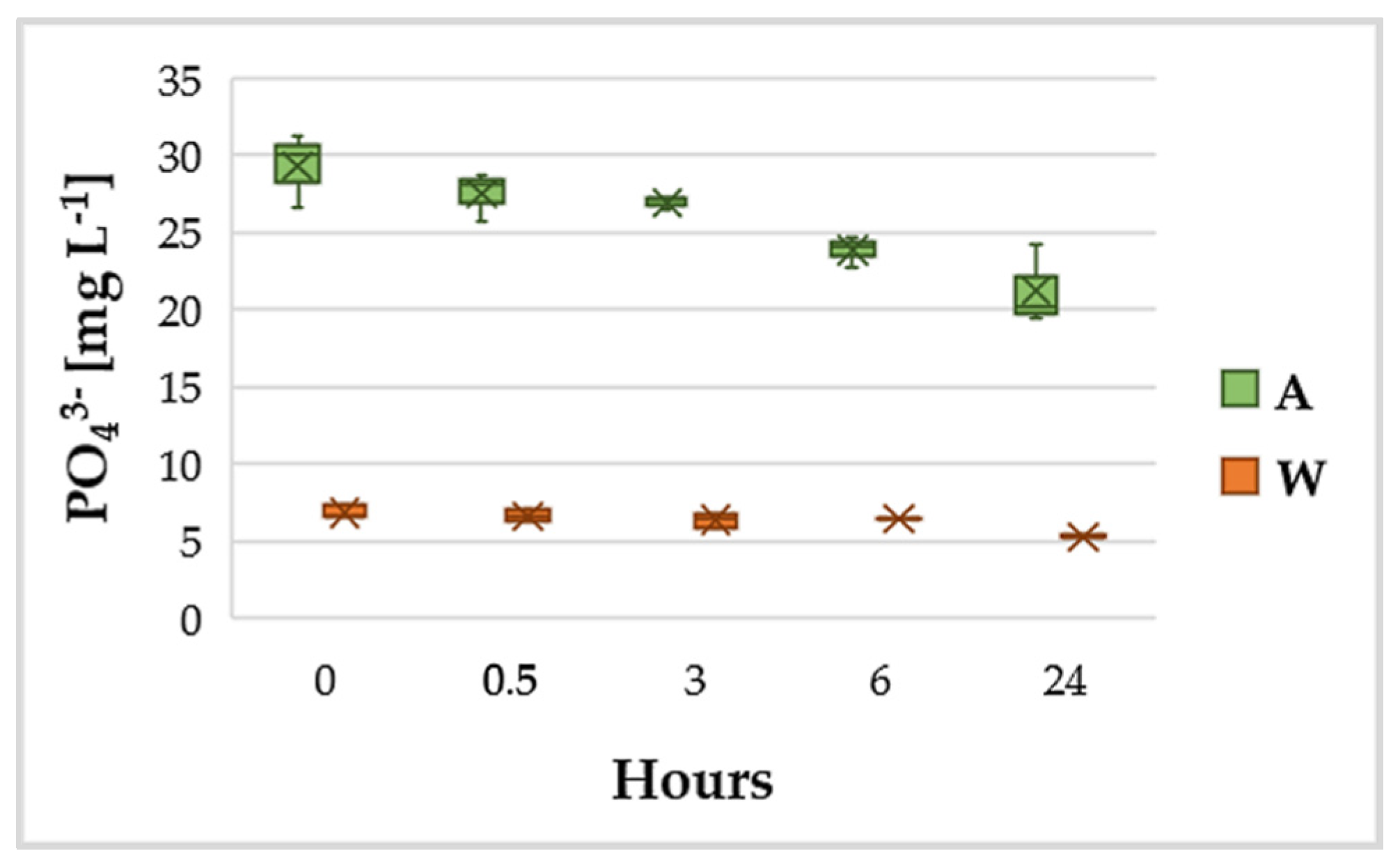
3.2. Variation of NH4+ and PO43−
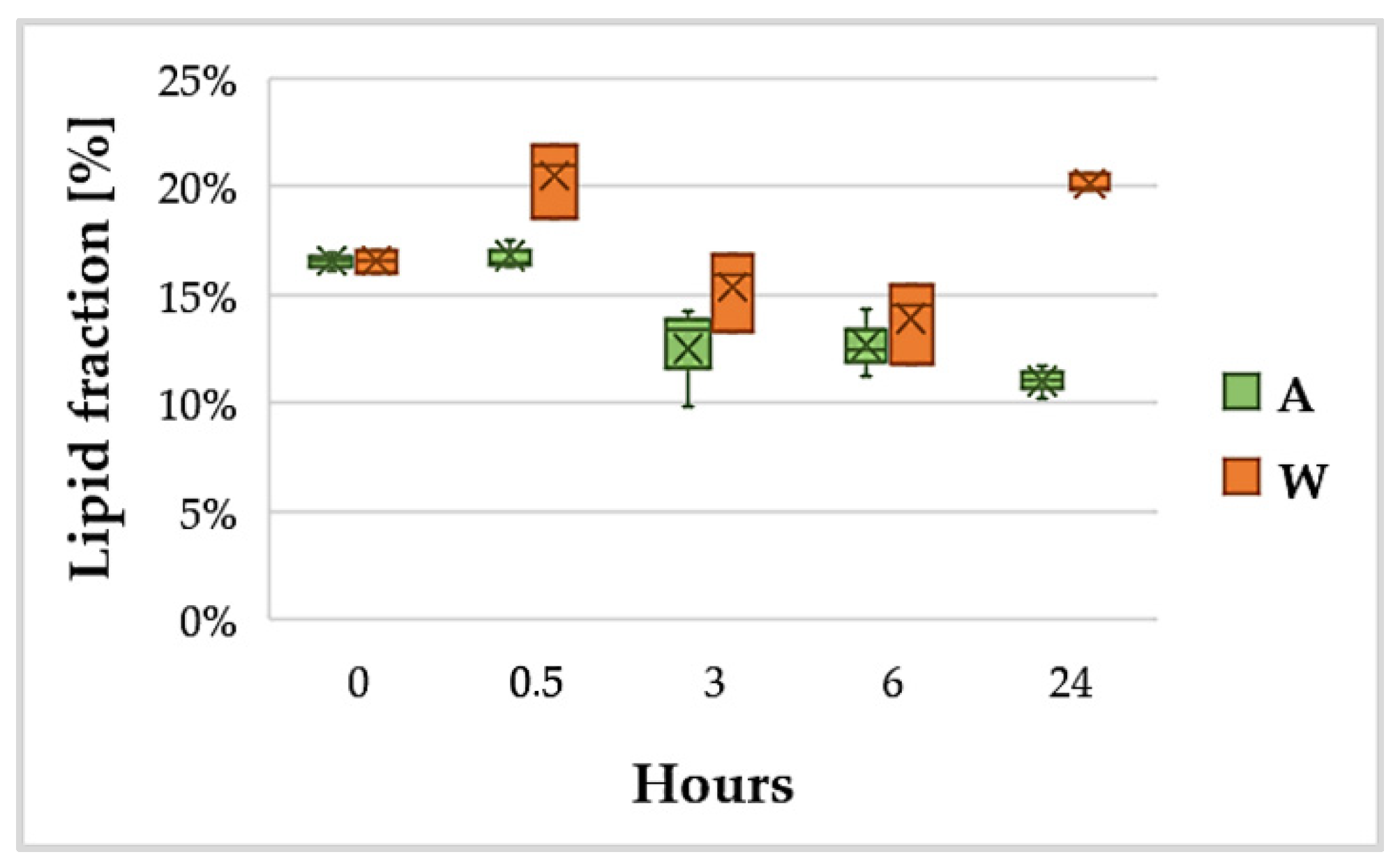
3.3. Lipid Content
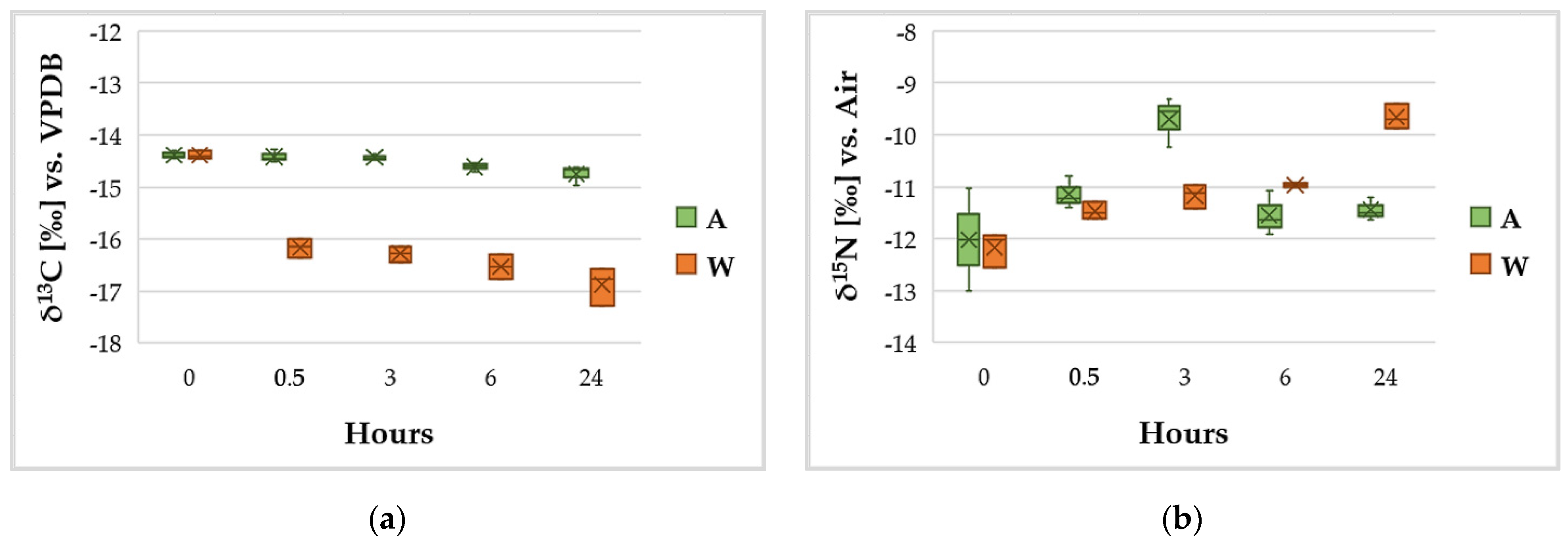
3.4. C and N Content and Variation of the Isotopic Ratios δ13C and δ15N
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kehrein, P.; van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants–market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Cabanelas, I.T.D.; Arbib, Z.; Chinalia, F.A.; Souza, C.O.; Perales, J.A.; Almeida, P.F.; Druzian, J.I.; Nascimento, I.A. From waste to energy: Microalgae production in wastewater and glycerol. Appl. Energy 2013, 109, 283–290. [Google Scholar] [CrossRef]
- Jeyanayagam, S. True Confessions of the Biological Nutrient Removal Process. Fla. Water Resour. J. 2005, 1, 37–46. [Google Scholar]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef]
- Tyler, H.; Fallgren, P.H.; Song, J.; Zhiyong Jason, R. Energy and Performance Comparison of Microbial Fuel Cell and Conventional Aeration Treating of Wastewater. J. Microb. Biochem. Technol. 2013, S6. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Liang, H.; Wang, J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Hwang, I.S.; Min, K.S. ANAMMOX and partial denitritation in anaerobic nitrogen removal from piggery waste. Water Sci. Technol. 2004, 49, 145–153. [Google Scholar] [CrossRef]
- Cui, Y.-X.; Biswal, B.K.; Guo, G.; Deng, Y.-F.; Huang, H.; Chen, G.-H.; Wu, D. Biological nitrogen removal from wastewater using sulphur-driven autotrophic denitrification. Appl. Microbiol. Biotechnol. 2019, 103, 6023–6039. [Google Scholar] [CrossRef]
- González, I.; Herrero, N.; Siles, J.Á.; Chica, A.F.; Martín, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Caskan, N.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Algal-based, single-step treatment of urban wastewaters. Bioresour. Technol. 2015, 189, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Schideman, L.C.; Canam, T.; Hudson, R.J.M. Pilot-scale demonstration of efficient ammonia removal from a high-strength municipal wastewater treatment sidestream by algal-bacterial biofilms affixed to rotating contactors. Algal Res. 2018, 34, 143–153. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.-U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process. Eng. 2020, 101747. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef] [PubMed]
- Sydney, E.B.; Schafranski, K.; Barretti, B.R.V.; Sydney, A.C.N.; Zimmerman, J.F.D.A.; Cerri, M.L.; Mottin Demiate, I. Biomolecules from extremophile microalgae: From genetics to bioprocessing of a new candidate for large-scale production. Process. Biochem. 2019, 87, 37–44. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Kormpa, A.; van der Maarel, M.J.E.C. The glycogen of Galdieria sulphuraria as alternative to starch for the production of slowly digestible and resistant glucose polymers. Carbohydr. Polym. 2017, 169, 75–82. [Google Scholar] [CrossRef]
- Carfagna, S.; Landi, V.; Coraggio, F.; Salbitani, G.; Vona, V.; Pinto, G.; Pollio, A.; Ciniglia, C. Different characteristics of C-phycocyanin (C-PC) in two strains of the extremophilic Galdieria phlegrea. Algal Res. 2018, 31, 406–412. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Ríos Pinto, L.F.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Chakraborty, M.; Miao, C.; McDonald, A.; Chen, S.L. Concomitant extraction of bio-oil and value added polysaccharides from Chlorella sorokiniana using a unique sequential hydrothermal extraction technology. Fuel 2012, 95, 63–70. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Lammers, P.J.; van Voorhies, W. Feasibility of algal systems for sustainable wastewater treatment. Renew. Energy 2015, 82, 71–76. [Google Scholar] [CrossRef]
- Hena, S.; Znad, H.; Heong, K.; Judd, S. Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Moreno Osorio, J.H.; Luongo, V.; Del Mondo, A.; Pinto, G.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. Nutrient removal from high strength nitrate containing industrial wastewater using Chlorella sp. strain ACUF_802. Ann. Microbiol. 2018, 68, 899–913. [Google Scholar] [CrossRef]
- Martínez, M.E.; Sánchez, S.; Jiménez, J.M.; El Yousfi, F.; Muñoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.; Li, W.; Rahaman, M.H.; Zhao, Y.; Wei, B.; Wei, H. Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol. Eng. 2017, 108, 83–92. [Google Scholar] [CrossRef]
- Čížková, M.; Vítová, M.; Zachleder, V. The Red Microalga Galdieria as a Promising Organism for Applications in Biotechnology. In Biotechnology, Microalgae–From Physiology to Application; Vítová, M., Ed.; IntechOpen: London, UK, 2019; pp. 105–122. [Google Scholar] [CrossRef]
- Biswal, B.K.; Mazza, A.; Masson, L.; Gehr, R.; Frigon, D. Impact of wastewater treatment processes on antimicrobial resistance genes and their co-occurrence with virulence genes in Escherichia coli. Water Res. 2014, 50, 245–253. [Google Scholar] [CrossRef]
- Cho, C.H.; Park, S.I.; Ciniglia, C.; Yang, E.C.; Graf, L.; Bhattacharya, D.; Yoon, H.S. Potential causes and consequences of rapid mitochondrial genome evolution in thermoacidophilic Galdieria (Rhodophyta). BMC Evol. Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Ciniglia, C.; Cennamo, P.; De Natale, A.; De Stefano, M.; Sirakov, M.; Iovinella, M.; Yoon, H.S.; Pollio, A. Cyanidium chilense (Cyanidiophyceae, Rhodophyta) from tuff rocks of the archeological site of Cuma, Italy. Phycol. Res. 2019, 67, 311–319. [Google Scholar] [CrossRef]
- Eren, A.; Iovinella, M.; Yoon, H.S.; Cennamo, P.; de Stefano, M.; de Castro, O.; Ciniglia, C. Genetic structure of Galdieria populations from Iceland. Polar Biol. 2018, 41, 1681–1691. [Google Scholar] [CrossRef]
- Ciniglia, C.; Yoon, H.S.; Pollio, A.; Pinto, G.; Bhattacharya, D. Hidden biodiversity of the extremophilic Cyanidiales red algae. Mol. Ecol. 2004, 13, 1827–1838. [Google Scholar] [CrossRef]
- Iovinella, M.; Carbone, D.A.; Diana, C.; Seth, J.D.; Michele, I.; Esposito, S.; Ciniglia, C. Prevalent pH Controls the Capacity of Galdieria maxima to Use Ammonia and Nitrate as a Nitrogen Source. Plants 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Cipolletta, S.; Vona, V.; Di Martino, C.; Carfagna, S. Heterotrophic Cultures of Galdieria phlegrea Shift to Autotrophy in the Presence or Absence of Glycerol. J. Plant. Growth Regul. 2020, 1–8. [Google Scholar] [CrossRef]
- Minoda, A.; Sawada, H.; Suzuki, S.; Miyashita, S.; Inagaki, K.; Yamamoto, T.; Tsuzuki, M. Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Appl. Microbiol. Biotechnol. 2015, 99, 1513–1519. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Reddy, H.; Kanapathipillai, N.; Nirmalakhandan, N.; Deng, S.; Lammers, P.J. Algal biofuels from urban wastewaters: Maximizing biomass yield using nutrients recycled from hydrothermal processing of biomass. Bioresour. Technol. 2015, 182, 232–238. [Google Scholar] [CrossRef]
- Tchinda, D.; Henkanatte-Gedera, S.M.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Zhang, Y.; Nirmalakhandan, N. Single-step treatment of primary effluent by Galdieria sulphuraria: Removal of biochemical oxygen demand, nutrients, and pathogens. Algal Res. 2019, 42, 101578. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Reddy, H.; Muppaneni, T.; Holguin, F.O.; Nirmalakhandan, N.; Lammers, P.J.; Deng, S. Optimizing energy yields from nutrient recycling using sequential hydrothermal liquefaction with Galdieria sulphuraria. Algal Res. 2015, 12, 74–79. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- Cheng, F.; Jarvis, J.M.; Yu, J.; Jena, U.; Nirmalakhandan, N.; Schaub, T.M.; Brewer, C.E. Bio-crude oil from hydrothermal liquefaction of wastewater microalgae in a pilot-scale continuous flow reactor. Bioresour. Technol. 2019, 294, 122184. [Google Scholar] [CrossRef]
- Li, Y.; Slouka, S.A.; Henkanatte-Gedera, S.M.; Nirmalakhandan, N.; Strathmann, T.J. Seasonal treatment and economic evaluation of an algal wastewater system for energy and nutrient recovery. Environ. Sci. Water Res. Technol. 2019, 5, 1545–1557. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef]
- Pinto, G.; Ciniglia, C.; Cascone, C.; Pollio, A. Species Composition of Cyanidiales Assemblages in Pisciarelli (Campi Flegrei, Italy) and Description of Galdieria Phlegrea SP. NOV. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 487–502. [Google Scholar] [CrossRef]
- Barcytė, D.; Elster, J.; Nedbalová, L. Plastid-encoded rbcL phylogeny suggests widespread distribution of Galdieria phlegrea (Cyanidiophyceae, Rhodophyta). Nord. J. Bot. 2018, 36, e01794. [Google Scholar] [CrossRef]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Corbo, G.; Lubritto, C. Energy Monitoring of a Wastewater Treatment Plant in Salerno, Campania Region (Southern Italy). In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability, Proceedings of the 2nd WaterEnergyNEXUS Conference, 14–17 November 2018, Salerno, Italy; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Corbo, G.; Lubritto, C. Assessing energy performance and critical issues of a large wastewater treatment plant through full-scale data Benchmarking. Water Sci. Technol. 2019, 80, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Lubritto, C. Energetic and environmental analysis of a wastewater treatment plant through static and dynamic monitoring activities. Int. J. Environ. Sci. Technol. 2020, 17, 4299–4312. [Google Scholar] [CrossRef]
- Allen, M.B. Studies with cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Mikrobiol. 1959, 32, 270–277. [Google Scholar] [CrossRef] [PubMed]
- LeGresley, M.; McDermott, G. Counting chamber methods for quantitative phytoplankton analysis—haemocytometer, Palmer-Maloney cell and Sedgewick-Rafter cell. UNESCO (IOC Man. Guides) 2010, 55, 25–30. [Google Scholar]
- Hernández-López, J.; Vargas-Albores, F. A microplate technique to quantify nutrients (NO₂−, NO₃−,NH₄⁺ and PO₄3−) in seawater. Aquac. Res. 2003, 34, 1201–1204. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.; Costa, J.A.; Burkert, C.A.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Rahman, D.Y.; Sarian, F.D.; van Wijk, A.; Martinez-Garcia, M.; van der Maarel, M. Thermostable phycocyanin from the red microalga Cyanidioschyzon merolae, a new natural blue food colorant. J. Appl. Phycol. 2017, 29, 1233–1239. [Google Scholar] [CrossRef]
- Onay, M.; Sonmez, C.; Oktem, H.A.; Yucel, M. Evaluation of various extraction techniques for efficient lipid recovery from thermo-resistant microalgae, Hindakia, Scenedesmus and Micractinium Species. Am. J. Anal. Chem. 2016, 07, 141–150. [Google Scholar] [CrossRef]
- Scirè-Calabrisotto, C.; Webb, J.M.; Frankel, D.; Ricci, P.; Altieri, S.; Lubritto, C. New evidence for diet and subsistence economy in Early and Middle Bronze Age Cyprus. J. Archaeol. Sci. Rep. 2020, 33, 102518. [Google Scholar] [CrossRef]
- Tiwari, M.; Singh, A.K.; Sinha, D.K. Chapter 3–Stable Isotopes: Tools for Understanding Past Climatic Conditions and Their Applications in Chemostratigraphy. In Chemostratigraphy; Ramkumar, M., Ed.; Elsevier: Oxford, UK, 2015; pp. 65–92. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Oberlin, E.A. Chapter 9–Volatiles Measured by the Phoenix Lander at the Northern Plains of Mars. In Volatiles in the Martian Crust; Filiberto, J., Schwenzer, S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–283. [Google Scholar] [CrossRef]
- Condie, K.C. 7–Living Systems. In Earth as an Evolving Planetary System; Condie, K.C., Ed.; Academic Press: Burlington, NJ, USA, 2005; pp. 223–263. [Google Scholar] [CrossRef]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Gröning, M.; Meijer, H.A.J.; Toman, B.; Verkouteren, R.M. New Guidelines for δ13C Measurements. Anal. Chem. 2006, 78, 2439–2441. [Google Scholar] [CrossRef] [PubMed]
- Bohlke, J.K.; Gwinn, C.J.; Coplen, T.B. New reference materials for nitrogen isotope ratio measurements. Geostand. Newsl. 1993, 17, 159–164. [Google Scholar] [CrossRef]
- Fuggi, A.; Di Martino Rigano, V.; Vona, V.; Rigano, C. Nitrate and ammonium assimilation in algal cell-suspensions and related pH variations in the external medium, monitored by electrodes. Plant. Sci. Lett. 1981, 23, 129–138. [Google Scholar] [CrossRef]
- Fuggi, A.; Rigano, V.D.M.; Vona, V.; Rigano, C. Pattern of inhibition of nitrate utilization by ammonium in the acidophilic thermophilic unicellular alga Cyanidium caldarium. Arch. Microbiol. 1981, 130, 349–352. [Google Scholar] [CrossRef]
- Rigano, C.; Di Martino Rigano, V.; Vona, V.; Fuggi, A. Nitrate reductase and glutamine synthetase activities, nitrate and ammonia assimilation, in the unicellular alga Cyanidium caldarium. Arch. Microbiol. 1981, 129, 110–114. [Google Scholar] [CrossRef]
- Woertz, I.; Feffer, A.; Lundquist, T.; Nelson, Y. Algae Grown on Dairy and Municipal Wastewater for Simultaneous Nutrient Removal and Lipid Production for Biofuel Feedstock. J. Environ. Eng. 2009, 135, 1115–1122. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Min, M.; Zhou, W.; Li, Y.; Mohr, M.; Cheng, Y.; Lei, H.; Liu, Y.; Lin, X.; Chen, P.; et al. Influence of Exogenous CO2 on Biomass and Lipid Accumulation of Microalgae Auxenochlorella protothecoides Cultivated in Concentrated Municipal Wastewater. Appl. Biochem. Biotechnol. 2012, 166, 1661–1673. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Factories 2019, 18, 178. [Google Scholar] [CrossRef]
- Zhu, L.D.; Li, Z.H.; Hiltunen, E. Strategies for Lipid Production Improvement in Microalgae as a Biodiesel Feedstock. Biomed Res. Int. 2016, 2016, 8792548. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as human food: Chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 2013, 4, 144–152. [Google Scholar] [CrossRef] [PubMed]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Aoki, M.; Ju, X.; Ueda, T.; Nakamura, Y.; Fujiwara, S.; Umemura, T.; Tsuzuki, M.; Minoda, A. Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Bioresour. Technol. 2016, 200, 861–866. [Google Scholar] [CrossRef]
- Minoda, A.; Sato, N.; Nozaki, H.; Okada, K.; Takahashi, H.; Sonoike, K.; Tsuzuki, M. Role of sulfoquinovosyl diacylglycerol for the maintenance of photosystem II in Chlamydomonas reinhardtii. Eur. J. Biochem. 2002, 269, 2353–2358. [Google Scholar] [CrossRef]
- Weber, A.P.M.; Horst, R.J.; Barbier, G.G.; Oesterhelt, C. Metabolism and Metabolomics of Eukaryotes Living Under Extreme Conditions. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 2007; Volume 256, pp. 1–34. [Google Scholar]
- Berto, D.; Calace, N.; Rampazzo, F.; Saccomandi, F.; Stellato, L. Isotopi: Dalla teoria alla pratica. Transl. “Isotopes: From theory to practice”; ISPRA–Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2018.
- Yu, G.; Zhang, Y.; Schideman, L.; Funk, T.; Wang, Z. Distributions of carbon and nitrogen in the products from hydrothermal liquefaction of low-lipid microalgae. Energy Environ. Sci. 2011, 4, 4587–4595. [Google Scholar] [CrossRef]
- Toyoda, S.; Suzuki, Y.; Hattori, S.; Yamada, K.; Fujii, A.; Yoshida, N.; Kouno, R.; Murayama, K.; Shiomi, H. Isotopomer Analysis of Production and Consumption Mechanisms of N2O and CH4 in an Advanced Wastewater Treatment System. Environ. Sci. Technol. 2011, 45, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, C.; Schmalzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant. J. 2007, 51, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Price, D.C.; Weber, A.P.M.; Reeb, V.; Chan Yang, E.; Lee, J.M.; Kim, S.Y.; Yoon, H.S.; Bhattacharya, D. Adaptation through horizontal gene transfer in the cryptoendolithic red alga Galdieria phlegrea. Curr. Biol. 2013, 23, R865–R866. [Google Scholar] [CrossRef] [PubMed]
| Sampling Times | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 3 h | 6 h | 24 h | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | ||
| Monitored parameters | OD750 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Cell counting | ✔ | ✔ | ✔ | ✔ | ||||||||||
| NH4+ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| PO43− | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| Phycocyanin | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| Lipid fraction | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| [C]-[N] fraction | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| δ13C-δ15N | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Cicco, M.R.; Palmieri, M.; Altieri, S.; Ciniglia, C.; Lubritto, C. Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. Int. J. Environ. Res. Public Health 2021, 18, 2291. https://doi.org/10.3390/ijerph18052291
di Cicco MR, Palmieri M, Altieri S, Ciniglia C, Lubritto C. Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. International Journal of Environmental Research and Public Health. 2021; 18(5):2291. https://doi.org/10.3390/ijerph18052291
Chicago/Turabian Styledi Cicco, Maria Rosa, Maria Palmieri, Simona Altieri, Claudia Ciniglia, and Carmine Lubritto. 2021. "Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields" International Journal of Environmental Research and Public Health 18, no. 5: 2291. https://doi.org/10.3390/ijerph18052291
APA Styledi Cicco, M. R., Palmieri, M., Altieri, S., Ciniglia, C., & Lubritto, C. (2021). Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. International Journal of Environmental Research and Public Health, 18(5), 2291. https://doi.org/10.3390/ijerph18052291










