Implementing Precision Medicine in Human Frailty through Epigenetic Biomarkers
Abstract
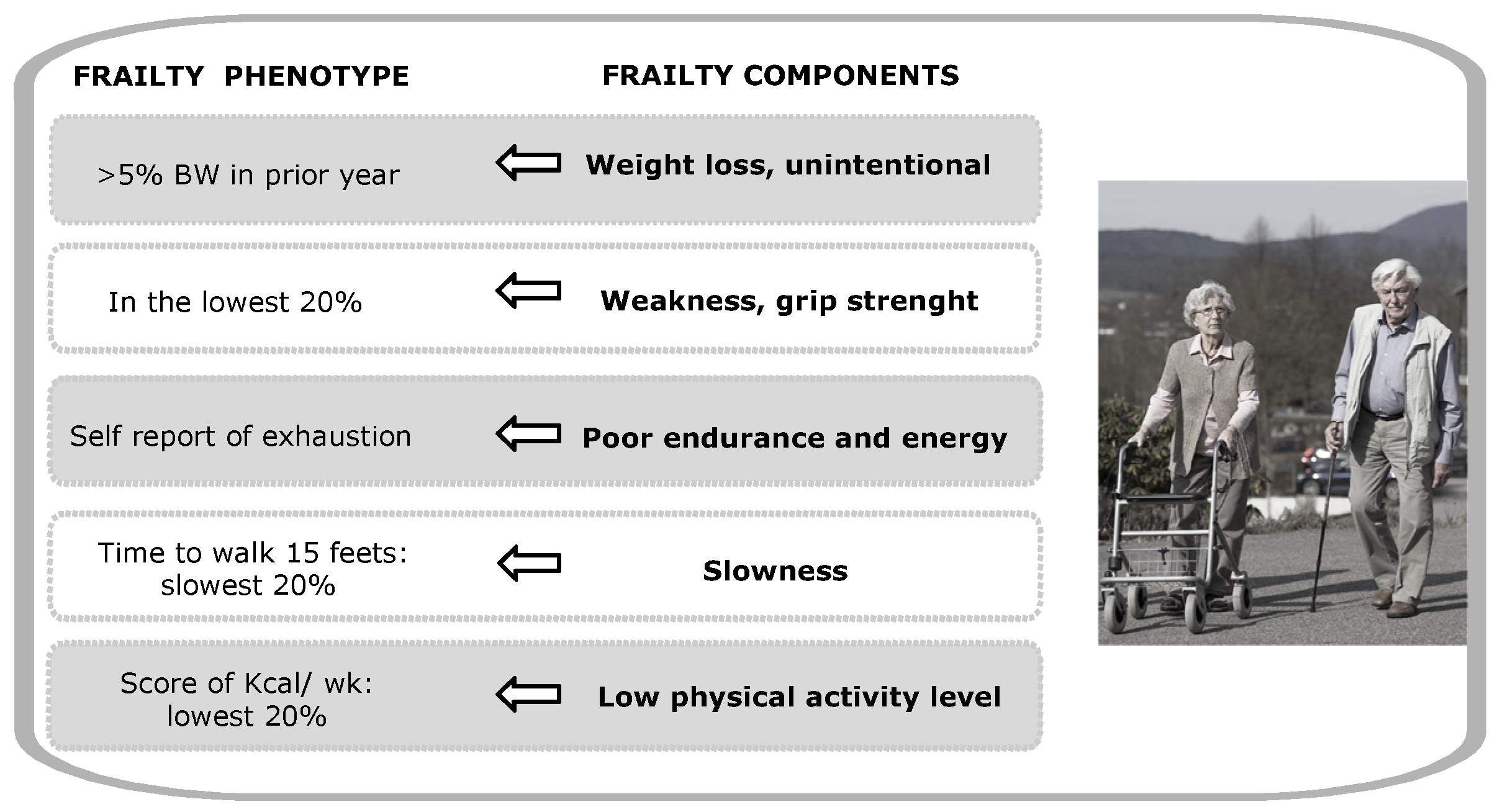
1. Introduction
2. Materials and Methods
3. Results
3.1. Epigenetic Mechanisms Related to Aging and Frailty
3.1.1. DNA Methylation
3.1.2. Histone Post-Translational Modifications and Histone Variants
3.1.3. Non-Coding RNA
3.2. Epigenetic Biomarkers Associated with Aging and Frailty
3.2.1. DNA Methylation as a Frailty Biomarker
3.2.2. Histone PTMs and Histone Variants as Frailty Biomarkers
3.2.3. Non-Coding RNAs as Frailty Biomarkers
3.3. Epigenetic Biomarkers to Follow-up the Interventions in Frailty
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L.; Fried, L.P. Frailty in the clinical scenario. Lancet 2015, 385, e7–e9. [Google Scholar] [CrossRef]
- Bisset, E.S.; Howlett, S.E. The biology of frailty in humans and animals: Understanding frailty and promoting translation. Aging Med. 2019, 2, 27–34. [Google Scholar] [CrossRef]
- Rockwood, K. Conceptual Models of Frailty: Accumulation of Deficits. Can. J. Cardiol. 2016, 32, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Tarazona-Santabalbina, F.J.; Pérez-Ros, P.; Martínez-Arnau, F.M.; Borras, C.; Olaso-Gonzalez, G.; Salvador-Pascual, A.; Gomez-Cabrera, M.C. Biology of frailty: Modulation of ageing genes and its importance to prevent age-associated loss of function. Mol. Asp. Med. 2016, 50, 88–108. [Google Scholar] [CrossRef]
- Nascimento, C.M.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.C.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free. Radic. Biol. Med. 2019, 132, 42–49. [Google Scholar] [CrossRef]
- Bock, J.-O.; König, H.-H.; Brenner, H.; Haefeli, W.E.; Quinzler, R.; Matschinger, H.; Saum, K.-U.; Schöttker, B.; Heider, D. Associations of frailty with health care costs—Results of the ESTHER cohort study. BMC Health Serv. Res. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Eeles, E.M.P.; White, S.V.; O’Mahony, S.M.; Bayer, A.J.; Hubbard, R.E. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012, 41, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Beck, S.; Havelock, W.; Eeles, E.; Hubbard, R.E.; Johansen, A. Predicting outcome after hip fracture: Using a frailty index to integrate comprehensive geriatric assessment results. Age Ageing 2014, 43, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; Andrew, M.K.; Fallah, N.; Rockwood, K. Comparison of the prognostic importance of diagnosed diabetes, co-morbidity and frailty in older people. Diabet. Med. 2010, 27, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Sipila, S.; Taaffe, D.R.; Cheng, S.; Puolakka, J.; Toivanen, J.; Suominen, H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: A randomized placebo-controlled study. Clin. Sci. 2001, 101, 147–157. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Marzetti, E.; Leeuwenburgh, C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp. Gerontol. 2006, 41, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Vaz Fragoso, C.A.; Enright, P.L.; McAvay, G.; Van Ness, P.H.; Gill, T.M. Frailty and Respiratory Impairment in Older Persons. Am. J. Med. 2012, 125, 79–86. [Google Scholar] [CrossRef]
- Lutski, M.; Haratz, S.; Weinstein, G.; Goldbourt, U.; Tanne, D. Impaired Cerebral Hemodynamics and Frailty in Patients with Cardiovascular Disease. J. Gerontol. Ser. A 2018, 73, 1714–1721. [Google Scholar] [CrossRef]
- Drew, W.; Wilson, D.; Sapey, E. Frailty and the immune system. J. Aging Res. Health 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Abel, G.A.; Buckstein, R. Integrating Frailty, Comorbidity, and Quality of Life in the Management of Myelodysplastic Syndromes. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e337–e344. [Google Scholar] [CrossRef]
- Walston, J.; Fedarko, N.; Yang, H.; Leng, S.; Beamer, B.; Espinoza, S.; Lipton, A.; Zheng, H.; Becker, K. The Physical and Biological Characterization of a Frail Mouse Model. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2008, 63, 391–398. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef]
- Lipton, M.L.; Ifrah, C.; Stewart, W.F.; Fleysher, R.; Sliwinski, M.J.; Kim, M.; Lipton, R.B. Validation of HeadCount-2w for estimation of two-week heading: Comparison to daily reporting in adult amateur player. J. Sci. Med. Sport 2018, 21, 363–367. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Mena-Mollá, S.; García-Giménez, J.L. Epigenetic IVD Tests for Personalized Precision Medicine in Cancer. Front. Genet. 2019, 10, 621. [Google Scholar] [CrossRef]
- Garcia-Gimenez, J.L.; Sanchis-Gomar, F.; Lippi, G.; Mena, S.; Ivars, D.; Gomez-Cabrera, M.C.; Vina, J.; Pallardo, F.V. Epigenetic biomarkers: A new perspective in laboratory diagnostics. Clin. Chim. Acta 2012, 413, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- García-Giménez, J.L.; Seco-Cervera, M.; Tollefsbol, T.O.; Romá-Mateo, C.; Peiró-Chova, L.; Lapunzina, P.; Pallardó, F.V. Epigenetic biomarkers: Current strategies and future challenges for their use in the clinical laboratory. Crit. Rev. Clin. Lab. Sci. 2017, 54, 529–550. [Google Scholar] [CrossRef]
- Sandoval, J.; Peiró-Chova, L.; Pallardó, F.V.; García-Giménez, J.L. Epigenetic biomarkers in laboratory diagnostics: Emerging approaches and opportunities. Expert Rev. Mol. Diagn. 2013, 13, 457–471. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Cavazzini, C.; Corsi, A.; Bartali, B.; Russo, C.R.; Lauretani, F.; Corsi, A.M.; Bandinelli, S.; Guralnik, J.M. Biomarkers of frailty in older persons. J. Endocrinol. Investig. 2002, 25, 10–15. [Google Scholar]
- van der Graaf, A.; Wardenaar, R.; Neumann, D.A.; Taudt, A.; Shaw, R.G.; Jansen, R.C.; Schmitz, R.J.; Colomé-Tatché, M.; Johannes, F. Rate, spectrum, and evolutionary dynamics of spontaneous epimutations. Proc. Natl. Acad. Sci. USA 2015, 112, 6676–6681. [Google Scholar] [CrossRef] [PubMed]
- Cruickshanks, H.A.; McBryan, T.; Nelson, D.M.; VanderKraats, N.D.; Shah, P.P.; van Tuyn, J.; Singh Rai, T.; Brock, C.; Donahue, G.; Dunican, D.S.; et al. Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 2013, 15, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Pfeifer, G.P. Aging and DNA methylation. BMC Biol. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Verjan, J.C.; Ramírez-Aldana, R.; Pérez-Zepeda, M.U.; Quiroz-Baez, R.; Luna-López, A.; Gutierrez Robledo, L.M. Systems biology and network pharmacology of frailty reveal novel epigenetic targets and mechanisms. Sci. Rep. 2019, 9, 10593. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suner, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. From The Cover: Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef]
- Veitia, R.A.; Govindaraju, D.R.; Bottani, S.; Birchler, J.A. Aging: Somatic Mutations, Epigenetic Drift and Gene Dosage Imbalance. Trends Cell Biol. 2017, 27, 299–310. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Gutarra, S.; García-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardí, M.; Ballestar, E.; González, S.; Serrano, A.L.; et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef]
- Bellizzi, D.; D’Aquila, P.; Montesanto, A.; Corsonello, A.; Mari, V.; Mazzei, B.; Lattanzio, F.; Passarino, G. Global DNA methylation in old subjects is correlated with frailty. AGE 2012, 34, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Montesanto, A.; Lagani, V.; Martino, C.; Dato, S.; De Rango, F.; Berardelli, M.; Corsonello, A.; Mazzei, B.; Mari, V.; Lattanzio, F.; et al. A novel, population-specific approach to define frailty. AGE 2010, 32, 385–395. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Roetker, N.S.; Pankow, J.S.; Bressler, J.; Morrison, A.C.; Boerwinkle, E. Prospective Study of Epigenetic Age Acceleration and Incidence of Cardiovascular Disease Outcomes in the ARIC Study (Atherosclerosis Risk in Communities). Circ. Genom. Precis. Med. 2018, 11, e001937. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Ambatipudi, S.; Horvath, S.; Perrier, F.; Cuenin, C.; Hernandez-Vargas, H.; Le Calvez-Kelm, F.; Durand, G.; Byrnes, G.; Ferrari, P.; Bouaoun, L.; et al. DNA methylome analysis identifies accelerated epigenetic ageing associated with postmenopausal breast cancer susceptibility. Eur. J. Cancer 2017, 75, 299–307. [Google Scholar] [CrossRef]
- Levine, M.E.; Hosgood, H.D.; Chen, B.; Absher, D.; Assimes, T.; Horvath, S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging 2015, 7, 690–700. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Bennett, D.A.; Horvath, S. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging 2015, 7, 1198–1211. [Google Scholar] [CrossRef]
- Chen, B.H.; Marioni, R.E.; Colicino, E.; Peters, M.J.; Ward-Caviness, C.K.; Tsai, P.-C.; Roetker, N.S.; Just, A.C.; Demerath, E.W.; Guan, W.; et al. DNA methylation-based measures of biological age: Meta-analysis predicting time to death. Aging 2016, 8, 1844–1865. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Ritchie, S.J.; Muniz-Terrera, G.; Harris, S.E.; Gibson, J.; Redmond, P.; Cox, S.R.; Pattie, A.; et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol. 2015, 44, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wilson, R.; Heiss, J.; Breitling, L.P.; Saum, K.-U.; Schöttker, B.; Holleczek, B.; Waldenberger, M.; Peters, A.; Brenner, H. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun. 2017, 8, 14617. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Leung, D.; Thrush, K.; Zhao, W.; Ratliff, S.; Tanaka, T.; Schmitz, L.L.; Smith, J.A.; Ferrucci, L.; Levine, M.E. Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell 2020, 19, e13229. [Google Scholar] [CrossRef] [PubMed]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Weidner, C.; Lin, Q.; Koch, C.; Eisele, L.; Beier, F.; Ziegler, P.; Bauerschlag, D.; Jöckel, K.-H.; Erbel, R.; Mühleisen, T.; et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014, 15, R24. [Google Scholar] [CrossRef]
- Vidal-Bralo, L.; Lopez-Golan, Y.; Gonzalez, A. Simplified Assay for Epigenetic Age Estimation in Whole Blood of Adults. Front. Genet. 2016, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wagner, W. Epigenetic Aging Signatures Are Coherently Modified in Cancer. PLoS Genet. 2015, 11, e1005334. [Google Scholar] [CrossRef]
- Bergsma, T.; Rogaeva, E. DNA Methylation Clocks and Their Predictive Capacity for Aging Phenotypes and Healthspan. Neurosci. Insights 2020, 15, 263310552094222. [Google Scholar] [CrossRef]
- Breitling, L.P.; Saum, K.-U.; Perna, L.; Schöttker, B.; Holleczek, B.; Brenner, H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin. Epigenet. 2016, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Marioni, R.E.; Harris, S.E.; Starr, J.M.; Deary, I.J. DNA methylation and the epigenetic clock in relation to physical frailty in older people: The Lothian Birth Cohort 1936. Clin. Epigenet. 2018, 10, 101. [Google Scholar] [CrossRef]
- Kim, D.H.; Schneeweiss, S.; Glynn, R.J.; Lipsitz, L.A.; Rockwood, K.; Avorn, J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J. Gerontol. Ser. A 2018, 73, 980–987. [Google Scholar] [CrossRef] [PubMed]
- García-Giménez, J.L.; Romá-Mateo, C.; Pallardó, F.V. Oxidative post-translational modifications in histones. BioFactors 2019, 45, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Pollina, E.A.; Brunet, A. Epigenetic regulation of ageing: Linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 2015, 16, 593–610. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; François, M.; Fenech, M.F.; Leifert, W.R. Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat. Res. Mutat. Res. 2015, 766, 1–19. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Horikawa, I.; Redon, C.; Nakamura, A.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell 2008, 7, 89–100. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Kobashigawa, J.; Dadhania, D.; Bhorade, S.; Adey, D.; Berger, J.; Bhat, G.; Budev, M.; Duarte-Rojo, A.; Dunn, M.; Hall, S.; et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am. J. Transplant. 2019, 19, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Sannicandro, A.J.; Soriano-Arroquia, A.; Goljanek-Whysall, K. Micro(RNA)-managing muscle wasting. J. Appl. Physiol. 2019, 127, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Smith-Vikos, T.; Liu, Z.; Parsons, C.; Gorospe, M.; Ferrucci, L.; Gill, T.M.; Slack, F.J. A serum miRNA profile of human longevity: Findings from the Baltimore Longitudinal Study of Aging (BLSA). Aging 2016, 8, 2971–2987. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Rusanova, I.; Fernández-Martínez, J.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Escames, G.; García-García, F.J.; Mañas, L.; Acuña-Castroviejo, D. Involvement of plasma miRNAs, muscle miRNAs and mitochondrial miRNAs in the pathophysiology of frailty. Exp. Gerontol. 2019, 124, 110637. [Google Scholar] [CrossRef]
- García-Giménez, J.L. Epigenetic Biomarkers and Diagnostics; Academic Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Theou, O.; Sluggett, J.K.; Bell, J.S.; Lalic, S.; Cooper, T.; Robson, L.; Morley, J.E.; Rockwood, K.; Visvanathan, R. Frailty, Hospitalization, and Mortality in Residential Aged Care. J. Gerontol. Ser. A 2018, 73, 1090–1096. [Google Scholar] [CrossRef]
- Collerton, J.; Gautrey, H.E.; van Otterdijk, S.D.; Davies, K.; Martin-Ruiz, C.; von Zglinicki, T.; Kirkwood, T.B.L.; Jagger, C.; Mathers, J.C.; Strathdee, G. Acquisition of aberrant DNA methylation is associated with frailty in the very old: Findings from the Newcastle 85+ Study. Biogerontology 2014, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.; Rockwood, K. The rate of aging: The rate of deficit accumulation does not change over the adult life span. Biogerontology 2016, 17, 199–204. [Google Scholar] [CrossRef]
- Vetter, V.M.; Meyer, A.; Karbasiyan, M.; Steinhagen-Thiessen, E.; Hopfenmüller, W.; Demuth, I. Epigenetic Clock and Relative Telomere Length Represent Largely Different Aspects of Aging in the Berlin Aging Study II (BASE-II). J. Gerontol. Ser. A 2019, 74, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.M.; Spira, D.; Banszerus, V.L.; Demuth, I. Epigenetic Clock and Leukocyte Telomere Length Are Associated with Vitamin D Status but not with Functional Assessments and Frailty in the Berlin Aging Study II. J. Gerontol. Ser. A 2020, 75, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I. Clock-drawing: Is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry 2000, 15, 548–561. [Google Scholar] [CrossRef]
- Gräff, J.; Tsai, L.H. Histone acetylation: Molecular mnemonics on the chromatin. Nat. Rev. Neurosci. 2013, 14, 97–111. [Google Scholar] [CrossRef]
- Burns, A.M.; Gräff, J. Cognitive epigenetic priming: Leveraging histone acetylation for memory amelioration. Curr. Opin. Neurobiol. 2020, 67, 75–84. [Google Scholar] [CrossRef]
- Yoshihara, T.; Machida, S.; Tsuzuki, T.; Kakigi, R.; Chang, S.W.; Sugiura, T.; Naito, H. Age-related changes in histone modification in rat gastrocnemius muscle. Exp. Gerontol. 2019, 125, 110658. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Sánchez-Flores, M.; Marcos-Pérez, D.; Lorenzo-López, L.; Maseda, A.; Millán-Calenti, J.C.; Pásaro, E.; Laffon, B. Exploring Genetic Outcomes as Frailty Biomarkers. J. Gerontol. Ser. A 2019, 74, 168–175. [Google Scholar] [CrossRef]
- Ipson, B.R.; Fletcher, M.B.; Espinoza, S.E.; Fisher, A.L. Identifying Exosome-Derived MicroRNAs as Candidate Biomarkers of Frailty. J. Frailty Aging 2018, 7, 100–103. [Google Scholar] [CrossRef]
- Rusanova, I.; Diaz-Casado, M.E.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Guerra-Librero, A.; García-García, F.J.; Escames, G.; Mañas, L.; Acuña-Castroviejo, D. Analysis of Plasma MicroRNAs as Predictors and Biomarkers of Aging and Frailty in Humans. Oxidative Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- García-Giménez, J.L.; Rubio-Belmar, P.A.; Peiró-Chova, L.; Hervás, D.; González-Rodríguez, D.; Ibañez-Cabellos, J.S.; Bas-Hermida, P.; Mena-Mollá, S.; García-López, E.M.; Pallardó, F.V.; et al. Circulating miRNAs as diagnostic biomarkers for adolescent idiopathic scoliosis. Sci. Rep. 2018, 8, 2646. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front. Endocrinol. 2019, 10, 255. [Google Scholar] [CrossRef]
- Wu, I.C.; Lin, C.C.; Hsiung, C.A. Emerging roles of frailty and inflammaging in risk assessment of age-related chronic diseases in older adults: The intersection between aging biology and personalized medicine. Biomedicine 2015, 5, 1. [Google Scholar] [CrossRef]
- Zheng, Y.; Kong, J.; Li, Q.; Wang, Y.; Li, J. Role of miRNAs in skeletal muscle aging. Clin. Interv. Aging 2018, 13, 2407–2419. [Google Scholar] [CrossRef]
- Sathyan, S.; Barzilai, N.; Atzmon, G.; Milman, S.; Ayers, E.; Verghese, J. Genetic Insights Into Frailty: Association of 9p21-23 Locus With Frailty. Front. Med. 2018, 5, 105. [Google Scholar] [CrossRef]
- Pasmant, E.; Sabbagh, A.; Vidaud, M.; Bièche, I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011, 25, 444–448. [Google Scholar] [CrossRef]
- Melzer, D.; Frayling, T.M.; Murray, A.; Hurst, A.J.; Harries, L.W.; Song, H.; Khaw, K.; Luben, R.; Surtees, P.G.; Bandinelli, S.S.; et al. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech. Ageing Dev. 2007, 128, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.M.; Noh, J.H.; Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Long noncoding RNAs in diseases of aging. Biochim. Biophys. Acta 2016, 1859, 209–221. [Google Scholar] [CrossRef]
- Dey, B.K.; Pfeifer, K.; Dutta, A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014, 28, 491–501. [Google Scholar] [CrossRef]
- McKay, B.R.; Ogborn, D.I.; Bellamy, L.M.; Tarnopolsky, M.A.; Parise, G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012, 26, 2509–2521. [Google Scholar] [CrossRef]
- Watts, R.; Johnsen, V.L.; Shearer, J.; Hittel, D.S. Myostatin-induced inhibition of the long noncoding RNA Malat1 is associated with decreased myogenesis. Am. J. Physiol. Cell Physiol. 2013, 304, C995–C1001. [Google Scholar] [CrossRef] [PubMed]
- Legnini, I.; Morlando, M.; Mangiavacchi, A.; Fatica, A.; Bozzoni, I. A feedforward regulatory loop between HuR and the long noncoding RNA linc-MD1 controls early phases of myogenesis. Mol. Cell 2014, 53, 506–514. [Google Scholar] [CrossRef]
- Pardo, P.S.; Boriek, A.M. The physiological roles of Sirt1 in skeletal muscle. Aging (Albany NY) 2011, 3, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Rodriguez-Mañas, L.; Salvador-Pascual, A.; Tarazona-Santabalbina, F.J.; Gomez-Cabrera, M.C. Exercise: The lifelong supplement for healthy ageing and slowing down the onset of frailty. J. Physiol. 2016, 594, 1989–1999. [Google Scholar] [CrossRef]
- Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; Correas, A.G.; Olaso-Gonzalez, G.; De la Rosa, A.; Carretero, A.; Gomez-Cabrera, M.C.; Viña, J. Reversal of age-associated frailty by controlled physical exercise: The pre-clinical and clinical evidences. Sports Med. Health Sci. 2019, 1, 33–39. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Izquierdo, M. Editorial: Precision Physical Activity and Exercise Prescriptions for Disease Prevention: The Effect of Interindividual Variability Under Different Training Approaches. Front. Physiol. 2019, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.E.; Jiwani, R.; Wang, J.; Wang, C.P. Review of Interventions for the Frailty Syndrome and the Role of Metformin as a Potential Pharmacologic Agent for Frailty Prevention. Clin. Ther. 2019, 41, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M. How healthy is the healthspan concept? Geroscience 2018, 40, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Moats, J.M.; Di Germanio, C.; Bernier, M.; de Cabo, R. Frailty index as a biomarker of lifespan and healthspan: Focus on pharmacological interventions. Mech. Ageing Dev. 2019, 180, 42–48. [Google Scholar] [CrossRef]
- Garcia-Valles, R.; Gomez-Cabrera, M.C.; Rodriguez-Manas, L.; Garcia-Garcia, F.J.; Diaz, A.; Noguera, I.; Olaso-Gonzalez, G.; Vina, J. Life-long spontaneous exercise does not prolong lifespan but improves health span in mice. Longev. Health 2013, 2, 14. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Garcia-Valles, R.; Rodriguez-Mañas, L.; Garcia-Garcia, F.J.; Olaso-Gonzalez, G.; Salvador-Pascual, A.; Tarazona-Santabalbina, F.J.; Viña, J. A New Frailty Score for Experimental Animals Based on the Clinical Phenotype: Inactivity as a Model of Frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 885–891. [Google Scholar] [CrossRef]
- Tarazona-Santabalbina, F.J.; Gomez-Cabrera, M.C.; Perez-Ros, P.; Martinez-Arnau, F.M.; Cabo, H.; Tsaparas, K.; Salvador-Pascual, A.; Rodriguez-Manas, L.; Vina, J. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2016, 17, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Salvador-Pascual, A.; Tarazona-Santabalbina, F.J.; Rodriguez-Mañas, L.; Gomez-Cabrera, M.C. Exercise training as a drug to treat age associated frailty. Free. Radic. Biol. Med. 2016, 98, 159–164. [Google Scholar] [CrossRef]
- Marques, E.; Carvalho, J.; Soares, J.M.; Marques, F.; Mota, J. Effects of resistance and multicomponent exercise on lipid profiles of older women. Maturitas 2009, 63, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gomez, M.; Rodriguez-Manas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr) 2014, 36, 773–785. [Google Scholar] [CrossRef]
- Gine-Garriga, M.; Roque-Figuls, M.; Coll-Planas, L.; Sitja-Rabert, M.; Salva, A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014, 95, 753–769.e753. [Google Scholar] [CrossRef]
- Gudlaugsson, J.; Gudnason, V.; Aspelund, T.; Siggeirsdottir, K.; Olafsdottir, A.S.; Jonsson, P.V.; Arngrimsson, S.A.; Harris, T.B.; Johannsson, E. Effects of a 6-month multimodal training intervention on retention of functional fitness in older adults: A randomized-controlled cross-over design. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 107. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Jeong, H.; Petrofsky, J.; Lee, H.; Yim, J. Effects of a randomized controlled recurrent fall prevention program on risk factors for falls in frail elderly living at home in rural communities. Med. Sci. Monit. 2014, 20, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Chang, K.C.; Tsauo, J.Y.; Hung, J.W.; Huang, Y.C.; Lin, S.I.; Fall Prevention Initiatives in Taiwan (FPIT) Investigators. Effects of a multifactorial fall prevention program on fall incidence and physical function in community-dwelling older adults with risk of falls. Arch. Phys. Med. Rehabil. 2013, 94, 606–615.e601. [Google Scholar] [CrossRef]
- Rosendahl, E.; Lindelöf, N.; Littbrand, H.; Yifter-Lindgren, E.; Lundin-Olsson, L.; Håglin, L.; Gustafson, Y.; Nyberg, L. High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: A randomised controlled trial. Aust. J. Physiother. 2006, 52, 105–113. [Google Scholar] [CrossRef]
- Serra-Rexach, J.A.; Bustamante-Ara, N.; Villarán, M.H.; Gil, P.G.; Ibáñez, M.J.S.; Bsc, N.B.S.; Bsc, V.O.S.; Bsc, N.G.S.; Bsc, A.B.M.P.; Gallardo, C.; et al. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: A randomized controlled trial. J. Am. Geriatr. Soc. 2011, 59, 594–602. [Google Scholar] [CrossRef]
- Zech, A.; Drey, M.; Freiberger, E.; Hentschke, C.; Bauer, J.M.; Sieber, C.C.; Pfeifer, K. Residual effects of muscle strength and muscle power training and detraining on physical function in community-dwelling prefrail older adults: A randomized controlled trial. BMC Geriatr. 2012, 12, 68. [Google Scholar] [CrossRef]
- Cesari, M.; Vellas, B.; Hsu, F.C.; Newman, A.B.; Doss, H.; King, A.C.; Manini, T.M.; Church, T.; Gill, T.M.; Miller, M.E.; et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Guralnik, J.M.; King, A.C.; Pahor, M.; McDermott, M.M.; Tudor-Locke, C.; Manini, T.M.; Glynn, N.W.; Marsh, A.P.; Axtell, R.S.; et al. Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: Results from the LIFE study randomized trial. PLoS ONE 2017, 12, e0182155. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Levine, M.E.; Kuo, P.L.; Simonsick, E.M. Time and the Metrics of Aging. Circ. Res. 2018, 123, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Derbre, F.; Gratas-Delamarche, A.; Gomez-Cabrera, M.C.; Viña, J. Inactivity-induced oxidative stress: A central role in sarcopenia? Eur. J. Sport Sci. 2012, 14, S98–S108. [Google Scholar] [CrossRef] [PubMed]
- Barrès, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef]
- Lindholm, M.E.; Marabita, F.; Gomez-Cabrero, D.; Rundqvist, H.; Ekström, T.J.; Tegnér, J.; Sundberg, C.J. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics 2014, 9, 1557–1569. [Google Scholar] [CrossRef]
- Sailani, M.R.; Halling, J.F.; Møller, H.D.; Lee, H.; Plomgaard, P.; Pilegaard, H.; Snyder, M.P.; Regenberg, B. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci. Rep. 2019, 9, 3272. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Bacalini, M.G.; Franceschi, C.; Meskers, C.G.M.; Maier, A.B.; Garagnani, P. Age-Related DNA Methylation Changes: Potential Impact on Skeletal Muscle Aging in Humans. Front. Physiol. 2019, 10, 996. [Google Scholar] [CrossRef] [PubMed]
| Epigenetic Mechanism | Epigenetic Biomarker Associated to Frailty | Biospecimen | Association between the Epigenetic Change and Frailty | Reference |
|---|---|---|---|---|
| DNA methylation | Horvath’s clock | Leukocytes | Epigenetic age acceleration was correlated with clinically relevant aging-related phenotypes | [54] |
| Histone PTMs | H3K9me3 and H3K9ac and H3K27ac decreases with age | Muscle samples in rat models | Muscle loss and sarcopenia | [79] |
| γH2AX | Leukocytes | Significantly higher γH2AX values observed in individuals positive for low physical activity (p < 0.001), slow waking (p < 0.01), and low grip strength (p < 0.01) criteria | [81] | |
| Non-coding RNAs | miR-10a-3p, miR-92a-3p, miR-185–3p, miR-194–5p, miR-326, miR-532–5p, miR-576–5p, miR-760 | Plasma exosome-derived miRNAs | Associated to frailty | [82] |
| miR-21, miR-223, miR-483 | Plasma | Increased expression in frail subjects compared to control subjects | [83] | |
| miR-146a | Plasma | Low levels in frail subjects compared to robust old adults | [83] | |
| miR-1, miR-21, miR-34a, miR-146a, miR-185, miR-206, miR-223 | Plasma | Increased levels in frail subjects compared to robust old adults | [69] | |
| miR-34a-5p miR-449b-5p | Muscle biopsy | Elevated in sarcopenic muscle compared with muscle tissue from controls | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Giménez, J.L.; Mena-Molla, S.; Tarazona-Santabalbina, F.J.; Viña, J.; Gomez-Cabrera, M.C.; Pallardó, F.V. Implementing Precision Medicine in Human Frailty through Epigenetic Biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 1883. https://doi.org/10.3390/ijerph18041883
García-Giménez JL, Mena-Molla S, Tarazona-Santabalbina FJ, Viña J, Gomez-Cabrera MC, Pallardó FV. Implementing Precision Medicine in Human Frailty through Epigenetic Biomarkers. International Journal of Environmental Research and Public Health. 2021; 18(4):1883. https://doi.org/10.3390/ijerph18041883
Chicago/Turabian StyleGarcía-Giménez, José Luis, Salvador Mena-Molla, Francisco José Tarazona-Santabalbina, Jose Viña, Mari Carmen Gomez-Cabrera, and Federico V. Pallardó. 2021. "Implementing Precision Medicine in Human Frailty through Epigenetic Biomarkers" International Journal of Environmental Research and Public Health 18, no. 4: 1883. https://doi.org/10.3390/ijerph18041883
APA StyleGarcía-Giménez, J. L., Mena-Molla, S., Tarazona-Santabalbina, F. J., Viña, J., Gomez-Cabrera, M. C., & Pallardó, F. V. (2021). Implementing Precision Medicine in Human Frailty through Epigenetic Biomarkers. International Journal of Environmental Research and Public Health, 18(4), 1883. https://doi.org/10.3390/ijerph18041883







