Effects of a Music-Based Rhythmic Auditory Stimulation on Gait and Balance in Subacute Stroke
Abstract
1. Introduction
2. Materials and Methods
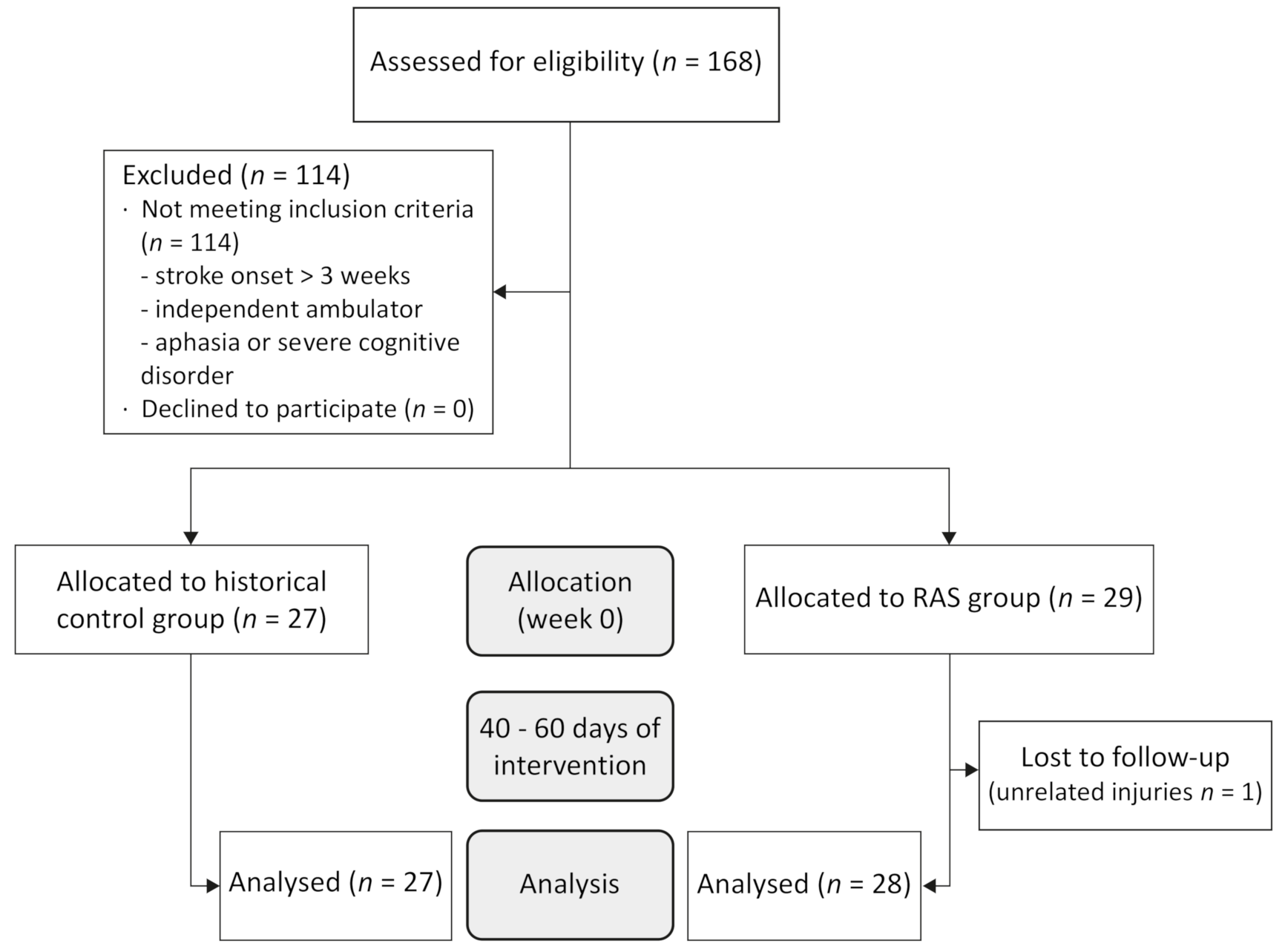
2.1. Study Design and Participants
2.2. Intervention
2.3. Outcome Assessment
2.4. Statistical Analysis
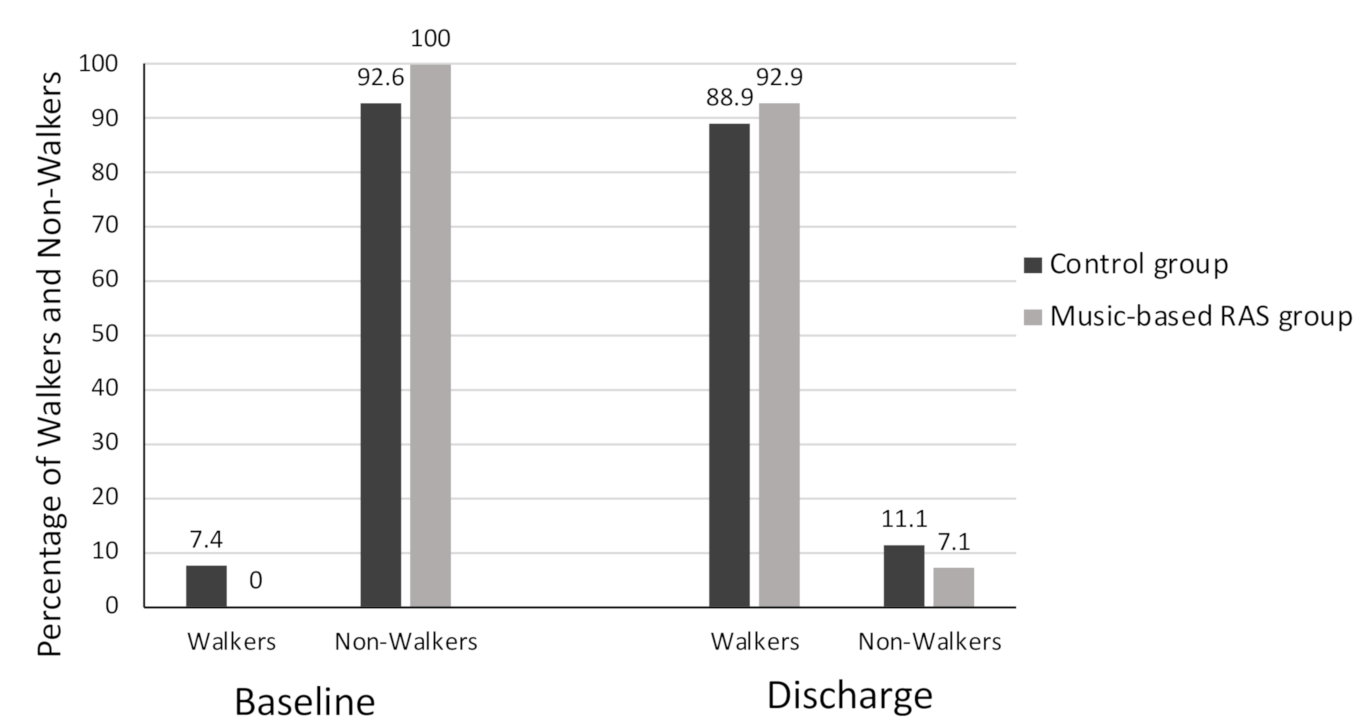
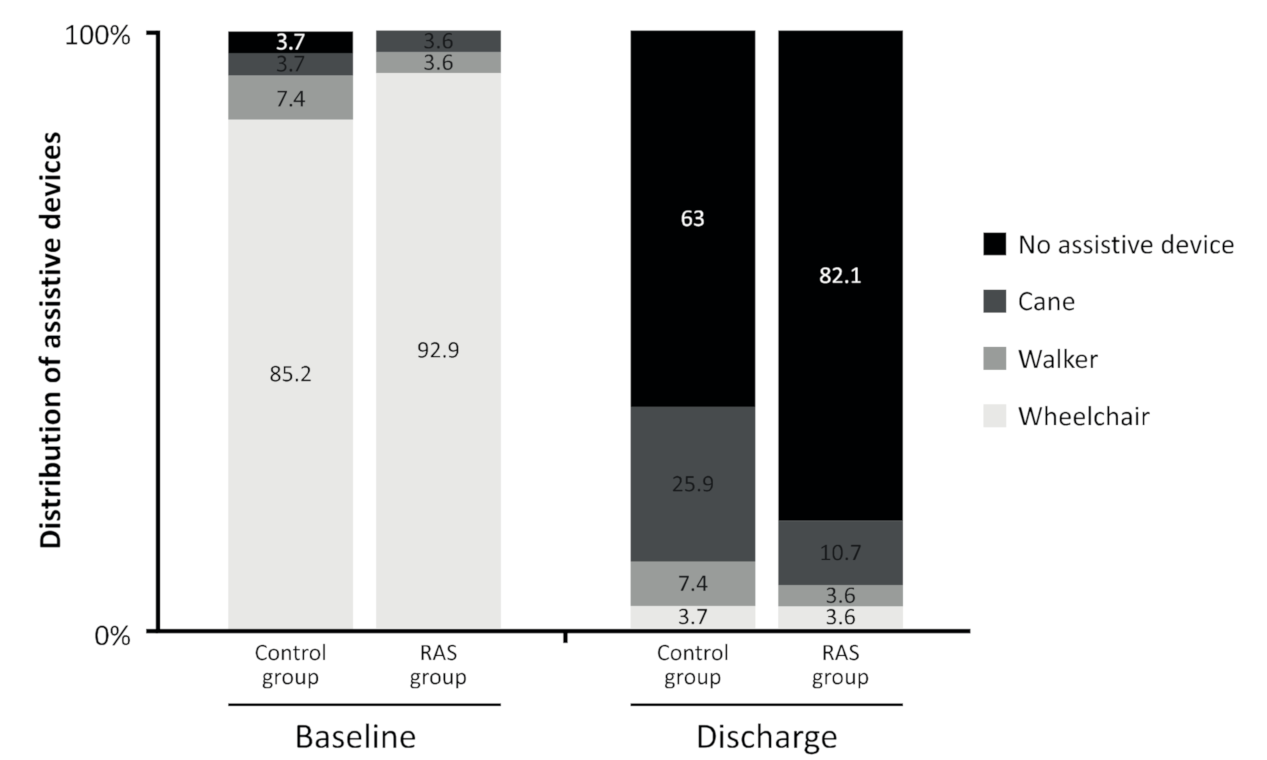
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- ∘
- Twinkle twinkle little star (68 bpm)
- ∘
- One love-Bob Marley (76 bpm)
- ∘
- No woman no cry-Bob Marley (78 bpm)
- ∘
- Lemon Tree-Fool’s Garden (143 bpm)
- ∘
- Tea for two cha cha (instrumental)-Tommy Dorsey Orchestra (167 bpm)
- ∘
- The lion sleeps tonight-The Tokens (68 bpm/121 bpm)
- ∘
- Colonel Hathi’s March, The Jungle Book-J. Pat Omalley (96 bpm)
- ∘
- Someone you loved-Lewis Capaldi (110)
- ∘
- Yellow submarine-The Beatles (111 bpm)
- ∘
- Radetzky march from Johann Strauss-performed by Peter Guth and the Royal Philharmonic Orchestra (111 bpm)
- ∘
- Ayo Technology-Milow (128 bpm)
- ∘
- Old Town Road-Lil Nas X (136 bpm)
- ∘
- Je veux-Zaz (155)
- ∘
- Lost on you-LP (174 bpm)
- ∘
- Qué vendrá-Zaz (176 bpm)
- ∘
- River flows in you-Yiruma
- ∘
- Canon D minor-Pachelbel
- ∘
- Enya-Only time
- ∘
- Intermezzo from Cavalleria Rusticana-Pietro Mascagni
References
- Thrift, A.G.; Thayabaranathan, T.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.L.; Norrving, B.; Donnan, G.A.; Cadilhac, D. Global stroke statistics. Int. J. Stroke 2017, 12, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Mercadal-Brotons, M.; Martí-Augé, P. Manual de Musicoterapia en Geriatría y Demencias; Monsa-Prayma: Barcelona, Spain, 2008. [Google Scholar]
- Poch, S. Compendio de Muiscoterapia, 2nd ed.; Herder: Barcelona, Spain, 2011; Volume 1. [Google Scholar]
- Grahn, J.A. Neural Mechanisms of Rhythm Perception: Current Findings and Future Perspectives. Top. Cogn. Sci. 2012, 4, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Alvin, J. Musicoterapia, 5th ed.; Paidós Iberica: Barcelona, Spain, 1997. [Google Scholar]
- Benenzon, R.O. Manual de Musicoterapia, 3rd ed.; Ediciones Paidós: Barcelona, Spain, 2011. [Google Scholar]
- Thaut, M.H. Rhythm, Music, and the Brain. Scientific Foundations and Clinical Applications; Taylor and Francis Group: New York, NY, USA, 2008; pp. 39–164. [Google Scholar]
- Maggee, W.L.; Clark, I.; Tamplin, J.; Bradt, J. Music interventions for acquired brain injury (Review). Cochrane Database Syst. Rev. 2017, 1, CD006787. [Google Scholar] [CrossRef]
- Mainka, S.; Wissel, J.; Völler, H.; Evers, S. The Use of Rhythmic Auditory Stimulation to Optimize Treadmill Training for Stroke Patients: A Randomized Controlled Trial. Front. Neurol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.E.; Kim, S.J. Rhythmic Auditory Cueing in Motor Rehabilitation for Stroke Patients: Systematic Review and Meta-Analysis. J. Music Ther. 2016, 53, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Carlsson, G.; Käll, L.B.; Nilsson, M.; Blomstrand, C. Experiences from a multimodal rhythm and music-based rehabilitation program in late phase of stroke recovery—A qualitative study. PLoS ONE 2018, 13, e0204215. [Google Scholar] [CrossRef]
- Moumdjian, L.; Sarkamo, T.; Leone, C.; Leman, M.; Feys, P. Effectiveness of music-based interventions on motricity or cognitive functioning in neurological populations: A systematic review. Eur. J. Phys. Rehabilit. Med. 2016, 53, 466–482. [Google Scholar]
- Shin, Y.-K.; Chong, H.J.; Kim, S.J.; Cho, S.-R. Effect of Rhythmic Auditory Stimulation on Hemiplegic Gait Patterns. Yonsei Med. J. 2015, 56, 1703–1713. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Song, C. Gait Training with Bilateral Rhythmic Auditory Stimulation in Stroke Patients: A Randomized Controlled Trial. Brain Sci. 2018, 8, 164. [Google Scholar] [CrossRef]
- Van Criekinge, T.; D’Août, K.; O’Brien, J.; Coutinho, E. The influence of sound-based interventions on motor behavior after stroke: A systematic review. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Flansbjer, U.B.; Downham, D.; Lexell, J. Knee muscle strength, gait performance, and perceived participation after stroke. Arch. Phys. Med. Rehabilit. 2006, 87, 974–980. [Google Scholar] [CrossRef]
- Neufeld, S.; Machacova, K.; Mossey, J.; Luborsky, M. Walking Ability and Its Relationship to Self-Rated Health in Later Life. Clin. Gerontol. 2013, 36, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Otokita, S.; Uematsu, H.; Kunisawa, S.; Sasaki, N.; Fushimi, K.; Imanaka, Y. Impact of rehabilitation start time on functional outcomes after stroke. J. Rehabilit. Med. 2020. [Google Scholar] [CrossRef]
- Gardiner, R. The Ronnie Gardiner Method. Okategoriserade: 2015. Available online: https://www.ronniegardinermethod.com/ (accessed on 24 September 2018).
- Canbek, J.; Fulk, G.; Nof, L.; Echternach, J. Test-Retest Reliability and Construct Validity of the Tinetti Performance-Oriented Mobility Assessment in People with Stroke. J. Neurol. Phys. Ther. 2013, 37, 14–19. [Google Scholar] [CrossRef]
- Hafsteinsdóttir, T.B.; Rensink, M.; Schuurmans, M. Clinimetric Properties of the Timed Up and Go Test for Patients With Stroke: A Systematic Review. Top. Stroke Rehabilit. 2014, 21, 197–210. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive Validity and Responsiveness of the Functional Ambulation Category in Hemiparetic Patients After Stroke. Arch. Phys. Med. Rehabilit. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Fujii, R.; Sugawara, H.; Ishikawa, M.; Fujiwara, T. Effects of Different Orthoses Used for Gait Training on Gait Function among Patients with Subacute Stroke. Prog. Rehabilit. Med. 2020, 5, 20200023. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, N.; Kai, C.; Hokotachi, Y.; Hasegawa, M.; Amagai, T. Determination of the cut-off point of the Functional Independence Measure as a predictor of adverse events in patients with acute stroke. J. Int. Med Res. 2018, 46, 4235–4245. [Google Scholar] [CrossRef]
- Duffy, L.; Gajree, S.; Langhorne, P.; Stott, D.J.; Quinn, T.J. Reliability (inter-rater agreement) of the Barthel Index for assessment of stroke survivors. Stroke 2013, 44, 462–468. [Google Scholar] [CrossRef]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and its use in future stroke trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I. Effects of (music-based) rhythmic auditory cueing training on gait and posture post-stroke: A systematic review & dose-response meta-analysis. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Schaffert, N.; Janzen, T.B.; Mattes, K.; Thaut, M.H. A review on the relationship between sound and movement in sports and rehabilitation. Front. Psychol. 2019, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, K.J.; Song, C.H. Effects of rhythmic auditory stimulation (RAS) on gait ability and symmetry after stroke. J. Phys. Ther. Sci. 2012, 24, 311–314. [Google Scholar] [CrossRef]
- Bunketorp-Käll, L.; Lundgren-Nilsson, Å.; Samuelsson, H.; Pekny, T.; Blomvé, K.; Pekna, M.; Pekny, M.; Blomstrand, C.; Nilsson, M. Long-Term Improvements After Multimodal Rehabilitation in Late Phase After Stroke. Stroke 2017, 48, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Wressle, E.; Lundin, F.; Enthoven, P.; Dizdar, N. Group-based music intervention in Parkinson’s disease—Findings from a mixed-methods study. Clin. Rehabilit. 2020, 1–12. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lim, H.; Park, G.-C.; Kim, M.-H.; Lee, B.-H. Effects of the combination of rhythmic auditory stimulation and task-oriented training on functional recovery of subacute stroke patients. J. Phys. Ther. Sci. 2012, 24, 1307–1313. [Google Scholar]
- Kwakkel, G.; Van Peppen, R.; Wagenaar, R.C.; Dauphinee, S.W.; Richards, C.; Ashburn, A.; Miller, K.; Lincoln, N.; Partridge, C.; Wellwood, I.; et al. Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke 2004, 35, 2529–2536. [Google Scholar] [CrossRef]
- Peurala, S.; Airaksinen, O.; Huuskonen, P.; Jäkälä, P.; Juhakoski, M.; Sandell, K.; Tarkka, I.; Sivenius, J. Effects of intensive therapy using gait trainer or floor walking exercises early after stroke. J. Rehabilit. Med. 2009, 41, 166–173. [Google Scholar] [CrossRef]
- Hunnicutt, J.L.; Gregory, C.M. Skeletal muscle changes following stroke: A systematic review and comparison to healthy individuals. Top. Stroke Rehabilit. 2017, 24, 463–471. [Google Scholar] [CrossRef]
- Selves, C.; Stoquart, G.; Lejeune, T. Gait rehabilitation after stroke: Review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol. Belg. 2020, 120, 783–790. [Google Scholar] [CrossRef]
- Scrivener, K.; Sherrington, C.; Schurr, K. A systematic review of the responsiveness of lower limb physical performance measures in inpatient care after stroke. BMC Neurol. 2013, 13, 4. [Google Scholar] [CrossRef]
- Lin, L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS ONE 2018, 13, e0204056. [Google Scholar] [CrossRef]
- Bour, A.M.J.J.; Rasquin, S.; Boreas, A.M.H.P.; Limburg, M.; Verhey, F.R.J. How predictive is the MMSE for cognitive performance after stroke? J. Neurol. 2009, 257, 630–637. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, W.-Y.; Li, F.; Ge, J.-S.; Zhang, X.; Luo, X.; Wang, Y.-L. Effect of Rhythm of Music Therapy on Gait in Patients with Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105544. [Google Scholar] [CrossRef]
- Forner-Cordero, A.; Pinho, J.P.; Umemura, G.; Lourenço, J.C.; Mezêncio, B.; Itiki, C.; Krebs, H.I. Effects of supraspinal feedback on human gait: Rhythmic auditory distortion. J. Neuroeng. Rehabilit. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Hessam, M.; Salehi, R.; Yazdi, M.J.S.; Negahban, H.; Rafie, S.; Mehravar, M. Relationship between functional balance and walking ability in individuals with chronic stroke. J. Phys. Ther. Sci. 2018, 30, 993–996. [Google Scholar] [CrossRef]
| Control Group (n = 27) | Music-Based RAS Group (n = 28) | p-Value | |
|---|---|---|---|
| Age (years) | 62.2 ± 8.9 | 65.7 ± 12.7 | 0.246 |
| Sex (n) | 0.335 | ||
| Male | 19 (70.4) | 16 (57.7) | |
| Female | 8 (29.5) | 12 (42.3) | |
| Stroke etiology (n) | 0.922 | ||
| Hemorrhage | 9 (33.3) | 9 (34.6) | |
| Infarction | 18 (66.7) | 17 (65.4) | |
| Side of hemiparesis (n) | – | – | 0.491 |
| Right | 20 (74.1) | 17 (65.4) | |
| Left | 7 (25.9) | 9 (34.6) | |
| Affected area (n) | – | – | 0.453 |
| Basal ganglia | 6 (22.2) | 6 (23.1) | |
| MCA | 7 (25.9) | 6 (23.1) | |
| Vertebrobasilar | 3 (11.1) | 7 (26.9) | |
| Lacunar | 5 (18.5) | 4 (15.4) | |
| MCA + ACA | 1 (3.7) | 1 (3.8) | |
| Thalamus | 1 (3.7) | 1 (3.8) | |
| Cerebellar | 4 (14.8) | 1 (3.8) | |
| Risk factors (n) | – | – | – |
| Arterial hypertension | 17 (63.0) | 19 (67.9) | 0.703 |
| Diabetes Mellitus type 2 | 11 (40.7) | 11 (39.3) | 0.912 |
| Dyslipidemia | 9 (33.3) | 12 (42.9) | 0.467 |
| Heart disease | 9 (33.3) | 7 (25) | 0.496 |
| Obesity | 5 (18.5) | 10 (35.7) | 0.152 |
| Toxic habits | – | – | – |
| Tobacco | 4 (14.8) | 5 (17.9) | 0.348 |
| Alcohol | 4 (14.8) | 1 (3.6) | |
| Mental health disorders | 3 (11.1) | 4 (14.3) | 0.724 |
| NIHSS (n) | – | – | 0.583 |
| Acute hospital admissions | – | – | |
| Josep Trueta Hospital | 9.1 ± 5.3 | 8.9 ± 6.4 | |
| Median (IQR) | 8 (5–13) | 8 (3.3–11) | |
| Days from onset (days) | 0.600 | ||
| 10.07 ± 4.27 | 10.46 ± 4.09 | ||
| Median (IQR) | 9 (7–11) | 10 (7–14) | |
| Days of stay (days) | – | – | 0.293 |
| Subacute and rehabilitation | – | – | |
| Mutuam Hospital | 52.3 ± 24.9 | 45.7 ± 20.6 | |
| Median (IQR) | 48 (36–73) | 43.5 (26–62.2) |
| Outcome | Baseline | Discharge | ||||
|---|---|---|---|---|---|---|
| Control Group (n = 27) | Music-Based RAS Group (n = 28) | p-Value | Control Group (n = 27) | Music-Based RAS Group (n = 28) | p-Value | |
| Tinetti score | 9.8 ± 7.5 | 8.3 ± 6.8 | 0.389 | 24.1 ± 4.3 | 23.1 ± 5.8 | 0.593 |
| (max score = 28) | 9 (3–16) | 8 (1–14) | 26 (21–27) | 24.5 (22–27) | ||
| Timed Up and Go | 16.5 ± 4.8 | 20.5 ± 11.9 | 0.79 | 12.6 ± 10.8 | 14.0 ± 6.1 | 0.058 |
| (seconds) | 16.4 (12.7–20.4) | 17.1 (3.6–24.3) | 10.4 (6.6–13.4) | 12.4 (10.1–16.0) | ||
| Gait Speed | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.314 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.314 |
| (meters per second) | 0.0 (0) | 0.0 (0) | 0.5 (0.3–0.6) | 0.6 (0.4–0.9) | ||
| FAC | 1.2 ± 0.6 | 0.4 ± 0.7 | 0.142 | 3.7 ± 1.2 | 3.8 ± 1.1 | 0.696 |
| (max score = 6) | 1 (1–1) | 0 (0–0.7) | 4 (3–5) | 4 (3–4) | ||
| FIM | 87.9 ± 17.2 | 85.5 ± 19.6 | 0.99 | 119 ± 9.2 | 120.0 ± 6.9 | 0.638 |
| (max score =126) | 86 (78–97) | 88 (72.7–98) | 122 (120–124) | 121 (120–124) | ||
| NIHSS # | 5.1 ± 3.0 | 5.6 ± 3.5 | 0.622 | 1.6 ± 1.8 | 0.7 ± 2.2 | 0.036 |
| (max score = 42) | 4 (3–7) | 5 (3–8) | 1 (0–2.5) | 2.5 (1–3) | ||
| Barthel index | 42.2 ± 14.7 | 48.1 ± 21.7 | 0.254 | 92.6 ± 10.3 | 91.1 ± 13.7 | 0.646 |
| (max score = 100) | 45 (30–55) | 45 (35–63.7) | 95 (90–100) | 92.5 (90–100) | ||
| Outcome | Control Group (n = 27) | Intervention Group (n = 28) | p-Value |
|---|---|---|---|
| Tinetti score | 14.30 ± 6.71 | 14.71 ± 7.37 | 0.840 |
| 15 (8–20) | 14 (8.5–20.75) | ||
| Gait speed | 0.36 ± 0.19 | 0.53 ± 0.26 | 0.621 |
| FAC score | 2.48 ± 1.09 | 3.43 ± 1.17 | 0.002 * |
| 3 (2–3) | 3.5 (3–4) | ||
| FIM score | 31.07 ± 17.58 | 34.57 ± 16.70 | 0.544 |
| 33 (22–43) | 35 (23–44.5) | ||
| Barthel Index | 50.37 ± 13.65 | 44.29 ± 20.98 | 0.326 |
| 50 (45–60) | 47.5 (31.25–55) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Hoelling, S.; Bertran-Noguer, C.; Reig-Garcia, G.; Suñer-Soler, R. Effects of a Music-Based Rhythmic Auditory Stimulation on Gait and Balance in Subacute Stroke. Int. J. Environ. Res. Public Health 2021, 18, 2032. https://doi.org/10.3390/ijerph18042032
Gonzalez-Hoelling S, Bertran-Noguer C, Reig-Garcia G, Suñer-Soler R. Effects of a Music-Based Rhythmic Auditory Stimulation on Gait and Balance in Subacute Stroke. International Journal of Environmental Research and Public Health. 2021; 18(4):2032. https://doi.org/10.3390/ijerph18042032
Chicago/Turabian StyleGonzalez-Hoelling, Samira, Carme Bertran-Noguer, Gloria Reig-Garcia, and Rosa Suñer-Soler. 2021. "Effects of a Music-Based Rhythmic Auditory Stimulation on Gait and Balance in Subacute Stroke" International Journal of Environmental Research and Public Health 18, no. 4: 2032. https://doi.org/10.3390/ijerph18042032
APA StyleGonzalez-Hoelling, S., Bertran-Noguer, C., Reig-Garcia, G., & Suñer-Soler, R. (2021). Effects of a Music-Based Rhythmic Auditory Stimulation on Gait and Balance in Subacute Stroke. International Journal of Environmental Research and Public Health, 18(4), 2032. https://doi.org/10.3390/ijerph18042032





