Cardiovascular Autonomic Control, Sleep and Health Related Quality of Life in Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study’s Design and Population
2.2. Physiological Recordings
2.3. Pain and Health-Related Quality of Life Assessment
2.4. Cardiovascular Autonomic Control Assessment
2.5. Statistical Analysis
3. Results
3.1. Demographic, Clinical and Cardiovascular Characteristics of the Study Population
3.2. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciaffi, J.; Morabito, M.F.; Ruscitti, P.; D’Angelo, S.; Mancarella, L.; Brusi, V.; Abignano, G.; Pucino, V.; Giacomelli, R.; Meliconi, R.; et al. Incidence, Prevalence and Mortality of Systemic Sclerosis in Italy: A Nationwide Population-Based Study Using Administrative Health Data. Rheumatol. Int. 2021, 41, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Merkel, P.A.; Herlyn, K.; Martin, R.W.; Anderson, J.J.; Mayes, M.D.; Bell, P.; Korn, J.H.; Simms, R.W.; Csuka, M.E.; Medsger, T.A.; et al. Measuring Disease Activity and Functional Status in Patients with Scleroderma and Raynaud’s Phenomenon. Arthritis Rheum. 2002, 46, 2410–2420. [Google Scholar] [CrossRef]
- Mouthon, L.; Mestre-Stanislas, C.; Bérezné, A.; Rannou, F.; Guilpain, P.; Revel, M.; Pagnoux, C.; Guillevin, L.; Fermanian, J.; Poiraudeau, S. Impact of Digital Ulcers on Disability and Health-Related Quality of Life in Systemic Sclerosis. Ann. Rheum. Dis. 2010, 69, 214–217. [Google Scholar] [CrossRef]
- Bassel, M.; Hudson, M.; Taillefer, S.S.; Schieir, O.; Baron, M.; Thombs, B.D. Frequency and Impact of Symptoms Experienced by Patients with Systemic Sclerosis: Results from a Canadian National Survey. Rheumatol. Oxf. Engl. 2011, 50, 762–767. [Google Scholar] [CrossRef]
- Willems, L.M.; Kwakkenbos, L.; Leite, C.C.; Thombs, B.D.; van den Hoogen, F.H.J.; Maia, A.C.; Vliet Vlieland, T.P.M.; van den Ende, C.H.M. Frequency and Impact of Disease Symptoms Experienced by Patients with Systemic Sclerosis from Five European Countries. Clin. Exp. Rheumatol. 2014, 32, 88–93. [Google Scholar]
- Paterniani, A.; Sperati, F.; Esposito, G.; Cognetti, G.; Pulimeno, A.M.L.; Rocco, G.; Diamanti, P.; Bertini, L.; Baldeschi, G.C.; Varrassi, G.; et al. Quality of Life and Disability of Chronic Non-Cancer Pain in Adults Patients Attending Pain Clinics: A Prospective, Multicenter, Observational Study. Appl. Nurs. Res. 2020, 56, 151332. [Google Scholar] [CrossRef]
- Reid, K.J.; Harker, J.; Bala, M.M.; Truyers, C.; Kellen, E.; Bekkering, G.E.; Kleijnen, J. Epidemiology of Chronic Non-Cancer Pain in Europe: Narrative Review of Prevalence, Pain Treatments and Pain Impact. Curr. Med. Res. Opin. 2011, 27, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Falvay, D.; Clamor, A.; Wagner, J.; Jarczok, M.N.; Ellis, R.J.; Weber, C.; Thayer, J.F. Pneumogastric (Vagus) Nerve Activity Indexed by Heart Rate Variability in Chronic Pain Patients Compared to Healthy Controls: A Systematic Review and Meta-Analysis. Pain Physician 2016, 19, E55–E78. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; Russell, J.W.; Hummers, L.K.; McMahan, Z.H. Symptoms of Autonomic Dysfunction in Scleroderma Assessed by the COMPASS-31 Questionnaire. J. Rheumatol. 2018, 45, 1145–1152. [Google Scholar] [CrossRef]
- DiRenzo, D.; Russell, J.; Bingham, C.O.I.; McMahan, Z. The Relationship Between Autonomic Dysfunction of the Gastrointestinal Tract and Emotional Distress in Patients With Systemic Sclerosis. JCR J. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.D.; Tobaldini, E.; Bellocchi, C.; Santaniello, A.; Caronni, M.; Severino, A.; Froldi, M.; Beretta, L.; da Silva Soares, P.P.; Montano, N. Cardiac Autonomic Modulation at Rest and during Orthostatic Stress among Different Systemic Sclerosis Subsets. Eur. J. Intern. Med. 2019, 66, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Naclerio, C.; Iengo, R.; D’Angelo, S.; Cuomo, G.; Valentini, G. Cardiac Autonomic Dysfunction Precedes the Development of Fibrosis in Patients with Systemic Sclerosis. Rheumatology 2002, 41, 586–588. [Google Scholar] [CrossRef][Green Version]
- Othman, K.M.; Assaf, N.Y.; Farouk, H.M.; Hassan, I.M.A. Autonomic Dysfunction Predicts Early Cardiac Affection in Patients with Systemic Sclerosis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010, 3, CMAMD.S4940. [Google Scholar] [CrossRef]
- Poliwczak, A.R.; Waszczykowska, E.; Dziankowska-Bartkowiak, B.; Dworniak-Pryca, K. Abnormalities of Heart Rate Turbulence and Heart Rate Variability as Indicators of Increased Cardiovascular Risk in Patients with Systemic Sclerosis. Postepy Derm. Alergol. 2019, 36, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Zlatanovic, M.; Tadic, M.; Celic, V.; Ivanovic, B.; Stevanovic, A.; Damjanov, N. Cardiac Mechanics and Heart Rate Variability in Patients with Systemic Sclerosis: The Association That We Should Not Miss. Rheumatol. Int. 2017, 37, 49–57. [Google Scholar] [CrossRef]
- Guzzetti, S.; Borroni, E.; Garbelli, P.E.; Ceriani, E.; Bella, P.D.; Montano, N.; Cogliati, C.; Somers, V.K.; Mallani, A.; Porta, A. Symbolic Dynamics of Heart Rate Variability: A Probe to Investigate Cardiac Autonomic Modulation. Circulation 2005, 112, 465–470. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Schieir, O.; Thombs, B.D.; Hudson, M.; Boivin, J.-F.; Steele, R.; Bernatsky, S.; Hanley, J.; Baron, M.; Canadian Scleroderma Research Group. Prevalence, Severity, and Clinical Correlates of Pain in Patients with Systemic Sclerosis. Arthritis Care Res. 2010, 62, 409–417. [Google Scholar] [CrossRef]
- Krishnan, E.; Sokka, T.; Häkkinen, A.; Hubert, H.; Hannonen, P. Normative Values for the Health Assessment Questionnaire Disability Index: Benchmarking Disability in the General Population. Arthritis Rheum. 2004, 50, 953–960. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Montano, N.; Porta, A.; Cogliati, C.; Costantino, G.; Tobaldini, E.; Casali, K.R.; Iellamo, F. Heart Rate Variability Explored in the Frequency Domain: A Tool to Investigate the Link between Heart and Behavior. Neurosci. Biobehav. Rev. 2009, 33, 71–80. [Google Scholar] [CrossRef]
- Porta, A.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N.; Gnecchi-Ruscone, T. Assessment of Cardiac Autonomic Modulation during Graded Head-up Tilt by Symbolic Analysis of Heart Rate Variability. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H702–H708. [Google Scholar] [CrossRef]
- Barbic, F.; Minonzio, M.; Cairo, B.; Shiffer, D.; Dipasquale, A.; Cerina, L.; Vatteroni, A.; Urechie, V.; Verzeletti, P.; Badilini, F.; et al. Effects of Different Classroom Temperatures on Cardiac Autonomic Control and Cognitive Performances in Undergraduate Students. Physiol. Meas. 2019, 40, 054005. [Google Scholar] [CrossRef]
- Zamunér, A.R.; Shiffer, D.; Barbic, F.; Minonzio, M.; Andrade, C.P.; Corato, M.; Lalli, S.; Dipaola, F.; Cairo, B.; Albanese, A.; et al. Mechanical Somatosensory Stimulation Decreases Blood Pressure in Patients with Parkinson’s Disease. J. Hypertens. 2019, 37, 1714–1721. [Google Scholar] [CrossRef]
- Tobaldini, E.; Nobili, L.; Strada, S.; Casali, K.R.; Braghiroli, A.; Montano, N. Heart Rate Variability in Normal and Pathological Sleep. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Jackowska, M.; Dockray, S.; Endrighi, R.; Hendrickx, H.; Steptoe, A. Sleep Problems and Heart Rate Variability over the Working Day. J. Sleep Res. 2012, 21, 434–440. [Google Scholar] [CrossRef]
- Tobaldini, E.; Fiorelli, E.M.; Solbiati, M.; Costantino, G.; Nobili, L.; Montano, N. Short Sleep Duration and Cardiometabolic Risk: From Pathophysiology to Clinical Evidence. Nat. Rev. Cardiol. 2019, 16, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Cogliati, C.; Fiorelli, E.M.; Nunziata, V.; Wu, M.A.; Prado, M.; Bevilacqua, M.; Trabattoni, D.; Porta, A.; Montano, N. One Night On-Call: Sleep Deprivation Affects Cardiac Autonomic Control and Inflammation in Physicians. Eur. J. Intern. Med. 2013, 24, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Bertinotti, L.; Bracci, S.; Nacci, F.; Colangelo, N.; Del Rosso, A.; Casale, R.; Pignone, A.; Matucci-Cerinic, M. The Autonomic Nervous System in Systemic Sclerosis. A Review. Clin. Rheumatol. 2004, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dessein, P.H.; Joffe, B.I.; Metz, R.M.; Millar, D.L.; Lawson, M.; Stanwix, A.E. Autonomic Dysfunction in Systemic Sclerosis: Sympathetic Overactivity and Instability. Am. J. Med. 1992, 93, 143–150. [Google Scholar] [CrossRef]
- Pancera, P.; Sansone, S.; Presciuttini, B.; Montagna, L.; Cerù, S.; Lunardi, C.; Lechi, A. Autonomic Nervous System Dysfunction in Sclerodermic and Primary Raynaud’s Phenomenon. Clin. Sci. 1999, 96, 49. [Google Scholar] [CrossRef]
- Milette, K.; Hudson, M.; Körner, A.; Baron, M.; Thombs, B.D. Sleep Disturbances in Systemic Sclerosis: Evidence for the Role of Gastrointestinal Symptoms, Pain and Pruritus. Rheumatology 2013, 52, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.F.; Allen, R.P.; Trevisani, V.M.F.; Toscano, V.G.; Earley, C.J. Sleep Disruption in Systemic Sclerosis (Scleroderma) Patients: Clinical and Polysomnographic Findings. Sleep Med. 2002, 3, 341–345. [Google Scholar] [CrossRef]
- Koenig, J.; Loerbroks, A.; Jarczok, M.N.; Fischer, J.E.; Thayer, J.F. Chronic Pain and Heart Rate Variability in a Cross-Sectional Occupational Sample: Evidence for Impaired Vagal Control. Clin. J. Pain 2016, 32, 218–225. [Google Scholar] [CrossRef]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-Analytic Evidence for Decreased Heart Rate Variability in Chronic Pain Implicating Parasympathetic Nervous System Dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Santos-de-Araújo, A.D.; Dibai-Filho, A.V.; dos Santos, S.N.; de Alcântara, E.V.; da Silva Souza, C.; de Paula Gomes, C.; de Souza, J.N.; Pinheiro, J.S.; Bassi, D. Correlation Between Chronic Neck Pain and Heart Rate Variability Indices at Rest: A Cross-Sectional Study. J. Manip. Physiol. 2019, 42, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Margiotta, D.; Navarini, L.; Liberatori, M.; Barbano, B.; Tubani, L.; Afeltra, A.; Rosato, E. Parasympathetic Activity Increases with Digital Microvascular Damage and Vascular Endothelial Growth Factor in Systemic Sclerosis. Clin. Exp. Rheumatol. 2018, 36, 24–27. [Google Scholar]
- Benarroch, E.E. Pain-Autonomic Interactions. Neurol. Sci. 2006, 27, s130–s133. [Google Scholar] [CrossRef]
- Cortelli, P.; Giannini, G.; Favoni, V.; Cevoli, S.; Pierangeli, G. Nociception and Autonomic Nervous System. Neurol. Sci. 2013, 34, 41–46. [Google Scholar] [CrossRef]
- Martucci, K.T.; Ng, P.; Mackey, S. Neuroimaging Chronic Pain: What Have We Learned and Where Are We Going? Future Neurol. 2014, 9, 615–626. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending Pain Modulation and Chronification of Pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Boettger, M.K.; Bär, K.-J.; Dohrmann, A.; Müller, H.; Mertins, L.; Brockmeyer, N.H.; Agelink, M.W. Increased Vagal Modulation in Atopic Dermatitis. J. Derm. Sci. 2009, 53, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Yokusoglu, M.; Ozturk, S.; Uzun, M.; Baysan, O.; Demirkol, S.; Caliskaner, Z.; Dundaroz, R.; Sag, C.; Karaayvaz, M.; Isik, E. Heart Rate Variability in Patients with Allergic Rhinitis. Mil. Med. 2007, 172, 98–101. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Median (IQR), N (%) |
|---|---|
| Age (years) | 56 (50–65) |
| Females n, (%) | 16 (80%) |
| BMI (kg/m2) | 24 (23–26) |
| Disease Duration (years) | 13 (10–22) |
| Disease Subset | |
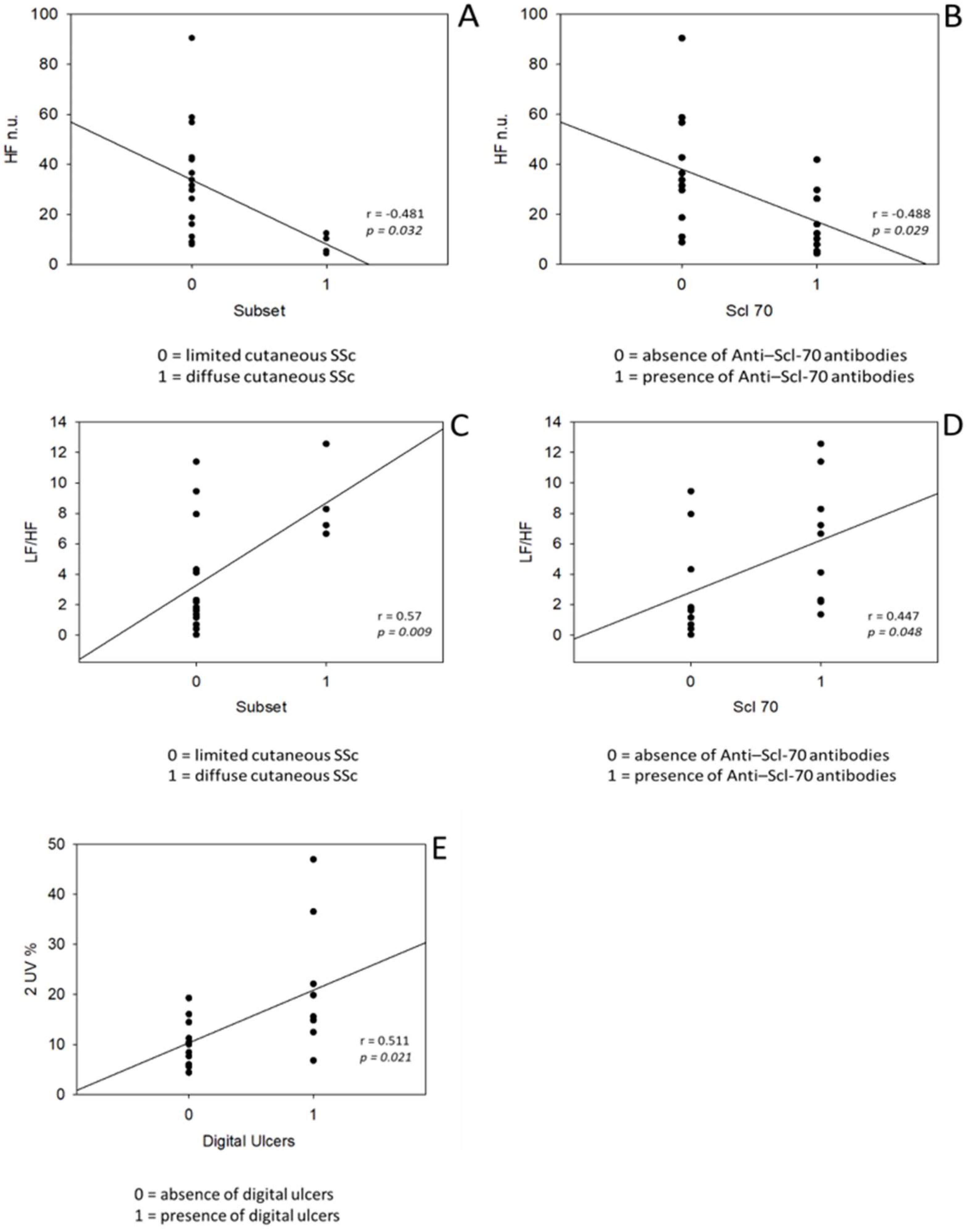
| - lcSSc | 16 (80%) |
| - dcSSc | 4 (20%) |
| Antibodies | |
| - ANA+ | 18 (90%) |
| - Scl70+ | 9 (45%) |
| - ACA+ | 9 (45%) |
| Digital ulcers | 9 (45%) |
| Pain Type | |
| - Articular | 9 (45%) |
| - Myalgia | 7 (35%) |
| - Ulcers | 4 (20%) |
| - Raynaud | 4 (20%) |
| - Migraine | 3 (15%) |
| Index | Median (IQR) |
|---|---|
| Cardiovascular Parameters | |
| sBP (mmHg) | 120 (109–140) |
| dBP (mmHg) | 75 (70–80) |
| HR (bpm) | 78 (70–86) |
| Spectral Analysis | |
| - TP (ms2) | 471 (198–863) |
| - LF (ms2) | 140 (54–341) |
| - HF (ms2) | 37 (18–221) |
| - LF nu | 59 (49–81) |
| - HF nu | 28 (11–38) |
| - LF/HF | 2.24 (1.55–7.40) |
| Symbolic Analysis | |
| - 0V% | 33 (27–44) |
| - 2LV% | 5 (2–8) |
| - 2UV% | 12 (8–17) |
| Respiratory Parameters | |
| RESP HF (Hz) | 0.27 (0.24–0.29) |
| K2 RR-RESP | 0.34 (0.21–0.75) |
| Questionnaires | |
| NRS | 7 (6–8) |
| HAQ | 1.38 (1.06–2.00) |
| PHQ-9 | 9 (5–11) |
| PSQI | |
| PSQI total score | 8 (7–10) |
| PSQI item 5 score | 11 (9–14) |
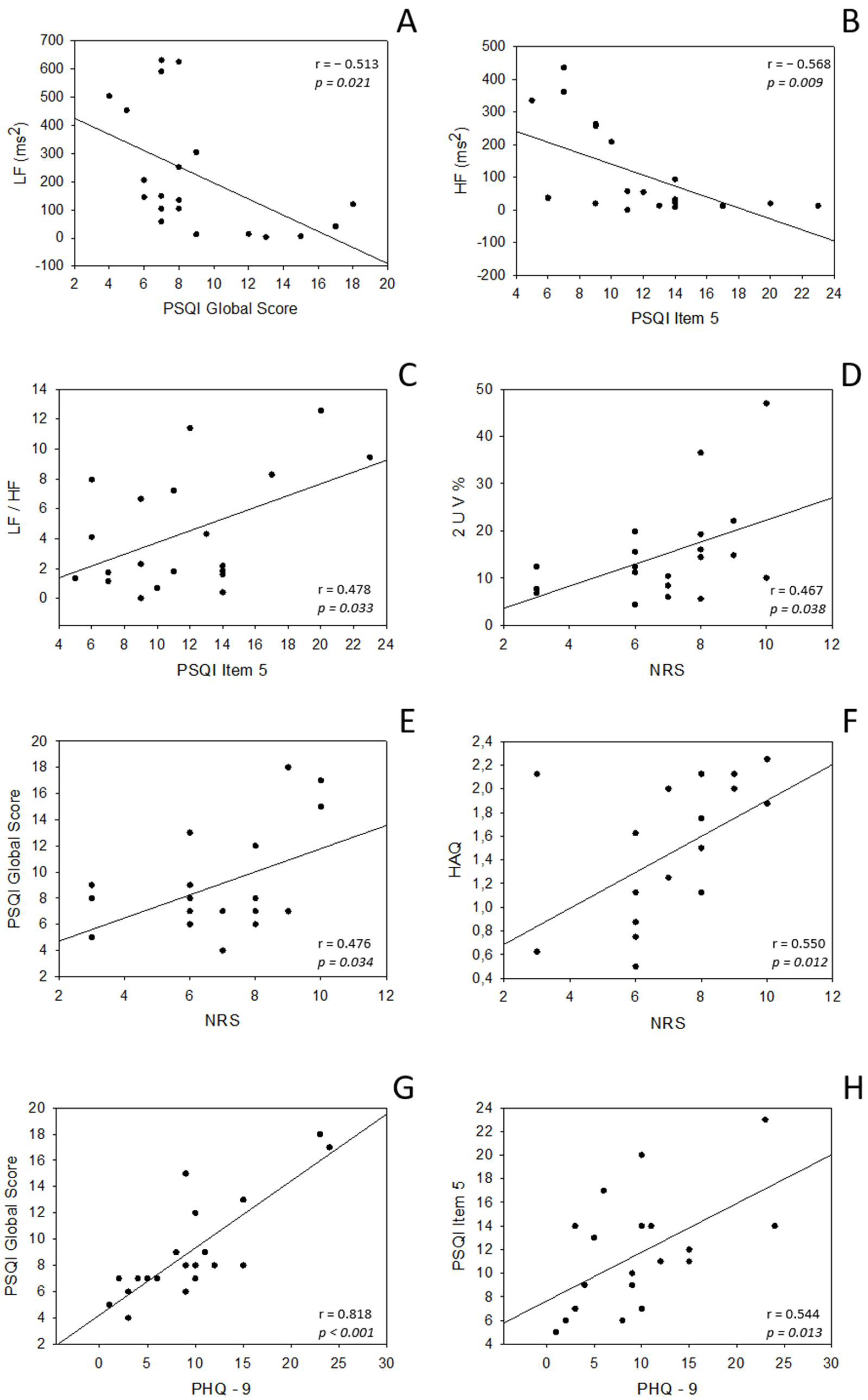
| Variables | NRS | PHQ-9 | HAQ | PSQI Global Score | PSQI Item 5 |
|---|---|---|---|---|---|
| HR | r = −0.029 p = 0.904 | r = 0.165 p = 0.488 | r = 0.204 p = 0.389 | r = 0.310 p = 0.184 | r = 0.263 p = 0.263 |
| HRV Spectral Analysis | |||||
| LF ms2 | r = −0.239 p = 0.310 | r = −0.287 p = 0.220 | r = −0.146 p = 0.538 | r = −0.513 p = 0.021 * | r = −0.392 p = 0.088 |
| HF ms2 | r = −0.028 p = 0.907 | r = −0.427 p = 0.061 | r = 0.214 p = 0.365 | r = −0.401 p = 0.080 | r = −0.568 p = 0.009 * |
| LF nu | r = −0.329 p = 0.156 | r = 0.039 p = 0.870 | r = −0.368 p = 0.110 | r = −0.191 p = 0.420 | r = 0.123 p = 0.606 |
| HF nu | r = 0.223 p = 0.345 | r = −0.162 p = 0.494 | r = 0.167 p = 0.481 | r = 0.024 p = 0.919 | r = −0.333 p = 0.151 |
| LF/HF | r = −0.171 p = 0.471 | r = 0.289 p = 0.217 | r = −0.231 p = 0.328 | r = 0.123 p = 0.606 | r = 0.478 p = 0.033 * |
| HRV Symbolic Analysis | |||||
| 0V% | r = −0.222 p = 0.346 | r = 0.081 p = 0.735 | r = −0.296 p = 0.205 | r = −0.158 p = 0.506 | r = 0.087 p = 0.716 |
| 2LV% | r = −0.131 p = 0.581 | r = −0.185 p = 0.436 | r = 0.145 p = 0.541 | r = −0.083 p = 0.730 | r = −0.301 p = 0.197 |
| 2UV% | r = 0.467 p = 0.038 * | r = −0.024 p = 0.922 | r = 0.425 p = 0.062 | r = 0.294 p = 0.208 | r = 0.106 p = 0.657 |
| Questionnaires | |||||
| NRS | r = 0.345 p = 0.137 | r = 0.550 p = 0.012 * | r = 0.476 p = 0.034 * | r = 0.402 p = 0.079 | |
| PHQ-9 | r = 0.153 p = 0.521 | r = 0.818 p < 0.001 * | r = 0.544 p = 0.013 * | ||
| HAQ | r = 0.418 p = 0.067 | r = 0.134 p = 0.573 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carandina, A.; Bellocchi, C.; Dias Rodrigues, G.; Beretta, L.; Montano, N.; Tobaldini, E. Cardiovascular Autonomic Control, Sleep and Health Related Quality of Life in Systemic Sclerosis. Int. J. Environ. Res. Public Health 2021, 18, 2276. https://doi.org/10.3390/ijerph18052276
Carandina A, Bellocchi C, Dias Rodrigues G, Beretta L, Montano N, Tobaldini E. Cardiovascular Autonomic Control, Sleep and Health Related Quality of Life in Systemic Sclerosis. International Journal of Environmental Research and Public Health. 2021; 18(5):2276. https://doi.org/10.3390/ijerph18052276
Chicago/Turabian StyleCarandina, Angelica, Chiara Bellocchi, Gabriel Dias Rodrigues, Lorenzo Beretta, Nicola Montano, and Eleonora Tobaldini. 2021. "Cardiovascular Autonomic Control, Sleep and Health Related Quality of Life in Systemic Sclerosis" International Journal of Environmental Research and Public Health 18, no. 5: 2276. https://doi.org/10.3390/ijerph18052276
APA StyleCarandina, A., Bellocchi, C., Dias Rodrigues, G., Beretta, L., Montano, N., & Tobaldini, E. (2021). Cardiovascular Autonomic Control, Sleep and Health Related Quality of Life in Systemic Sclerosis. International Journal of Environmental Research and Public Health, 18(5), 2276. https://doi.org/10.3390/ijerph18052276








