Towards a Semi-Automatic Early Warning System for Vector-Borne Diseases
Abstract
:1. Introduction
2. Methods
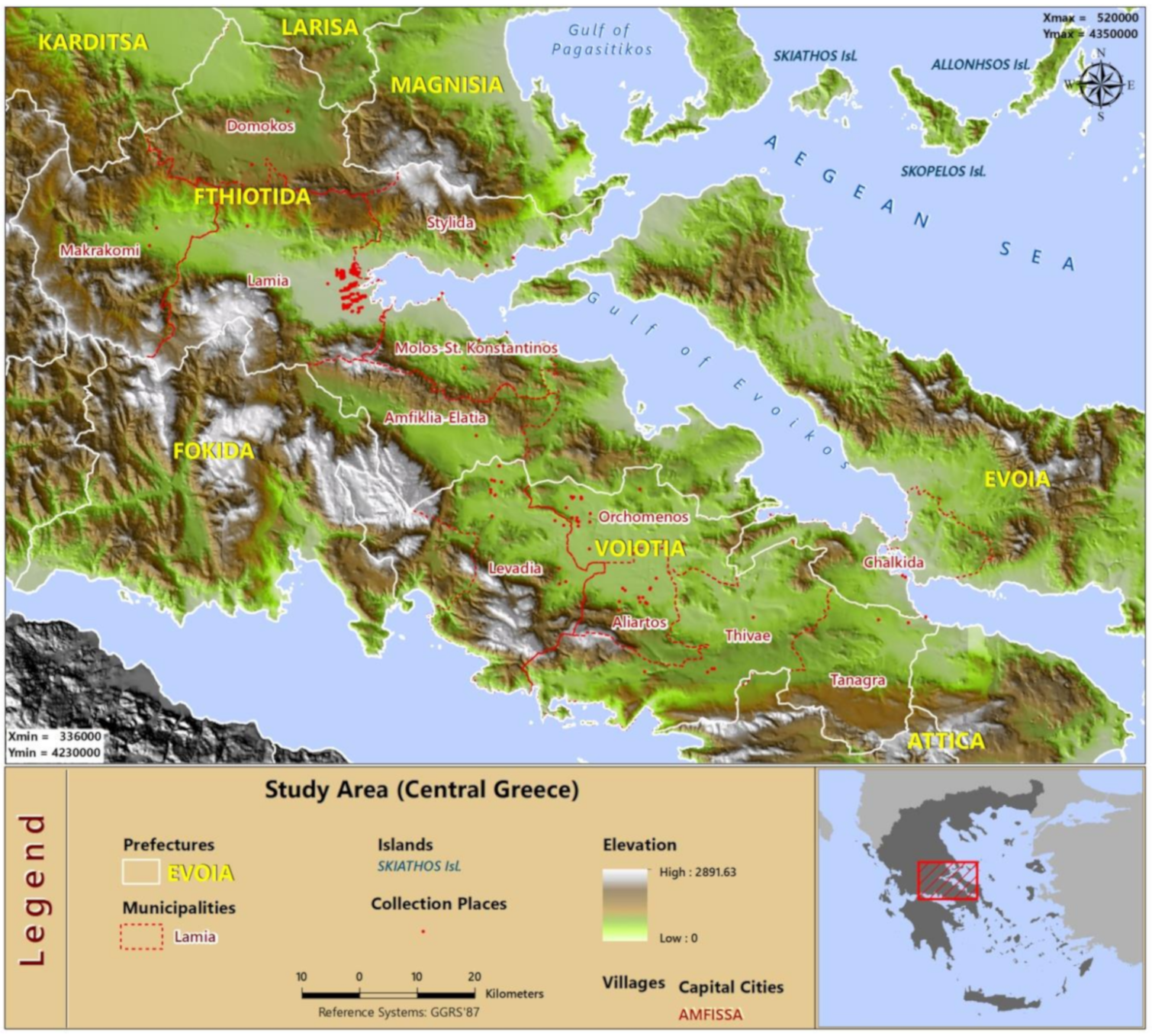
2.1. Study Area and Data Collection
2.2. The Spatial Predictive Model
2.3. Towards a Semi-Automatic Early Warning System Tool
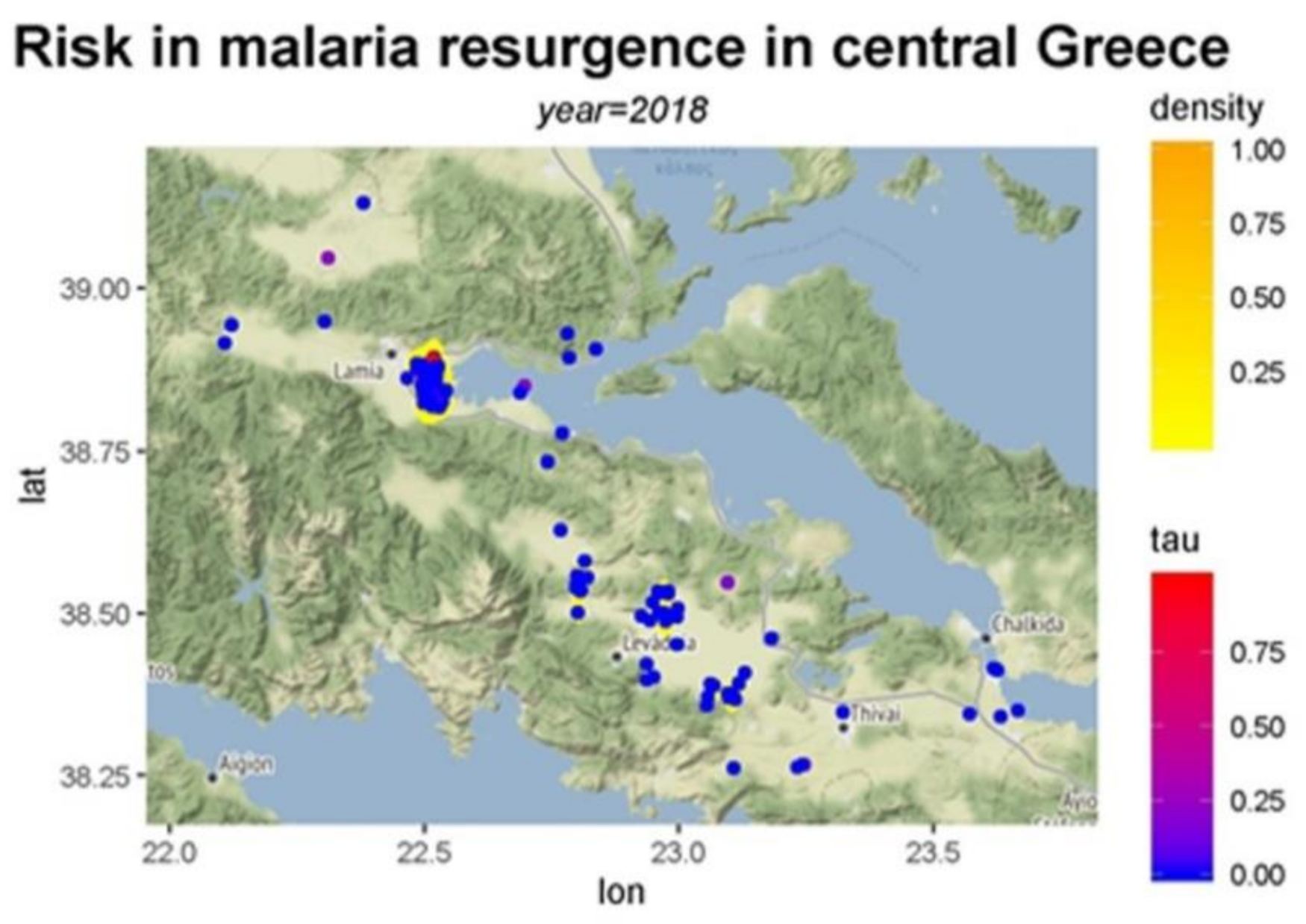
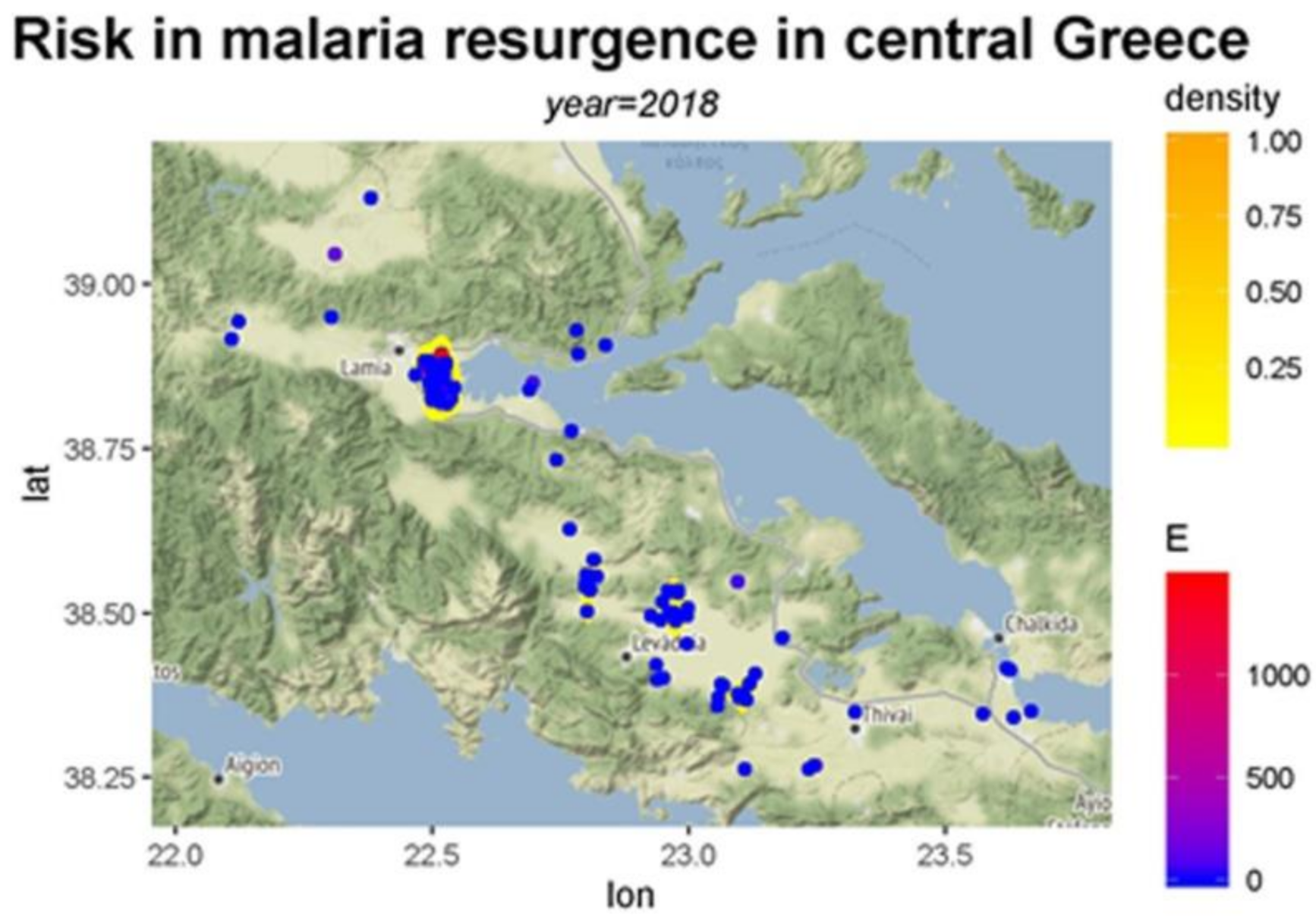
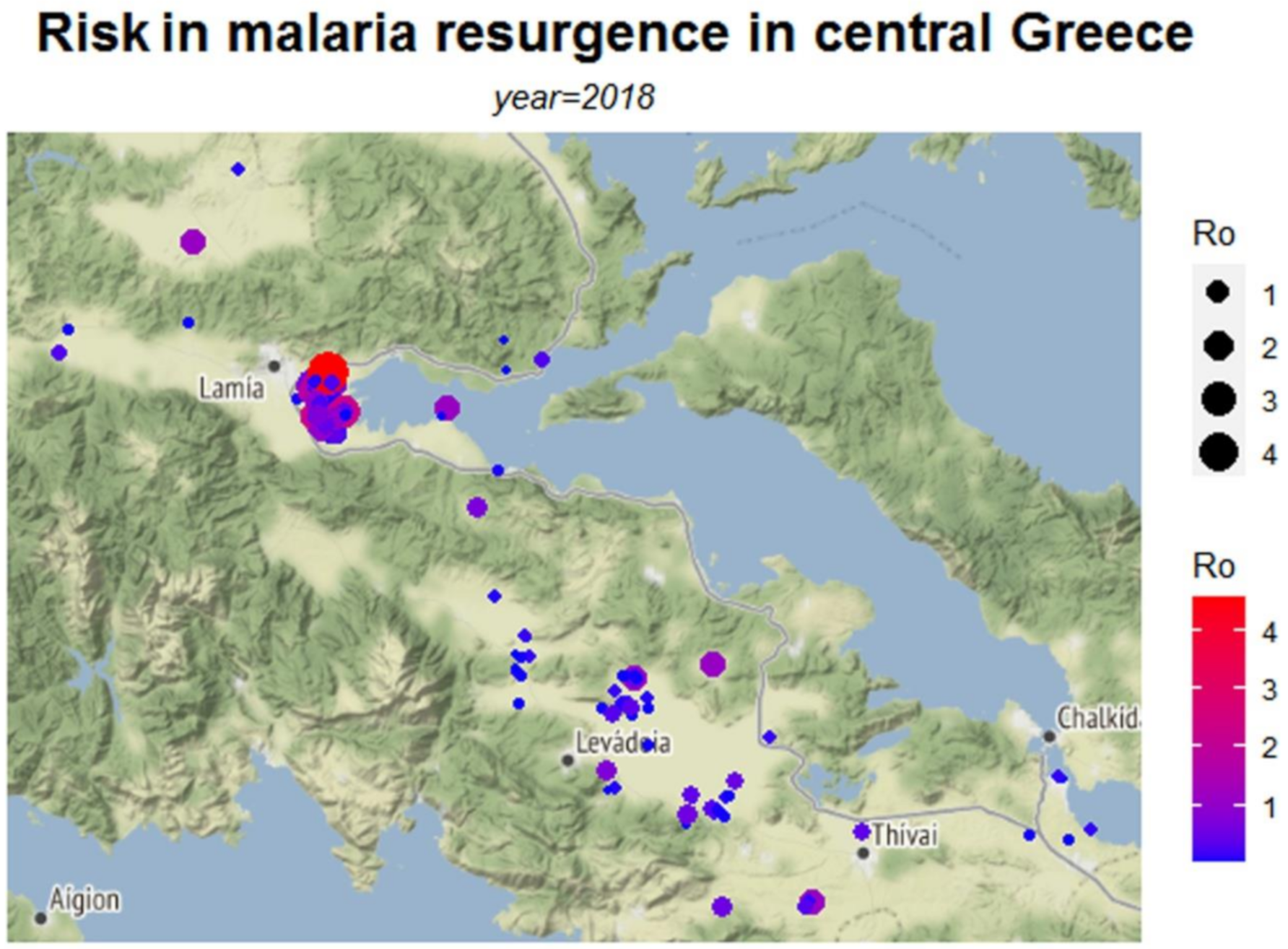
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
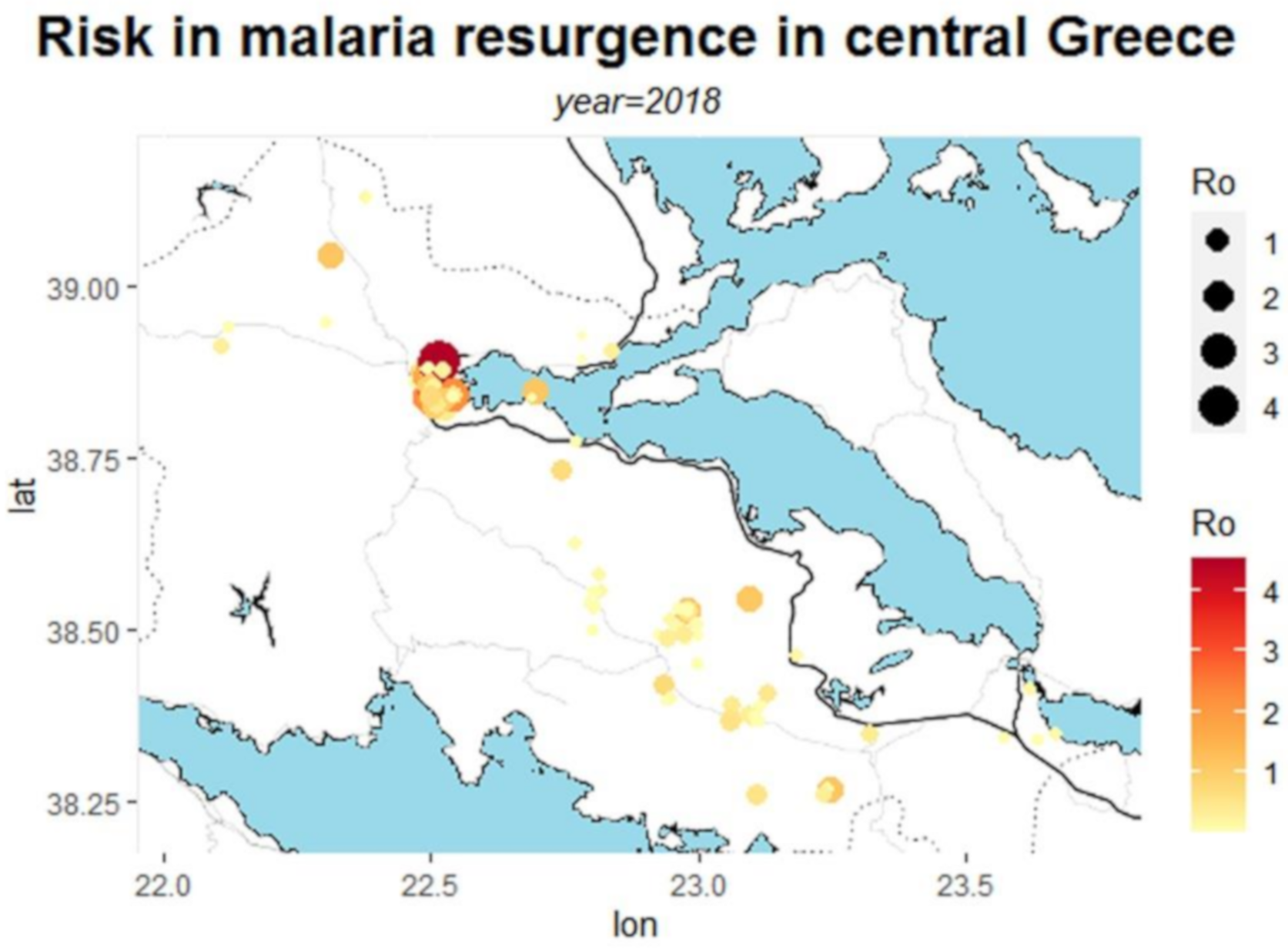
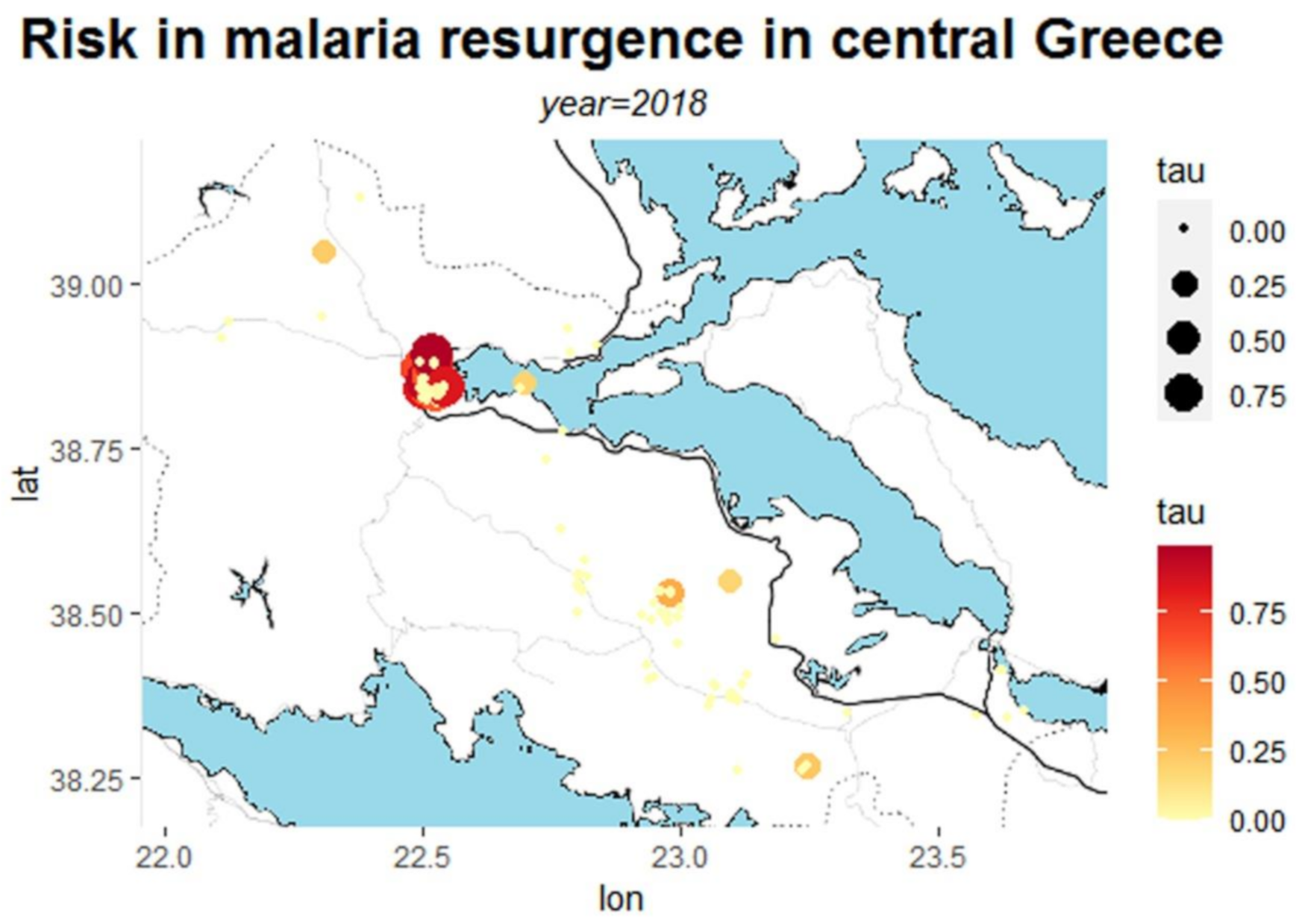
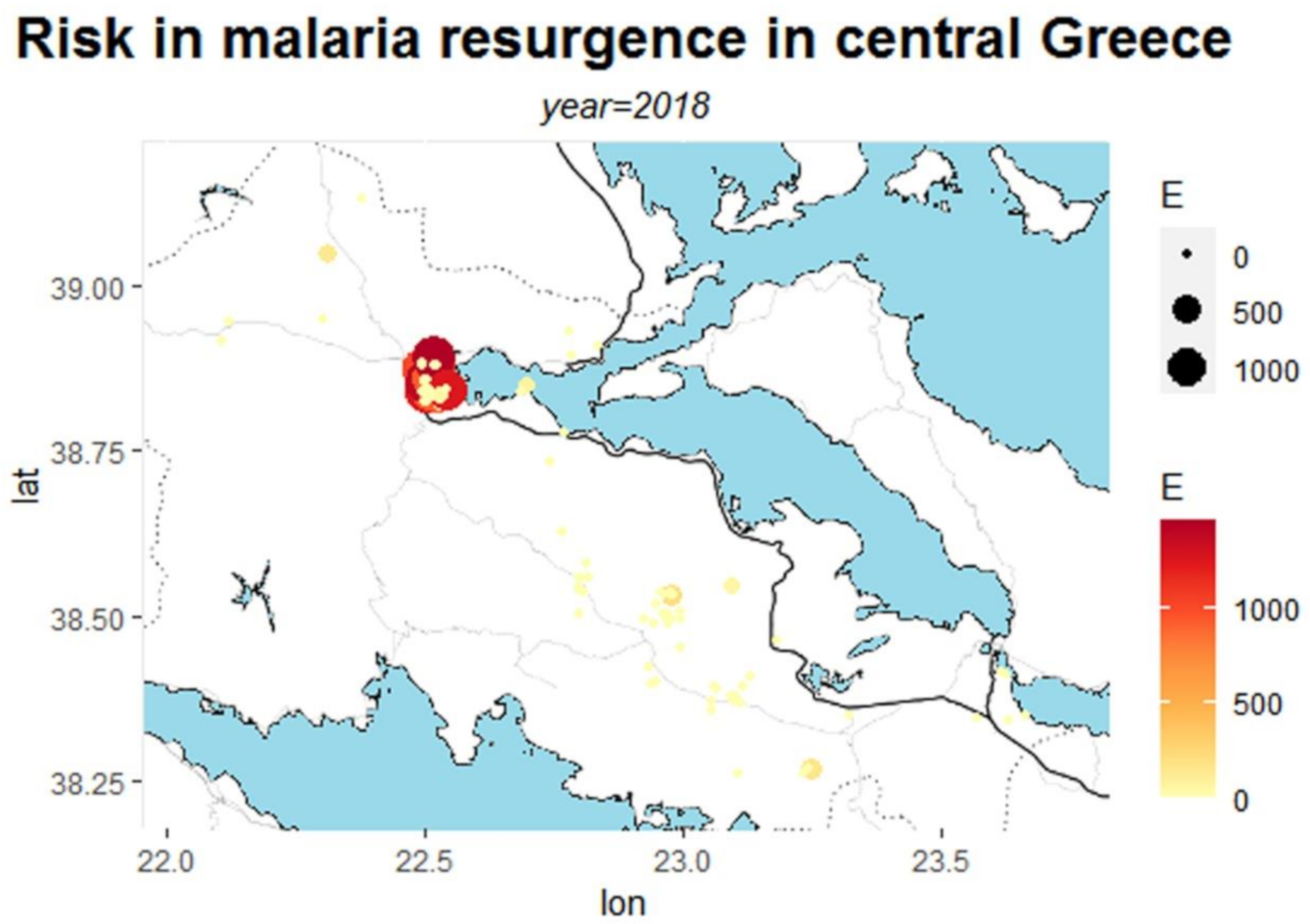
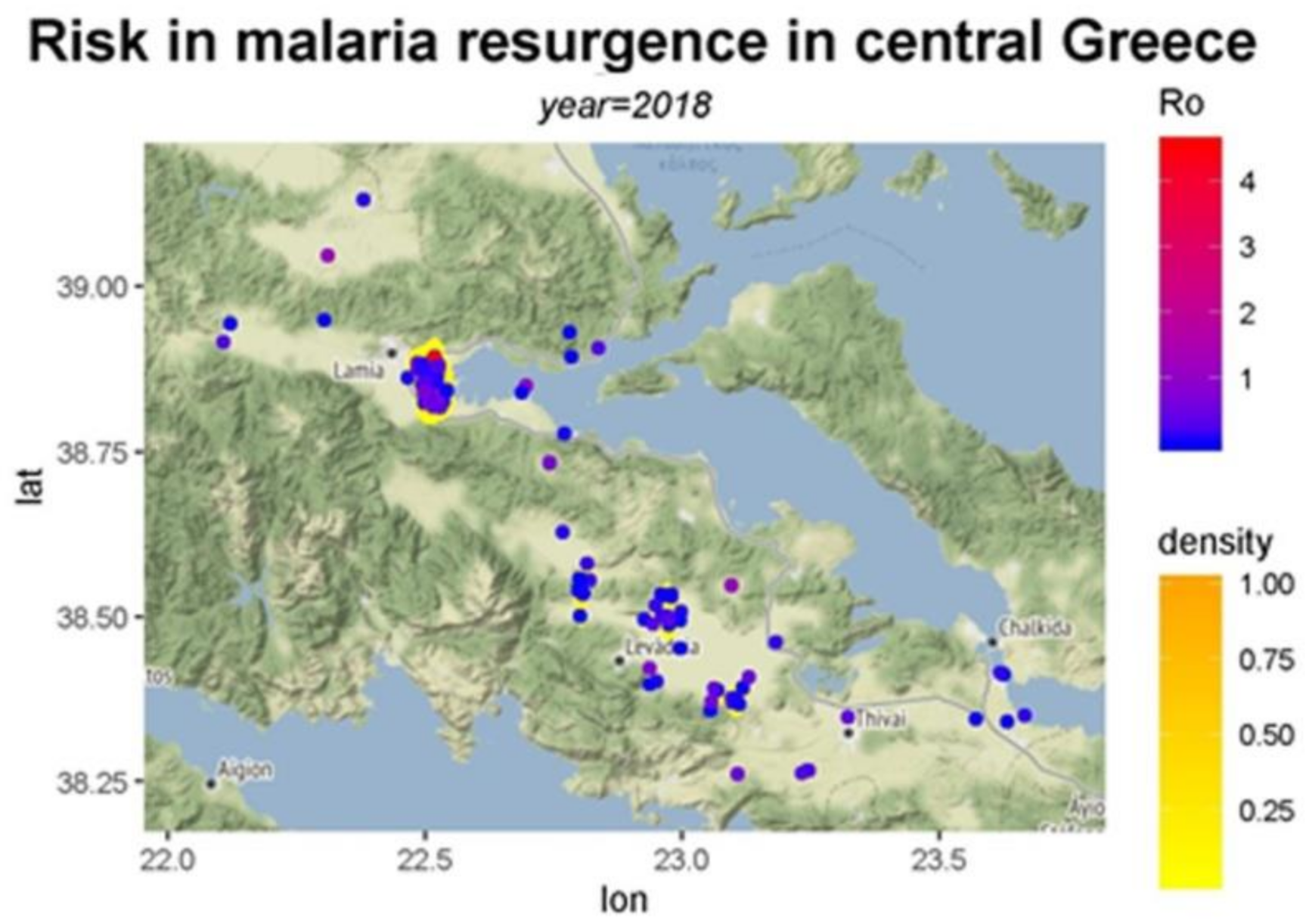
| Municipality | Area | Population | Population Density | Urban Area |
|---|---|---|---|---|
| Aliartos | 258,449,230.96 | 11,645 | 45.06 | 10,329,054 |
| Amfiklia-Elatia | 533,074,049.91 | 11,902 | 22.33 | 7,157,762 |
| Domokos | 708,266,142.17 | 15,227 | 21.50 | 3,088,636 |
| Thivae | 832,281,806.11 | 34,401 | 41.33 | 16,878,756 |
| Lamia | 946,708,662.20 | 71,006 | 75.00 | 27,276,528 |
| Levadia | 690,056,941.60 | 33,425 | 48.44 | 11,000,844 |
| Makrakomi | 837,249,336.29 | 19,211 | 22.95 | 6,073,913 |
| Molos-St. Konstantinos | 339,219,708.58 | 13,048 | 38.46 | 8,691,250 |
| Orchomenos | 418,237,273.08 | 13,107 | 31.34 | 10,791,795 |
| Stylida | 462,005,680.14 | 13,851 | 29.98 | 6,335,263 |
| Tanagra | 460,288,037.47 | 15,086 | 32.78 | 32,420,422 |
| Chalkida | 425,298,043.46 | 86,393 | 203.14 | 56,371,907 |
| Data Type | Detail | Source | References |
|---|---|---|---|
| Entomological | Number of mosquitoes | Trap survey | This study |
| Social | Number of immigrants | Information collected by Bioapplications Ltd. | https://www.bioapplications.gr/?lang=en (accessed on 6 December 2020) |
| Environmental | Temperature | Meteorological stations (National Observatory of Athens) | https://www.meteo.gr/meteomaps/ (accessed on 6 December 2020) |
| Geographical | Maps | National Bank of Meteorological and Hydrographic Data (Figure 1) | www.hydroscope.gr (accessed on 6 December 2020) |
| R Package: ggmap using Stamen open-source maps (Figure 2, Figure 3, Figure 4 and Figure 5, Figure A1, Figure A2, Figure A3, Figure A4, Figure A5 and Figure A6) | maps.stamen.com (accessed on 6 December 2020) |
| Parameter or Variable | Interpretation | Distribution/Value | Reference |
|---|---|---|---|
| % of mosquitoes that feed on humans/day | Uniform (0.01, 0.5) | [17,20,21], | |
| Probability a bite produces infection to a human | Uniform (0.2, 0.5) | [17,20,21] | |
| c | Probability a bite turns a susceptible mosquito to infected | 0.5 | [17,20,21] |
| Average daily recovery rate/day | Uniform (0.01, 0.5) | [17,20,21] | |
| # of days from infection to infectiousness in the mosquito | Uniform (5, 15) | [17,20,21] | |
| The ratio of mosquitoes to humans in region i | Observed data | ||
| Mosquito mortality rate/day | [20] | ||
| Average temperatures | Observed data | ||
| Spatial parameter for modeling the spatial component of migrant transmission | Observed data | ||
| Distances of migrant population from the larvae areas | Observed data |
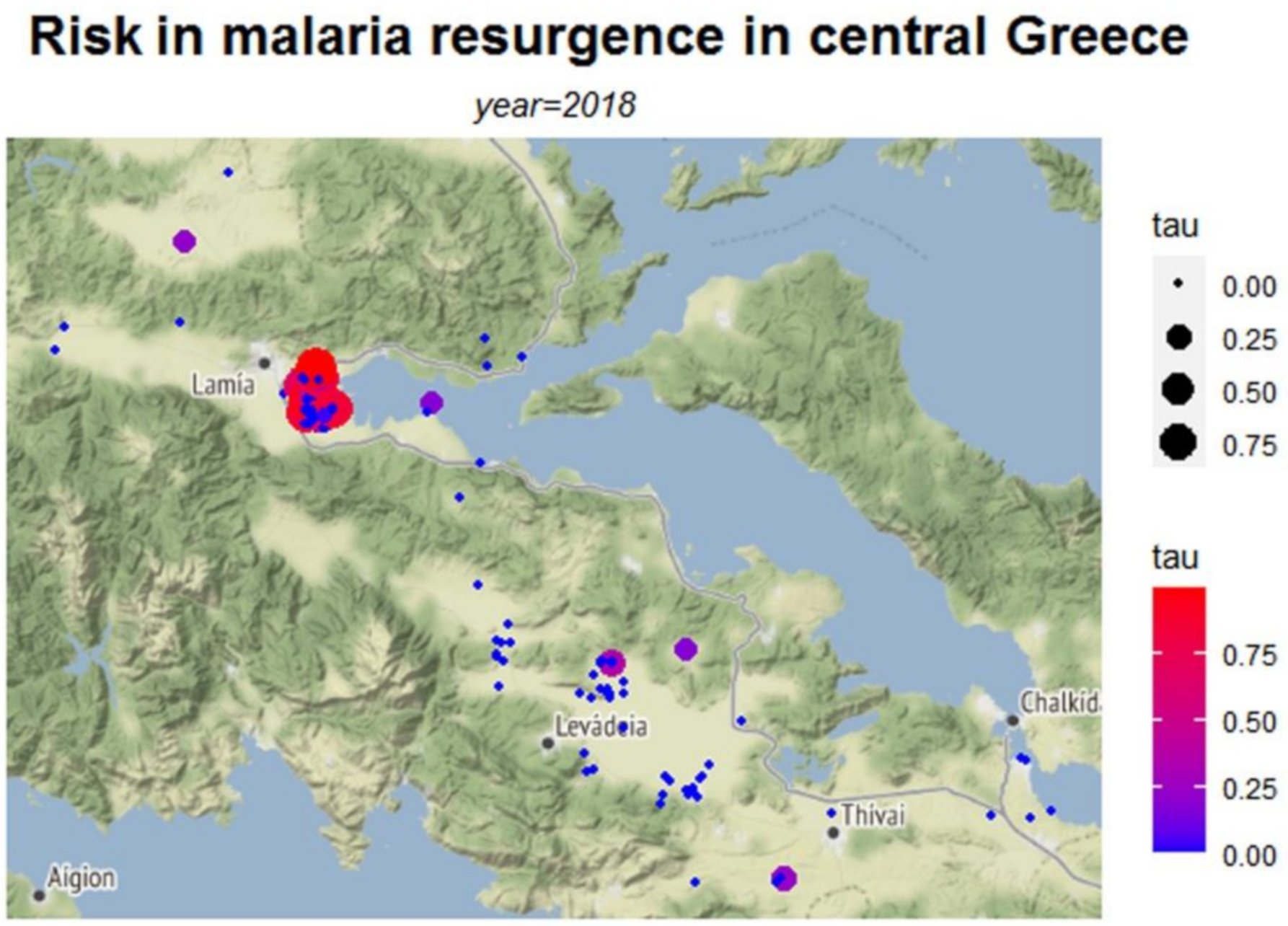
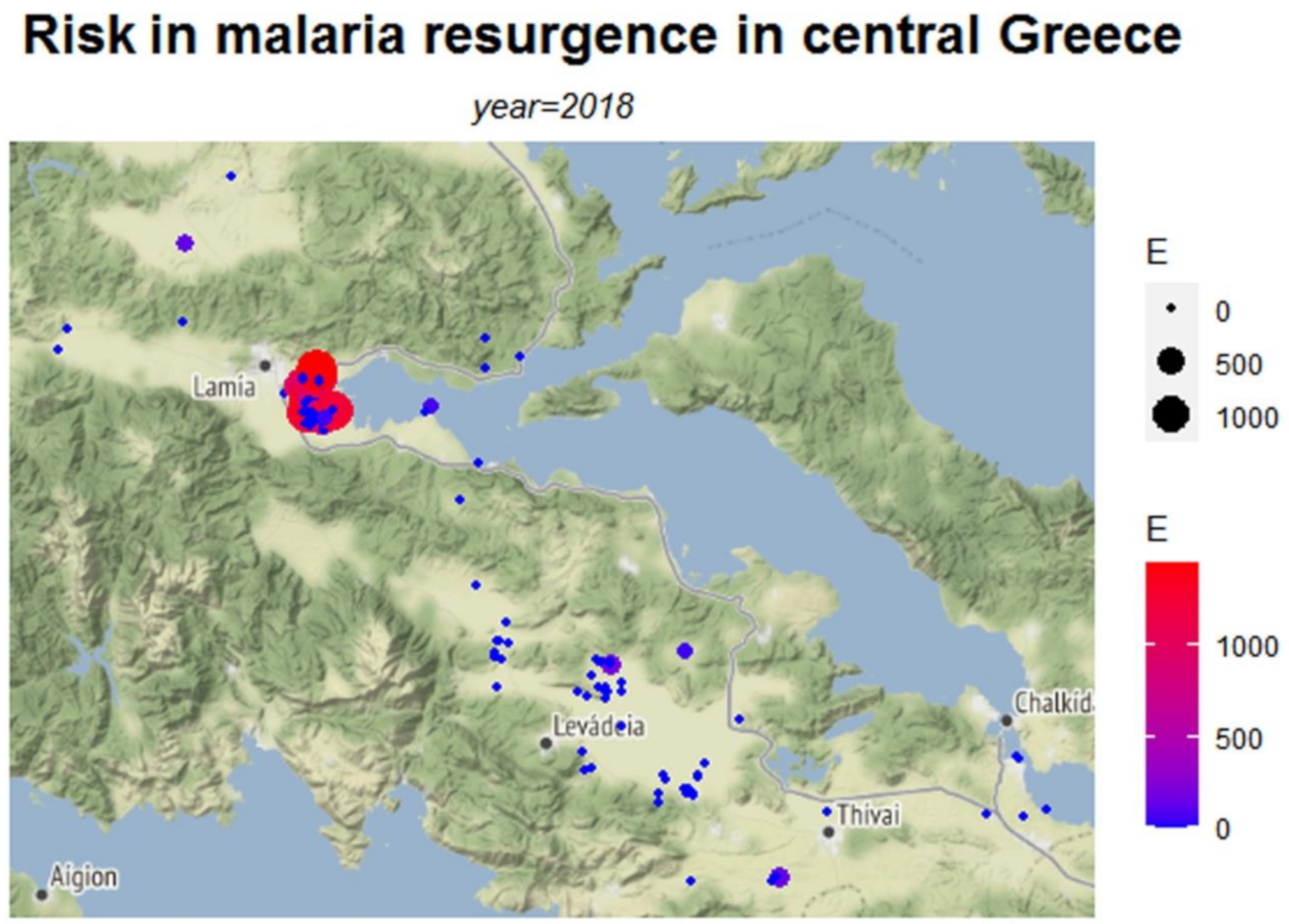
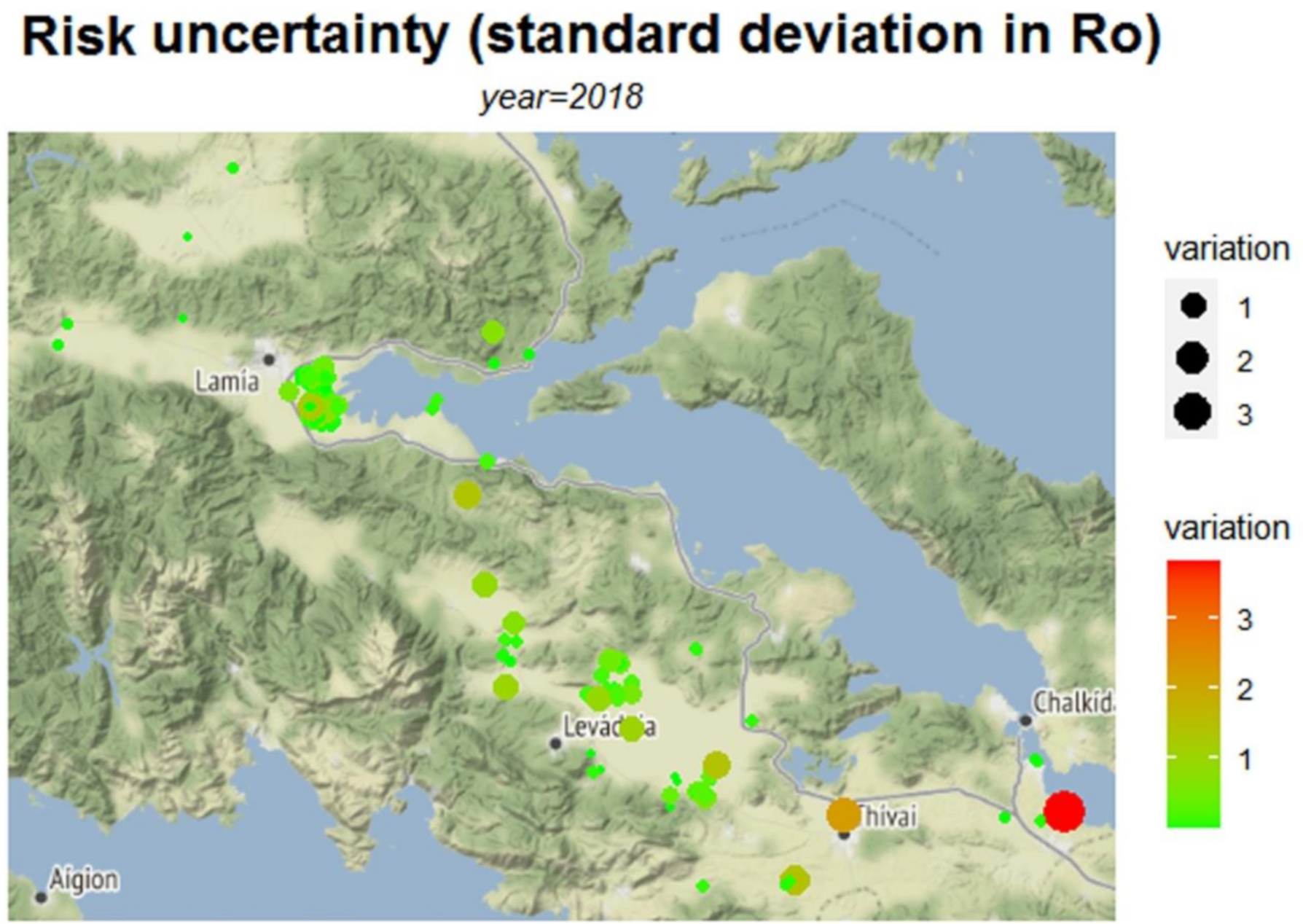
| Municipality | Ro | τ | E | Variation of Ro | |
|---|---|---|---|---|---|
| Aliartos | Mean | 0.162 | 0 | 0 | 0.125 |
| Median | 0.048 | 0 | 0 | 0 | |
| Std. Deviation | 0.190 | 0 | 0 | 0.342 | |
| Minimum | 0.010 | 0 | 0 | 0 | |
| Maximum | 0.563 | 0 | 0 | 1 | |
| Amfiklia-Elatia | Mean | 0.071 | 0 | 0 | 1 |
| Median | 0071 | 0 | 0 | 1 | |
| Std. Deviation | 0 | 0 | |||
| Minimum | 0.071 | 0 | 0 | 1 | |
| Maximum | 0.071 | 0 | 0 | 1 | |
| Chalkida | Mean | 0.004 | 0 | 0 | 0 |
| Median | 0.004 | 0 | 0 | 0 | |
| Std. Deviation | 0 | 0 | 0 | ||
| Minimum | 0.004 | 0 | 0 | 0 | |
| Maximum | 0.004 | 0 | 0 | 0 | |
| Domokos | Mean | 0.602 | 0.110 | 69,400 | 0 |
| Median | 0.602 | 0.110 | 69,400 | 0 | |
| Std. Deviation | 0.741 | 0.155 | 98,146 | 0 | |
| Minimum | 0.078 | 0 | 0 | 0 | |
| Maximum | 1.126 | 0.220 | 138,800 | 0 | |
| Lamia | Mean | 0.258 | 0.033 | 49,316 | 0.035 |
| Median | 0.096 | 0 | 0 | 0 | |
| Std. Deviation | 0.474 | 0.148 | 218,071 | 0.185 | |
| Minimum | 0.003 | 0 | 0 | 0 | |
| Maximum | 4.55 | 0.989 | 1,458,600 | 1 | |
| Levadia | Mean | 0.111 | 0 | 0 | 0.188 |
| Median | 0.036 | 0 | 0 | 0 | |
| Std. Deviation | 0.184 | 0 | 0 | 0.403 | |
| Minimum | 0.007 | 0 | 0 | 0 | |
| Maximum | 0.729 | 0 | 0 | 1 | |
| Makrakomi | Mean | 0.161 | 0 | 0 | 0 |
| Median | 0.161 | 0 | 0 | 0 | |
| Std. Deviation | 0.191 | 0 | 0 | 0 | |
| Minimum | 0.026 | 0 | 0 | 0 | |
| Maximum | 0.296 | 0 | 0 | 0 | |
| Molos-St. Konstantinos | Mean | 0.381 | 0.037 | 13,960 | 0.2 |
| Median | 0.061 | 0 | 0 | 0 | |
| Std. Deviation | 0.492 | 0.083 | 31,216 | 0.447 | |
| Minimum | 0.011 | 0 | 0 | 0 | |
| Maximum | 1.105 | 0.185 | 69,800 | 1 | |
| Orchomenos | Mean | 0.172 | 0.019 | 9146 | 0.143 |
| Median | 0.062 | 0 | 0 | 0 | |
| Std. Deviation | 0.303 | 0.073 | 39,138 | 0.356 | |
| Minimum | 0.005 | 0 | 0 | 0 | |
| Maximum | 1.228 | 0.353 | 201,900 | 1 | |
| Stylida | Mean | 0.098 | 0 | 0 | 0.333 |
| Median | 0.011 | 0 | 0 | 0 | |
| Std. Deviation | 0.153 | 0 | 0 | 0.577 | |
| Minimum | 0.009 | 0 | 0 | 0 | |
| Maximum | 0.274 | 0 | 0 | 1 | |
| Tanagra | Mean | 0.054 | 0 | 0 | 1.333 |
| Median | 0.026 | 0 | 0 | 0 | |
| Std. Deviation | 0.057 | 0 | 0 | 2.309 | |
| Minimum | 0.016 | 0 | 0 | 0 | |
| Maximum | 0.119 | 0 | 0 | 4 | |
| Thivae | Mean | 0.492 | 0.048 | 32,040 | 0.6 |
| Median | 0.385 | 0 | 0 | 0 | |
| Std. Deviation | 0.384 | 0.107 | 71,644 | 0.894 | |
| Minimum | 0.118 | 0 | 0 | 0 | |
| Maximum | 1.129 | 0.239 | 160,200 | 2 | |
| Total | Mean | 0.243 | 0.029 | 40,491 | 0.076 |
| Median | 0.085 | 0 | 0 | 0 | |
| Std. Deviation | 0.443 | 0.134 | 194,946 | 0.326 | |
| Minimum | 0.003 | 0 | 0 | 0 | |
| Maximum | 4.550 | 0.989 | 1,458,600 | 4 | |
References
- WHO (World Health Organization). Vector-Borne Diseases. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 20 September 2019).
- Müller, R.; Reuss, F.; Kendrovski, V.; Montag, D. 2019 Vector-Borne Diseases in Biodiversity and Health in the Face of Climate Change; Marselle, M.R., Stadler, J., Korn, H., Irvine, K.N., Bonne, A., Eds.; Springer Nature: London, UK, 2019; pp. 67–90. [Google Scholar]
- Semenza, J.C. Prototype early warning systems for vector-borne diseases in Europe. Int. J. Environ. Res. Public Health 2015, 12, 6333–6351. [Google Scholar] [CrossRef] [Green Version]
- Degallier, N.; Favier, C.; Menkes, C.; Lengaigne, M.; Ramalho, W.M.; Souza, R.; Servain, J.; Boulanger, J.-P. Toward an early warning system for dengue prevention: Modeling climate impact on dengue transmission. Clim. Chang. 2010, 98, 581–592. [Google Scholar] [CrossRef]
- Kuhn, K.; Campbell-Lendrum, D.; Haines, A.; Cox, J. Using Climate to Predict Infectious Disease Epidemics; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Let. 2019, 22, 1690–1708. [Google Scholar] [CrossRef] [Green Version]
- Calzolari, M. Mosquito-borne diseases in Europe: An emerging public health threat. Rep. Parasitol. 2016, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Schaffner, F.; Medlock, J.M.; Van Bortel, W. Public health significance of invasive mosquitoes in Europe. Clin. Microbiol. Infec. 2013, 19, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Marques, J.; Cardoso, J.R.; Felix, R.C.; Santana, R.A.G.; das Graças Barbosa Guerra, M.; Power, D.; Silveira, H. Fresh-blood-free diet for rearing malaria mosquito vectors. Sci. Rep. 2018, 8, 17807. [Google Scholar] [CrossRef]
- Kerkow, A.; Wieland, R.; Früh, L.; Hölker, F.; Jeschke, J.M.; Werner, D.; Kampen, H. Can data from native mosquitoes support determining invasive species habitats? Modelling the climatic niche of Aedes japonicus japonicus (Diptera, Culicidae) in Germany. Parasitol. Res. 2020, 119, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Pergantas, P.; Tsatsaris, A.; Malesios, C.; Kriparakou, G.; Demiris, N.; Tselentis, Y. A spatial predictive model for malaria resurgence in central Greece integrating entomological, environmental and social data. PLoS ONE 2017, 12, e0178836. [Google Scholar]
- Gubler, D.J.; Reiter, P.; Ebi, K.L.; Yap, W.; Nasci, R.; Patz, J.A. Climate variability and change in the United States: Potential impacts on vector- and rodent-borne diseases. Environ. Health Perspect. 2001, 109 (Suppl. 2), 223–233. [Google Scholar]
- Zeller, H.; Marrama, L.; Sudre, B.; Van Bortel, W.; Warns-Petit, E. Mosquito-borne disease surveillance by the European Centre for Disease Prevention and Control. Clin. Microbiol. Infect. 2013, 19, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HCDCP (Hellenic Center for Disease Control and Prevention). Department of Epidemiological Surveillance and Intervention. Epidemiological data for malaria in Greece. 2012. Available online: http://www.keelpno.gr/Portals/0/Files/English%20files/Malaria%20reports/Malaria%20Report_2012_FINAL_23-82013_EN.pdf (accessed on 10 March 2020).
- WHO. World Malaria Report; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Łukaszyk, S. A new concept of probability metric and its applications in approximation of scattered data sets. Comput. Mech. 2004, 33, 299–304. [Google Scholar] [CrossRef]
- Macdonald, G. The analysis of equilibrium in malaria. Trop. Dis. Bull. 1952, 49, 813–829. [Google Scholar]
- Smith, D.L.; McKenzie, F.E. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar. J. 2004, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Malaria Report and WHO Global Health Observatory data Repository. 2019. Available online: https://apps.who.int/gho/data/node.main.A1372?lang=en (accessed on 10 September 2019).
- Smith, D.L.; Dushoff, J.; McKenzie, F.E. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2004, 2, 1957–1963. [Google Scholar] [CrossRef] [Green Version]
- Ruan, S.; Xiao, D.; Beier, J.C. On the Delayed Ross–Macdonald Model for Malaria Transmission. Bull. Math. Biol. 2008, 70, 1098–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Hartemink, N.A.; Purse, B.V.; Meiswinkel, R.; Brown, H.E.; de Koeijer, A.; Elbers, A.R.W.; Boender, G.-J.; Rogers, D.J.; Heesterbeek, J.A.P. Mapping the basic reproduction number (R0) for vector-borne diseases: A case study on bluetongue virus. Epidemics 2009, 1, 153–161. [Google Scholar] [CrossRef]
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. [Google Scholar] [CrossRef] [Green Version]
- Ripley, B.D.; Venables, W.N. Modern Applied Statistics; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Eurostat. Asylum applications (non-EU) in the EU-28 Member States, 2008–2018. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Asylum_statistics.Data (accessed on 16 March 2020).
- Fotakis, E.A.; Giantsis, I.A.; Sierra, J.C.; Tanti, F.; Balaska, S.; Mavridis, K.; Kourtidis, S.; Vontas, J.; Chaskopoulou, A. Population dynamics, pathogen detection and insecticide resistance of mosquito and sand fly in refugee camps, Greece. Infect. Dis. Poverty 2020, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Diuk-Wasser, M.A.; Brown, H.E.; Andreadis, T.G.; Fish, D. Modeling the Spatial Distribution of Mosquito Vectors for West Nile Virus in Connecticut, USA. Vector Borne Zoonotic Dis. 2006, 6, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Ibañez-Justicia, A.; Cianci, D. Modelling the spatial distribution of the nuisance mosquito species Anopheles plumbeus (Diptera: Culicidae) in The Netherlands. Parasit. Vectors 2015, 8, 258. [Google Scholar] [CrossRef] [Green Version]
- Minakawa, N.; Mutero, C.M.; Githure, J.I.; Beier, J.C.; Yan, G. Spatial distribution and habitat characterization of Anopheline mosquito larvae in western Kenya. Am. J. Trop. Med. Hyg. 1999, 61, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Yamasita, W.M.S.; Das, S.S.; Chapiro, G. Numerical modeling of mosquito population dynamics of Aedes aegypti. Parasit. Vectors 2018, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.B.; Berrigan, D. Temperature, demography and ectotherm fitness. Am. Nat. 2001, 158, 204–210. [Google Scholar] [CrossRef]
- Huffaker, C.B.; Messenger, P.S.; DeBach, P. The natural enemy component in natural control and the theory of biological control. In Biological Control; Huffaker, C.B., Ed.; Springer: Boston, MA, USA, 1971. [Google Scholar]
- Messenger, P.S. Bioclimatic studies with insects. Annu. Rev. Entomol. 1959, 4, 183–206. [Google Scholar] [CrossRef]
- Kontodimas, D.C.; Eliopoulos, P.A.; Stathas, G.J.; Economou, L.P. Comparative temperature-dependent development of Nephus includens (Kirsch) and Nephus bisignatus (Boheman) (Coleoptera: Coccinellidae) preying on Planococcus citri (Risso) (Homoptera: Pseudococcidae): Evaluation of a linear and various nonlinear models using specific criteria. Environ. Entomol. 2004, 33, 1–11. [Google Scholar]
- Jalali, M.A.; Tirry, L.; Arbab, A.; De Clercq, P. Temperature-dependent development of the two-spotted ladybeetle, Adalia bipunctata, on the green peach aphid, Myzus persicae, and a factitious food under constant temperatures. J. Insect Sci. 2010, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, N.E.; Milonas, P.G.; Kontodimas, D.C.; Demiris, N.; Matsinos, Y.G. Temperature-dependent development, survival, longevity and fecundity of Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). Ann. Entomol. Soc. Am. 2013, 106, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Kontodimas, D.C.; Milonas, P.G.; Stathas, G.J.; Economou, L.P.; Kavallieratos, N.G. Life table parameters of the pseudococcid predators Nephus includens and Nephus bisignatus (Coleoptera: Coccinelidae). Eur. J. Entomol. 2007, 104, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, N.E.; Milonas, P.G.; Kontodimas, D.C.; Demiris, N.; Matsinos, Y.G. Life table analysis of Propylea quatuordecimpunctata (Coleoptera: Coccinellidae) at constant temperatures. Ann. Entomol. Soc. Am. 2014, 107, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, N.E.; Kavallieratos, N.G.; Kondakis, N.; Boukouvala, M.C.; Nika, E.P.; Demiris, N. Elucidating fitness components of the invasive dermestid beetle Trogoderma granarium Everts (Coleoptera: Dermestidae) at constant temperatures, combining deterministic and stochastic demography. PLoS ONE 2019, 14, e0212182. [Google Scholar] [CrossRef] [Green Version]
- Bardosh, K.L.; Ryan, S.; Ebi, K.; Welburn, S.; Singer, B. Addressing vulnerability, building resilience: Community-based adaptation to vector-borne diseases in the context of global change. Infect. Dis. Poverty 2017, 6, 166. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergantas, P.; Papanikolaou, N.E.; Malesios, C.; Tsatsaris, A.; Kondakis, M.; Perganta, I.; Tselentis, Y.; Demiris, N. Towards a Semi-Automatic Early Warning System for Vector-Borne Diseases. Int. J. Environ. Res. Public Health 2021, 18, 1823. https://doi.org/10.3390/ijerph18041823
Pergantas P, Papanikolaou NE, Malesios C, Tsatsaris A, Kondakis M, Perganta I, Tselentis Y, Demiris N. Towards a Semi-Automatic Early Warning System for Vector-Borne Diseases. International Journal of Environmental Research and Public Health. 2021; 18(4):1823. https://doi.org/10.3390/ijerph18041823
Chicago/Turabian StylePergantas, Panagiotis, Nikos E. Papanikolaou, Chrisovalantis Malesios, Andreas Tsatsaris, Marios Kondakis, Iokasti Perganta, Yiannis Tselentis, and Nikos Demiris. 2021. "Towards a Semi-Automatic Early Warning System for Vector-Borne Diseases" International Journal of Environmental Research and Public Health 18, no. 4: 1823. https://doi.org/10.3390/ijerph18041823
APA StylePergantas, P., Papanikolaou, N. E., Malesios, C., Tsatsaris, A., Kondakis, M., Perganta, I., Tselentis, Y., & Demiris, N. (2021). Towards a Semi-Automatic Early Warning System for Vector-Borne Diseases. International Journal of Environmental Research and Public Health, 18(4), 1823. https://doi.org/10.3390/ijerph18041823









