Comprehensive Risk Assessment of Schistosomiasis Epidemic Based on Precise Identification of Oncomelania hupensis Breeding Grounds—A Case Study of Dongting Lake Area
Abstract
1. Introduction
2. Materials and Methods
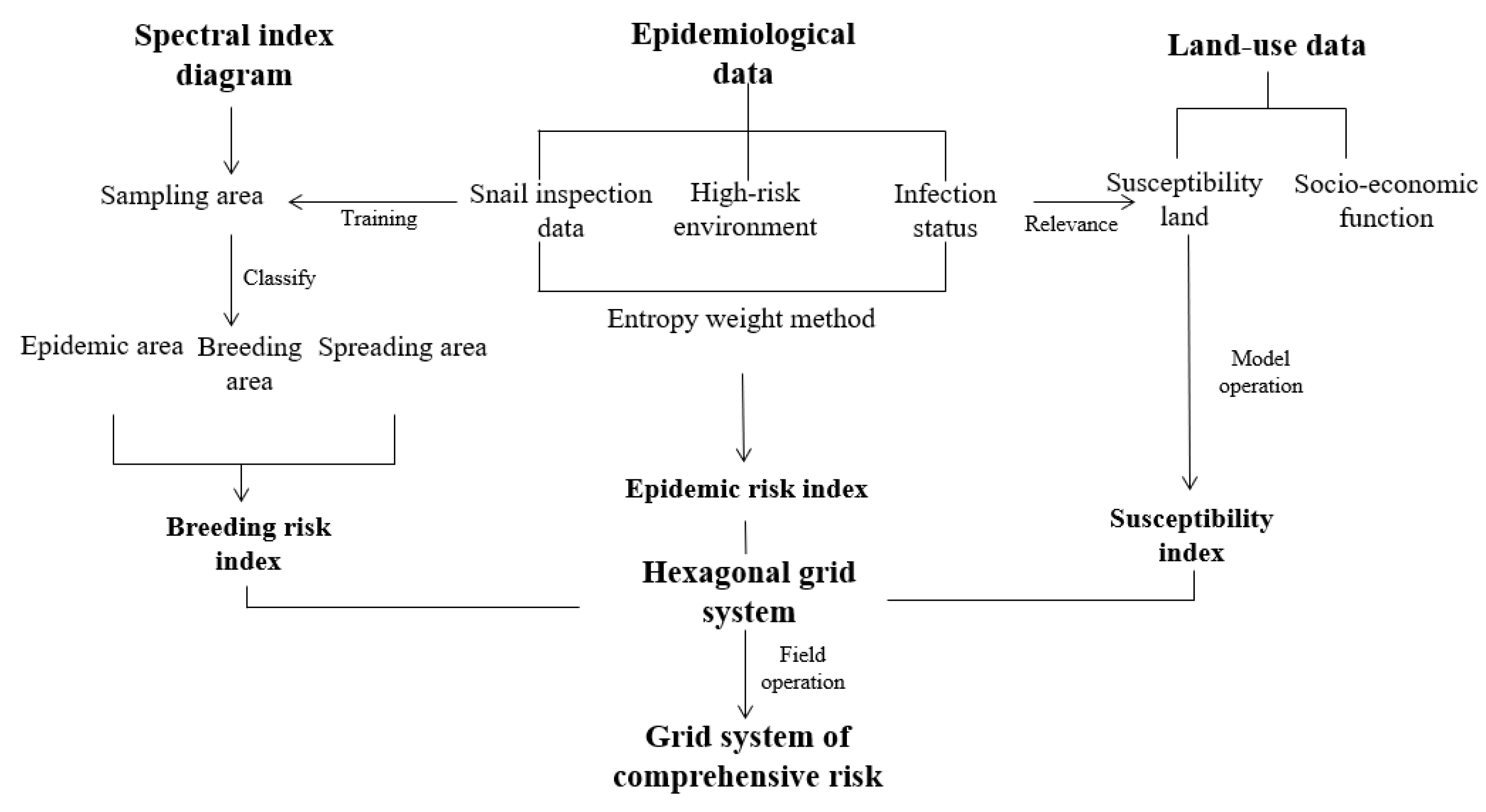
2.1. Research Framework
- (1)
- Since O. hupensis, patients, livestock, and contaminated water can be potentially exposed to infection and have strong spatiotemporal dynamics, evaluating the complex relationships of these factors is important in understanding the schistosomiasis risks [17,18]. Simply studying a single parameter’s activity patterns cannot accurately reveal the potential risks for an epidemic outbreak. However, the breeding grounds of O. hupensis can be monitored as the epidemic source using the environmental detection of important sources for the breeding and spread of O. hupensis.
- (2)
- The development and change of an epidemic is a long-term, spatio-temporal, cause-and-effect process and is closely related to snail status, patients, and sick animals [19]. The epidemic risk data, comprehensively defined based on epidemiological data, can be used to measure the influence of various factors including snail distribution, snail density, number of patients, number of sick animals, and their activities.
- (3)
- Epidemic factors are both complex and changeable. Schistosomiasis distribution characteristics, patterns, and trends vary considerably for different regions, prevalence types, and socio-economic attributes [20,21]. Hence, the sensitivity of different types of ground objects to changes in the epidemic was determined. We also measured the susceptibility for the different regions based on the combination of land-use types to quantify the potential risks of epidemic carriers.
2.2. Research Methods
2.2.1. Construction of the Grid System
2.2.2. Identification and Extraction of Snail Breeding Grounds
- (1)
- Identification and extraction of environmental types of potential O. hupensis breeding grounds based on spectral features
- (2)
- Identification of potential O. hupensis breeding grounds in the DTL area from 2006 to 2016
2.2.3. Evaluation and Calculation of Potential Epidemic Risk of Schistosomiasis
- (1)
- 2006–2016 epidemic index evaluation and calculation in potential risk areas
- (2)
- 2006–2016 susceptibility index calculation of potential risk areas
2.2.4. Extraction and Storage of Data in Grid System
2.2.5. Quantification and Visualization of Comprehensive Risks in Epidemic Areas
2.3. Data Source and Extraction of the Potential Risk Study Area
3. Results
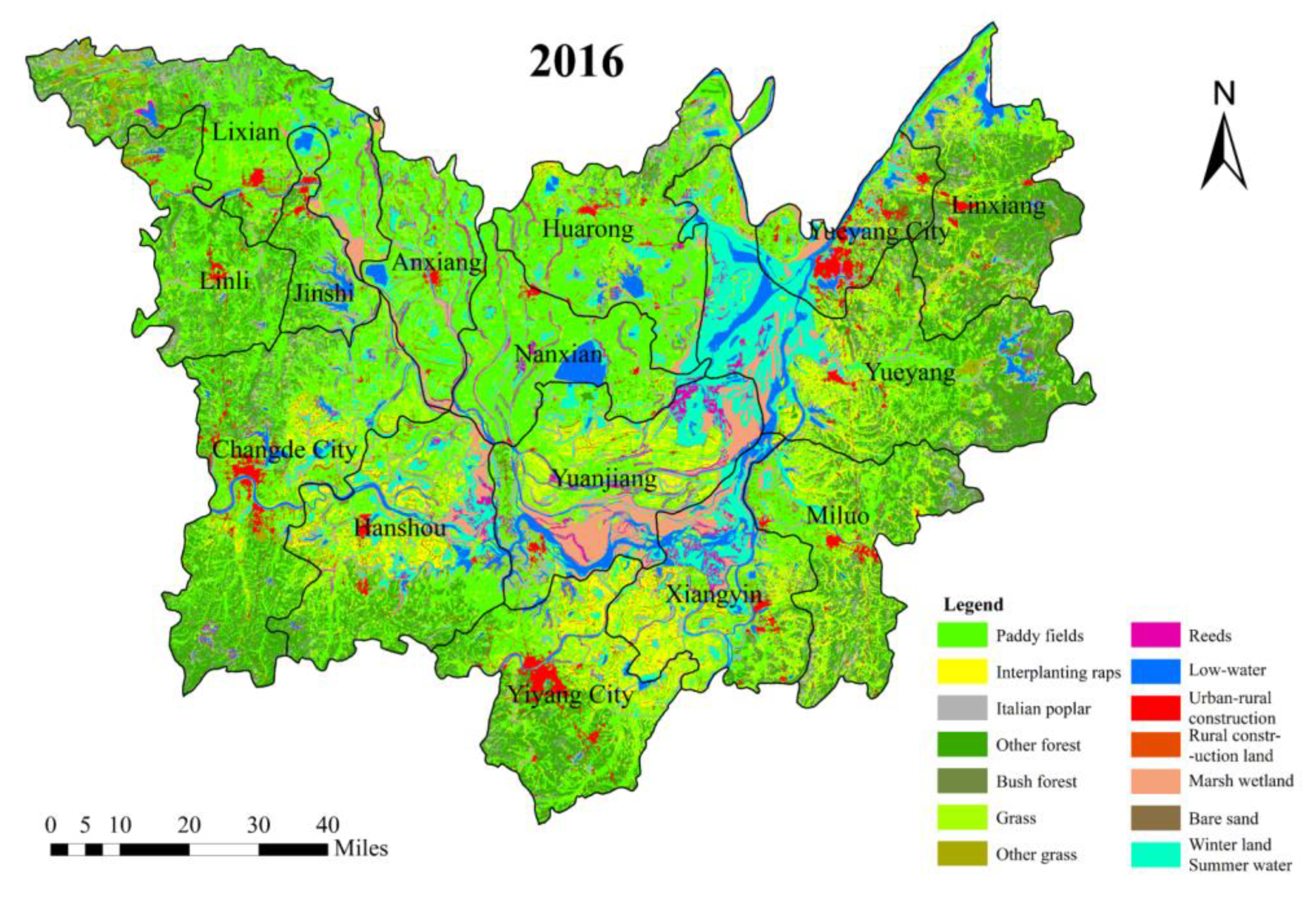
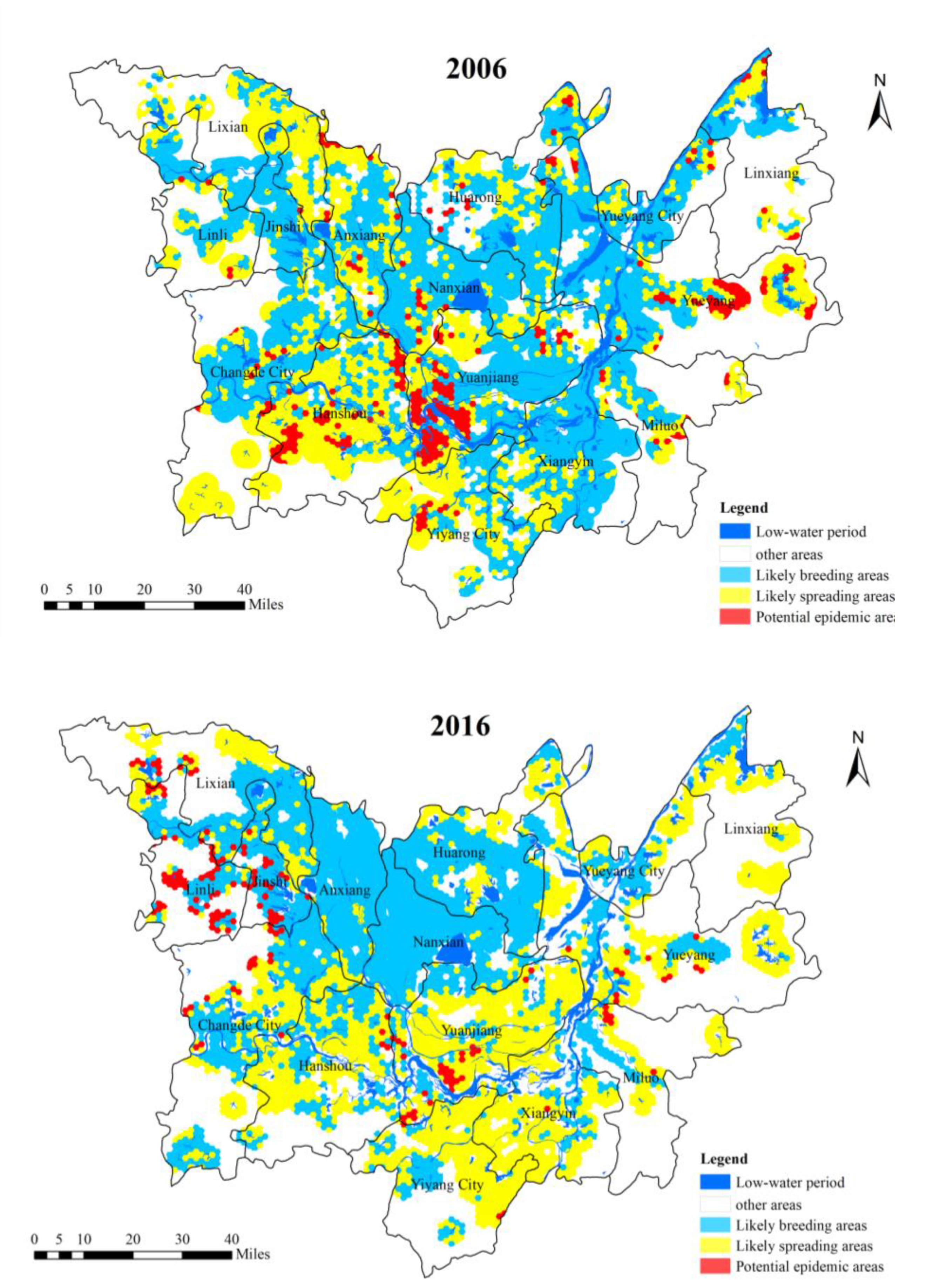
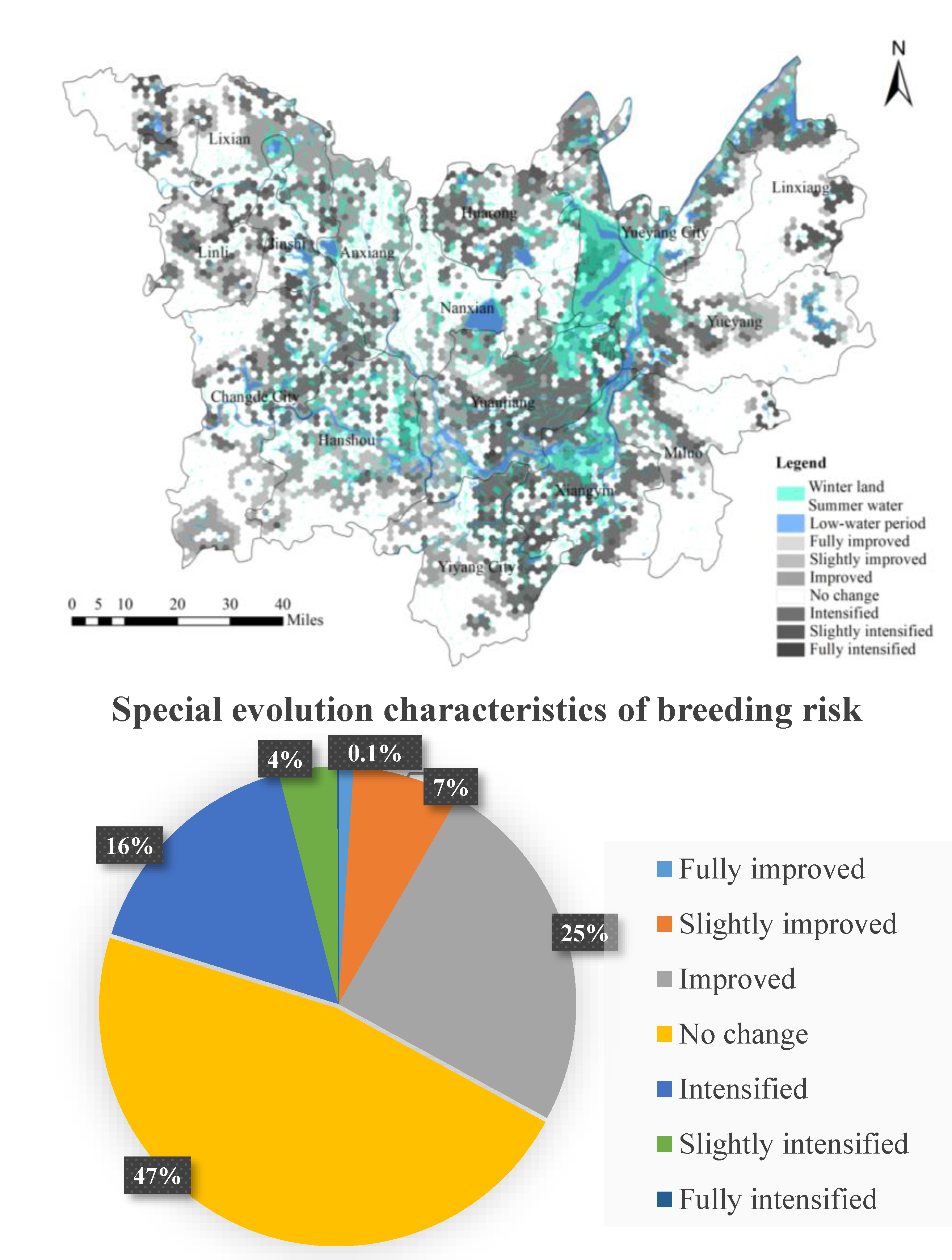
3.1. Spatial Distribution Analysis of Potential O. hupensis Breeding Grounds
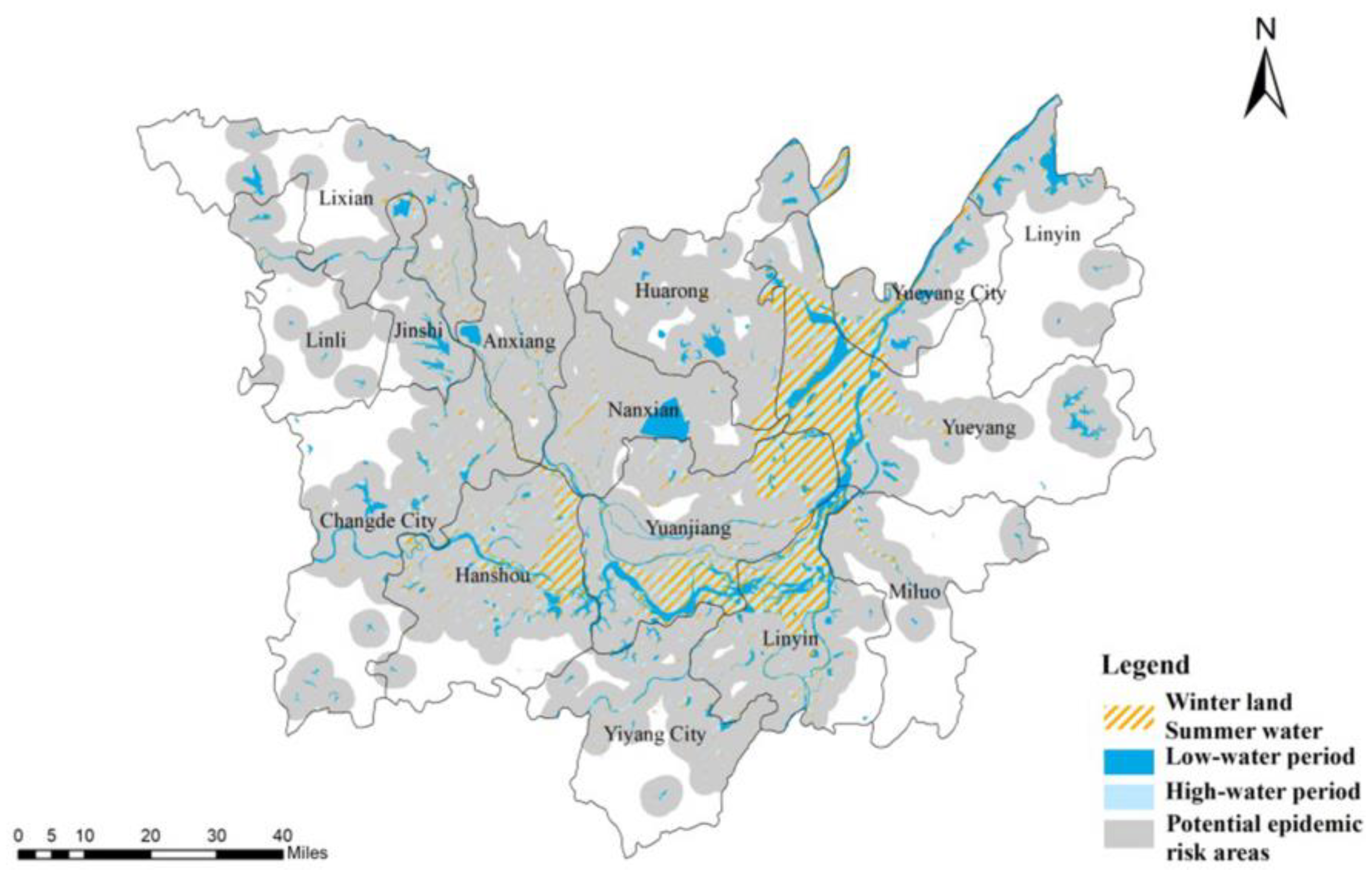
3.2. Analysis of Comprehensive Risk Evolution Characteristics of Schistosomiasis Epidemic
3.3. Comprehensive Risk Classification Criteria of Schistosomiasis in DTL Area
4. Discussion
4.1. Regional Characteristics and Control Strategies for Medium- and High-Risk Areas
- (1)
- Level I risk areas
- (2)
- Level II risk areas
- (3)
- Level III risk areas
4.2. Driving Factors of Comprehensive Risk
5. Conclusions
- (1)
- From 2006 to 2016, the spatial change of potential O. hupensis breeding grounds showed a weakening trend from the eastern and northern areas of DTL to the southwestern area. In the four types of risk areas, most of the improved areas exhibited a decrease in risk over time. For those that exhibited some change in breeding, the changes were mostly minor. More potential O. hupensis breeding areas emerged in east DTL, exhibiting some lakeside and hydrophilic agglomeration characteristics. The snail breeding areas evolved from fragmented to centralized distribution and had distinct regional (spatial) differentiation. The results also indicate the weakening of the snail population’s spatial mobility, the increasing independence of single snail groups, and the growing dependence of snail populations on their local environment.
- (2)
- The spatial risk distribution in potential risk areas in DTL exhibited an overall pattern of high in the core area, low in the peripheral area, high in the periphery of large lakes, low in other areas, high in the west Dongting area, and low in the east Dongting area. The cold-spot areas had Huarong County and Anxiang County as the core, with scattered distributions in peripheral areas. From 2006 to 2016, the core cold-spot region declined, the marginal cold-spot patches shrank, but the number of patches significantly increased. The risk distribution’s center shifted to the northwest, and the distribution axis extended from northeast to southwest. The evolution was initially in the east–west direction and then shifted to the north–south direction. The spatial risk distribution exhibited enhanced concentricity along the major axis and increased dispersion along the minor axis.
- (3)
- Using the epidemiological, socio-economic, and environmental characteristics of extreme high-risk, high-risk, and modern risk areas, we put forward targeted and differentiated strategies to prevent and control the occurrence and spread of the schistosomiasis epidemic. These strategies and measures can reduce the O. hupensis brewing risk, aesthetic risk, and susceptibility of land use in various regions. They can also be used to promote socio-economic development and environmental protection in different regions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Z.Q. Problems and countermeasures in the construction of emergency plan system for public health emergencies in China. Mod. Prev. Med. 2008, 12, 2400–2402. [Google Scholar]
- Qi, L.L.; Zhou, S.H.; Yan, X.P.; Gu, J. Development trend and current hot spot of medical geography. Adv. Geogr. Sci. 2013, 8, 1276–1285. [Google Scholar]
- Guimarães, R.J.; Freitas, C.C.; Dutra, L.V.; Moura, A.C.; Amaral, R.S.; Drummond, S.C.; Scholte, R.G.; Carvalho, O.S. Schistosomiasis risk estimation in Minas Gerais State, Brazil, using enviornmental data and GIS techniques. Acta Trop. 2008, 108, 234–241. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, C.; Zhang, Z.; Luo, C.; Ward, M.; Gao, J.; Zhang, L.; Jiang, Q. Dynamics of spatial clustering of schistosomiasis in the Yangtze River Valley at the end of and following the World Bank Loan Project. Parasitol. Int. 2014, 63, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Leal-Neto, O.B.; Albuquerque, J.; da Silva, H.; Barbosa, C.S. Schistosomiasis transmission and environmental change: A spatio-temporal analysis in Porto de Galinhas, Pernambuco-Brazil. Int. J. Health Geogr. 2012, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Ngassam, R.K.; Kouninki, H.; Monglo, B.; Djekin, E.; Liang, S.; Tchuente, L.T. Identification and mapping of some potential transmission foci of schistosomasis in Maroua, Far North Region, Cameroon. Int. J. Innov. Appl. Stud. 2014, 7, 65. [Google Scholar]
- Wang, Z.L.; Gao, J.; Tao, B.; Jiang, Q.L.; Zhang, Z.J.; Jiang, Q.W. Preliminary study on applying high resolution CBERS images to identify Oncomelania snail habitats in lake and marshland regions. Chin. J. Schistosomiasis Control. 2012, 6, 640–644. [Google Scholar]
- Xia, C.C.; Lu, C.F.; Li, S.; Zhang, T.J.; Lin, S.H.; Hu, Y.; Liu, Y.; Zhang, Z.J. Maxinum entropy model versus remote sensing-based methods for extracting Oncomelania hupensis snail habitats. Chin. J. Schistosomiasis Control. 2017, 29, 12–17. [Google Scholar]
- Zhang, Z.; Ward, M.; Gao, J.; Wang, Z.; Yao, B.; Zhang, T.; Jiang, Q. Remote sensing and disease control in China: Past, present and future. Parasites Vectors 2013, 6, 11. [Google Scholar] [CrossRef]
- Hu, Y.; Li, R.; Bergquist, R.; Lynn, H.; Gao, F.; Wang, Q.; Zhang, S.; Sun, L.; Zhang, Z.; Jiang, Q. Spatio-temporal Transmission and Environmental Determinants of Schistosomiasis Japonica in Anhui Province, China. PLoS Negl. Trop. Dis. 2015, 9, e0003470. [Google Scholar] [CrossRef]
- Balen, J.; Zhao, Z.-Y.; Williams, G.M.; McManus, D.P.; Raso, G.; Utzinger, J.; Zhou, J.; Li, Y.-S. Prevalence, intensity and associated morbidity of Schistosoma japonicum infection in the Dongting Lake region, China. Bull. World Health Organ. 2007, 85, 519–526. [Google Scholar] [CrossRef]
- Shi, X. Research progress on ecological snail control technology. J. Trop. Dis. Parasitol 2018, 16, 117–121. [Google Scholar]
- Du, Y.; Xue, H.-P.; Wu, S.-J.; Ling, F.; Xiao, F.; Wei, X.-H. Lake area changes in the middle Yangtze region of China over the 20th century. J. Environ. Manag. 2011, 92, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-Q.; Hu, B.-J.; Zhou, Y.-C.; Wang, X.-R.; Liu, K.-J.; Wang, Y.-M.; Meng, S.-H.; Li, G.-P.; Ren, G.-H. Retrospective investigation of schistosomiasis endemic situation in Hunan Province. Retrospective investigation of schistosomiasis endemic situation in Hunan Province. Chin. J. Schistosomiasis Control. 2014, 26, 491–493. [Google Scholar]
- Yang, K.; Li, S.-Z. Application of Big Data Mining Technology in Monitoring and Early-warning of Schistosomiasis. Chin. J. Parasitol. Parasit. Dis. 2015, 6, 461–465. [Google Scholar]
- Sun, M.; Xu, N.; Li, C.; Wu, D.; Zou, J.; Wang, Y.; Hao, M. The public health emergency management system in China: Trends from 2002 to 2012. BMC Public Health. 2018, 18, 474. [Google Scholar] [CrossRef]
- Shi, Y.; Li, R.; Xu, X.; Qiu, J.; Liu, K.; Chang, B.; Yi, F. Analysis on the distribution and succession of Oncomelania snails in Hubei Province since 1980. Resour. Environ. Yangtze Basin 2015, 24, 1744–1750. [Google Scholar]
- Gong, P.; Xu, B.; Liang, S. Remote sensing and geographic information systems in the spatial temporal dynamics modeling of infectious diseases. Sci. China Ser. C Life Sci. 2006, 49, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, S.; Liu, J.; Xiao, Y.; Li, G.; Shan, X.; Zhang, J. Epidemic and spatial distribution of Schistosomiasis in Hubei province from 2008 to 2012. Chin. J. Epidemiol. 2014, 35, 1366–1370. [Google Scholar]
- Hong, X.-C.; Xu, X.-J.; Chen, X.; Li, Y.-S.; Yu, C.-H.; Yuan, Y.; Chen, Y.-Y.; Li, R.-D.; Qiu, J.; Liu, Z.-C.; et al. Assessing the Effect of an Integrated Control Strategy for Schistosomiasis Japonica Emphasizing Bovines in a Marshland Area of Hubei Province, China: A Cluster Randomized Trial. PLoS Negl. Trop. Dis. 2013, 7, e2122. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Shi, Z.H.; Chong, F.C. Assessing regional environmental quality by integrated use of remote sensing, GIS, and spatial multi-criteria evaluation for prioritization of environmental restoration. Environ. Monit. Assess 2014, 186, 6993–7009. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; He, Q.Y.; Hu, B.J. Identification and risk monitoring of potential risk areas of schistosomiasis in Dongting Lake area. Chin. J. Dis. Control Prev. 2019, 23, 555–560. [Google Scholar]
- Xu, J.; He, Q.Y.; Zhu, Z. The Evolution Characteristics of the Industrial Lands of the Changsha-Zhuzhou-Xiangtan Urban Aglomeration. Econ. Geogr. 2018, 38, 89–97. [Google Scholar]
- Ouyang, X.; Wang, Z.; Zhu, X. Construction of the Ecological Security Pattern of Urban Agglomeration under the Framework of Supply and Demand of Ecosystem Services Using Bayesian Network Machine Learning: Case Study of the Changsha–Zhuzhou–Xiangtan Urban Agglomeration, China. Sustainability 2019, 11, 6416. [Google Scholar] [CrossRef]
- Ouyang, X.; Wei, X.; Li, Y.; Wang, X.-C.; Klemeš, J.J. Impacts of urban land morphology on PM2.5 concentration in the urban agglomerations of China. J. Environ. Manag. 2021, 283, 112000. [Google Scholar] [CrossRef]
- Zhu, Z.; He, Q.Y. Spatial Effect of the Dams and Sluices on the Risk of Schistomiasis in Dongting Lake refion of Hunan Province. Chin. J. Parasitol. Parasit. Dis. Oct. 2015, 33, 328–337. [Google Scholar]
- Zhu, Z. Spatial Structure of the Changsha–Zhuzhou–Xiangtan Urban Agglomeration Based on Dynamic Simulation Analysis. J. Urban Plan. Dev. 2015, 141, 357–369. [Google Scholar]
- He, M.Z.; Peng, W.X.; Jiang, Q.W. Application of Landsat TM images on the snail habitats monitoring in mountainous regions. Fudan Univ. J. Med. Sci. 2010, 5, 510–513. [Google Scholar]
- Zhang, S.Q.; Jiang, Q.W.; Wang, T.P.; Zhao, G.M.; Ge, J.H. Ecological surveillance on breeding ground for Oncomelania hupensis snails in the areas prevalent with islet-type schistosomiasis using remote sensing technology. Chin. J. Prev. Med. 2003, 5, 31–34. [Google Scholar]
- Tan, Z.F. Study on the Environment Factor and Spatial Characteristic of Oncomelania Snails’ Distribution in Marshlands of Dongting Lake Based on RS and GIS; Hunan Normal University: Changsha, China, 2006. [Google Scholar]
- Qin, J.X.; Tan, Z.F.; Zhang, C. Environment factors and spatial characters of distribution of oncomelania snails in islet and beach of Dongting Lake area. J. Nat. Disasters 2008, 4, 19–27. [Google Scholar]
- Gong, Z.; Luo, Q.-Z.; Lin, L.; Su, Y.-P.; Peng, H.-B.; Du, K.; Yu, P.; Wang, S.-P. Association of MICA gene polymorphisms with liver fibrosis in schistosomiasis patients in the Dongting Lake region. Braz. J. Med. Biol. Res. 2012, 45, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.D.S.; Leal-Neto, O.B.; De Oliveira, F.J.M.; Campos, J.V.; Souza-Santos, R.; Barbosa, C.S. Risk analysis for occurrences of schistosomiasis in the coastal area of Porto de Galinhas, Pernambuco, Brazil. BMC Infect. Dis. 2014, 14, 101. [Google Scholar]
- Wang, T.P.; Zhao, F.; Zhang, S.Q.; Zhang, Z.J.; Gao, F.H.; Zhou, Y.B.; He, J.X.; Jiang, Q.W. Spatial-temporal clustering analysis of schistosomiasis in Anhui from 2000 to 2008. J. Trop. Dis. Parasitol. 2011, 9, 127–130. [Google Scholar]
- Sun, L.-P.; Wang, W.; Liang, Y.-S.; Tian, Z.-X.; Hong, Q.-B.; Yang, K.; Yang, G.-J.; Dai, J.-R.; Gao, Y. Effect of an integrated control strategy for schistosomiasis japonica in the lower reaches of the Yangtze River, China: An evaluation from 2005 to 2008. Parasites Vectors 2011, 4, 243. [Google Scholar] [CrossRef] [PubMed]
- Kloos, H.; Correa-Oliveira, R.; Carlos dos, R.D.; Rodrigues, W.E.; Monteiro, A.S.L.; Gazzinelli, A. The role of population movement in the epidemiology and control of schistosomiasis in Brazil: A preliminary typology of population move-ment. Memórias Inst. Oswaldo Cruz. 2010, 105, 578–586. [Google Scholar] [CrossRef]
- Utzinger, J.; Goran, E.; Caffrey, C.; Keiser, J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011, 120, 121–137. [Google Scholar] [CrossRef]
- Li, S.S.; Luo, X.J.; Xiao, B.Z.; Wu, C.G. Study on AHP-based Early-warning Index System and Their Corresponding Weights of Schistosomiasis in the Three Gorges Reservoir Areas. J. Trop. Med. 2010, 10, 1442–1444. [Google Scholar]
- Xu, J.; He, Q.Y.; Cai, J.X.; Le, D.L. Study of schistosomiasis control based on Entropy and Grey Correlation Mode. Mod. Prev. Med. 2015, 12, 2113–2115. [Google Scholar]
- Lv, Y.W.; Li, P.A. Clustering Analysis Method of Weighted Principal Component Distance. Stat. Res. 2016, 33, 102–108. [Google Scholar]
- Ramsey, A.F. Probability Distributions of Crop Yields: A Bayesian Spatial Quantile Regression Approach. Am. J. Agric. Econ. 2020, 102, 220–239. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Tian, T.; Zeng, P.; Zhang, X.-Y.; Che, Y. Surface water change characteristics of Taihu Lake from 1984–2018 based on Google Earth Engine. Engine. Chin. J. Appl. Ecol. 2020, 31, 3163–3172. [Google Scholar]
- Liu, R.; Dong, H.-F.; Jiang, M.-S. The new national integrated strategy emphasizing infection sources control for schistosomiasis control in China has made remarkable achievements. Parasitol. Res. 2013, 112, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Walz, Y.; Wegmann, M.; Dech, S.; Vounatsou, P.; Poda, J.N.; N’Goran, E.K.; Utzinger, J.; Raso, G. Modeling and Validation of Environmental Suitability for Schistosomiasis Transmission Using Remote Sensing. PLOS Negl. Trop. Dis. 2015, 9, 4217. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Li, R.; Qiu, J.; Xu, X.; Huang, D.; Qu, Y. Geographical Clustering and Environmental Determinants of Schistosomiasis from 2007 to 2012 in Jianghan Plain, China. Int. J. Environ. Res. Public Health 2018, 15, 1481. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Li, R.; Qiu, J.; Xu, X.; Huang, D.; Shao, Q.; Cui, Y. Identifying and Predicting the Geographical Distribution Patterns of Oncomelania hupensis. Int. J. Environ. Res. Public Health 2019, 16, 2206. [Google Scholar] [CrossRef]
- Gurarie, D.; Lo, N.C.; Ndeffo-Mbah, M.L.; Durham, D.P.; King, C.H. The human-snail transmission environment shapes long term schistosomiasis control outcomes: Implications for improving the accuracy of predictive modeling. PLoS Negl. Trop. Dis. 2018, 12, e0006514. [Google Scholar] [CrossRef]
- Kalinda, C.; Chimbari, M.; Mukaratirwa, S. Implications of Changing Temperatures on the Growth, Fecundity and Survival of Intermediate Host Snails of Schistosomiasis: A Systematic Review. Int. J. Environ. Res. Public Health 2017, 14, 80. [Google Scholar] [CrossRef]
- Lo, N.C.; Gurarie, D.; Yoon, N.; Coulibaly, J.T.; Bendavid, E.; Andrews, J.R.; King, C.H. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc. Natl. Acad. Sci. USA 2018, 115, 584–591. [Google Scholar] [CrossRef]
- Paredes, H.; Souza-Santos, R.; Resendes, A.P.D.C.; Souza, M.A.A.D.; Albuquerque, J.; Bocanegra, S.; Gomes, E.C.D.S.; Barbosa, C.S. Spatial pattern, water use and risk levels associated with the trans-mission of schistosomiasis on the north coast of Pernambuco, Brazil. Cadernos Saúde Pública 2010, 26, 1013–1023. [Google Scholar] [CrossRef]
- Tayo, A.A.; Raphael, T.A.; Babatunji, E.O.; Kazeem, O.O.; Abidemi, P.K. The Effect of Climate Change and the Snail-Schistosome Cycle in Transmission and Bio-Control of Schistosomiasis in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2020, 17, 181. [Google Scholar]
- Ding, L.; Zhang, M.; Zhao, Z.T. The application of spatial analytic methods in epidemiologic studies of natural focus infection diseases. Chin. J. Dis. Control Prev. 2012, 16, 897–901. [Google Scholar]
- Yang, J.; Zhou, J.; Jin, J.; Sun, Q. The Stakeholders’ Views on Planting Trees to Control Schistosomiasis in China. Int. J. Environ. Res. Public Health 2020, 17, 939. [Google Scholar] [CrossRef] [PubMed]



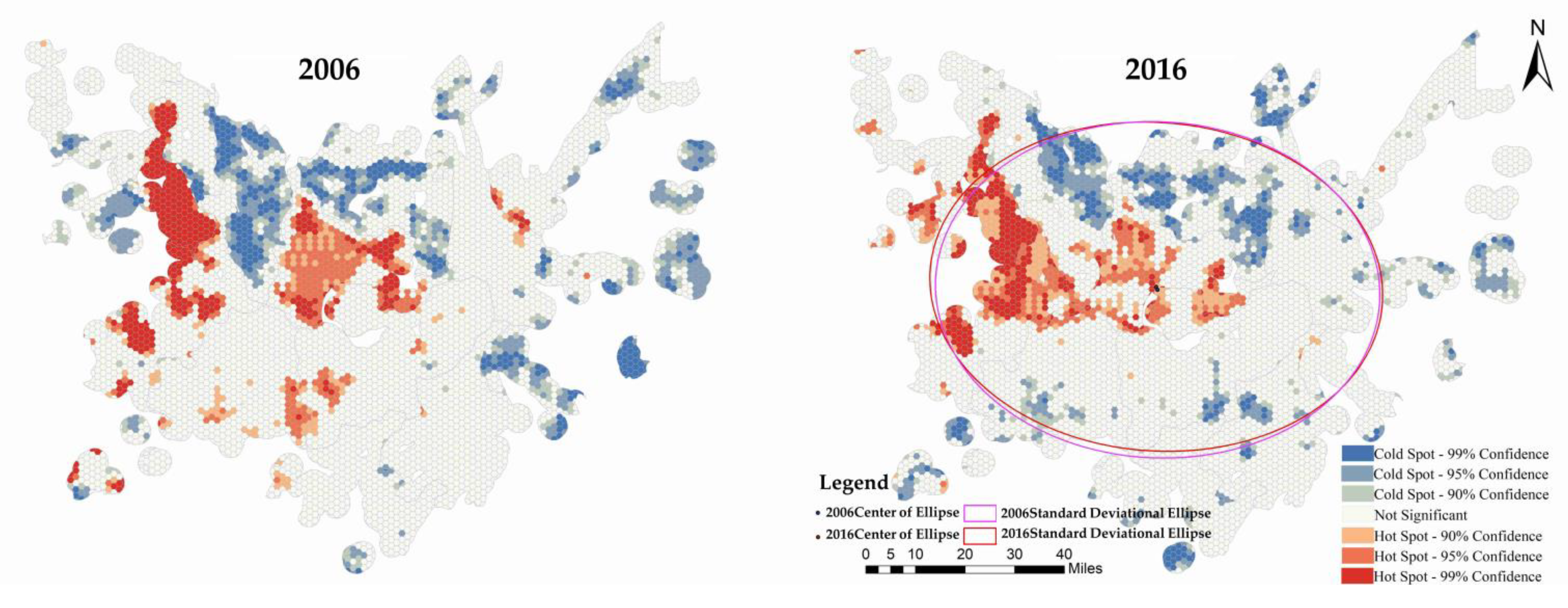
| Attributes | 2006 | 2016 |
|---|---|---|
| Shape_Length | 399.203 km | 400.390 km |
| Shape_Area | 12,314.978 km2 | 12,270.277 km2 |
| Center X | 112.418° E | 112.415° E |
| Center Y | 29.174° N | 29.183° N |
| XStdDist | 72.042 km | 73.471 km |
| YStdDist | 54.415 km | 53.163 km |
| Rotation | 92.817° | 94.468° |
| Oblateness | 0.245 | 0.276 |
| Comprehensive Risk Level | Comprehensive Risk Value | Environmental Spectral Characteristics | Epidemic Index Characteristics | Susceptibility Index Characteristics |
|---|---|---|---|---|
| Level I (Extremely high-risk area) | 8.92–12.09 | BI: 29.31–39.45 GVI: 11.69–42.51 NDVI: 0.15–0.35 | ≥0.44 | ≥3.92 |
| Level II (High-risk area) | 6.10–8.81 | BI: 27.30–44.64 GVI: 3.45–59.37 NDVI: 0.09–0.40 | ≥0.35 | ≥1.5 |
| Level III (Moderate-risk area) | 4.68–6.05 | BI: 18.46–29.31 ∪ 39.45–46.36 GVI: −19.27–11.69 ∪ 42.51–62.55 NDVI: −0.14–0.15 ∪ 0.35–0.61 | 0.07–0.46 | 0–2.5 |
| Level IV (Low-risk area) | 2.63–4.64 | BI: 18.46–27.30 ∪ 42.69–46.36 GVI: −19.27–15.68 ∪ 59.51–62.55 NDVI: −0.04–0.09 ∪ 0.39–0.61 | ≤0.35 | ≤1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Ouyang, X.; He, Q.; Wei, G. Comprehensive Risk Assessment of Schistosomiasis Epidemic Based on Precise Identification of Oncomelania hupensis Breeding Grounds—A Case Study of Dongting Lake Area. Int. J. Environ. Res. Public Health 2021, 18, 1950. https://doi.org/10.3390/ijerph18041950
Xu J, Ouyang X, He Q, Wei G. Comprehensive Risk Assessment of Schistosomiasis Epidemic Based on Precise Identification of Oncomelania hupensis Breeding Grounds—A Case Study of Dongting Lake Area. International Journal of Environmental Research and Public Health. 2021; 18(4):1950. https://doi.org/10.3390/ijerph18041950
Chicago/Turabian StyleXu, Jun, Xiao Ouyang, Qingyun He, and Guoen Wei. 2021. "Comprehensive Risk Assessment of Schistosomiasis Epidemic Based on Precise Identification of Oncomelania hupensis Breeding Grounds—A Case Study of Dongting Lake Area" International Journal of Environmental Research and Public Health 18, no. 4: 1950. https://doi.org/10.3390/ijerph18041950
APA StyleXu, J., Ouyang, X., He, Q., & Wei, G. (2021). Comprehensive Risk Assessment of Schistosomiasis Epidemic Based on Precise Identification of Oncomelania hupensis Breeding Grounds—A Case Study of Dongting Lake Area. International Journal of Environmental Research and Public Health, 18(4), 1950. https://doi.org/10.3390/ijerph18041950







