Motor Developmental Outcomes in Children Exposed to Maternal Diabetes during Pregnancy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Question and Eligibility
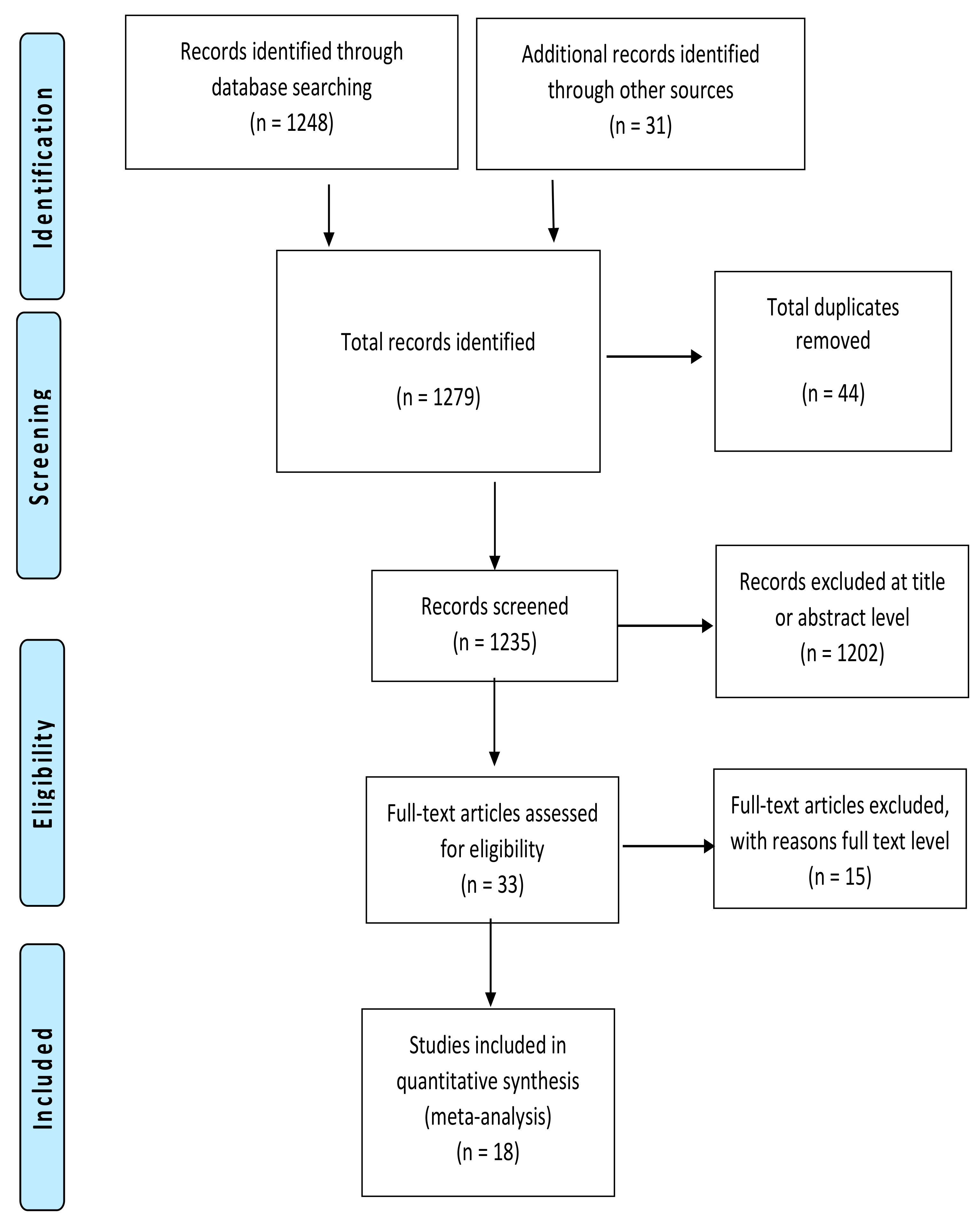
2.2. Search Strategy and Study Selection
2.3. Data Extraction and Assessment of Methodological Quality
2.4. Data Synthesis and Analysis
3. Results
3.1. Methodological Quality
3.2. Characteristics of Included Studies
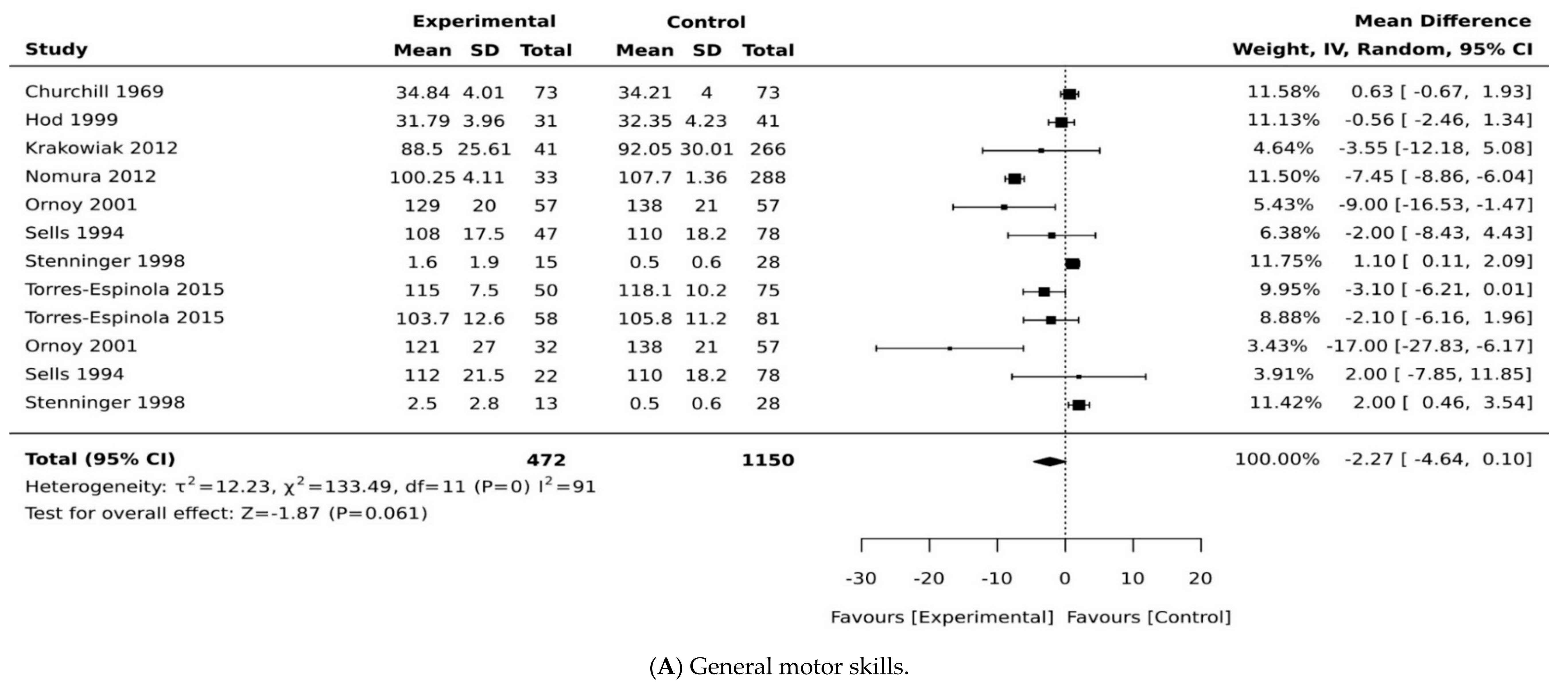
3.3. Exposure to Maternal Diabetes and Motor Development
3.4. Exposure to Maternal Diabetes and Gross Motor Development
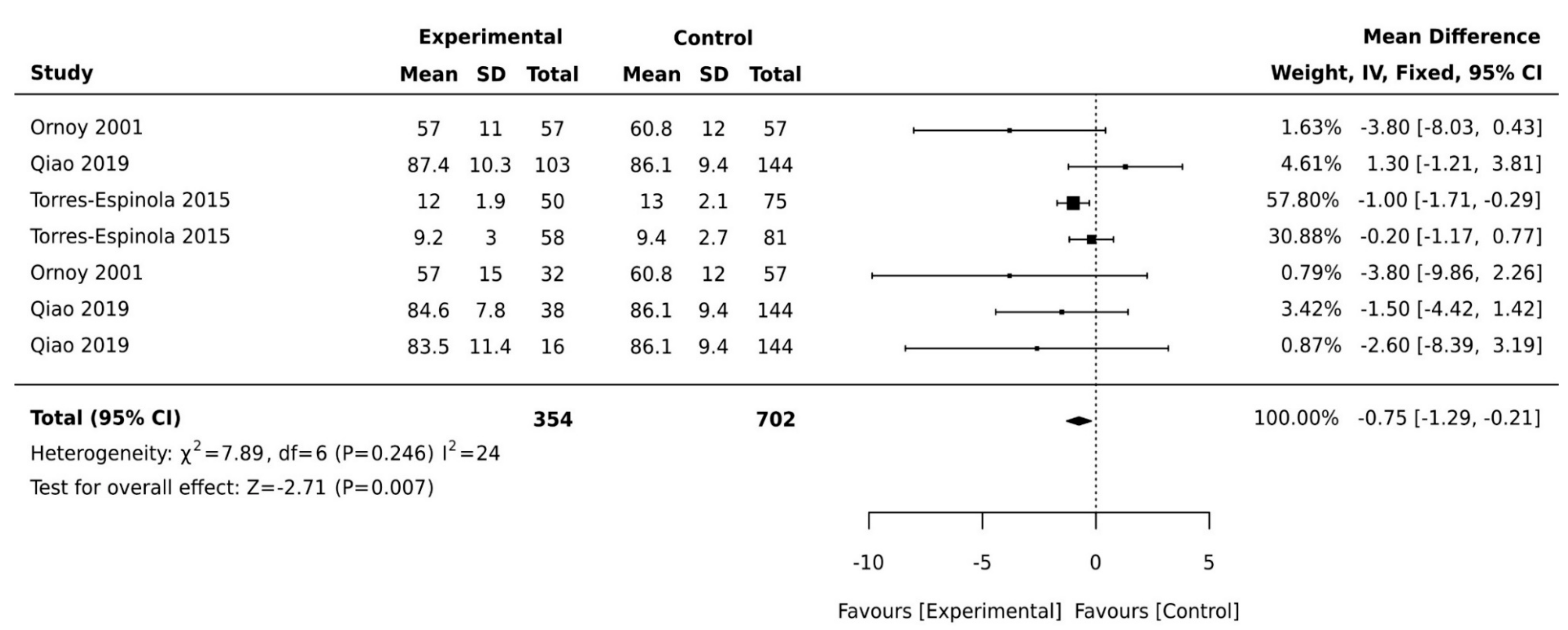
3.5. Exposure to Maternal Diabetes and Fine Motor Development
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinero-Pinto, E.; Perez-Cabezas, V.; De-Hita-Cantalejo, C.; Ruiz-Molinero, C.; Gutiérrez-Sánchez, E.; Jimenez-Rejano, J.J.; Sánchez-González, J.; Sánchez-González, M.C. Vision Development Differences between Slow and Fast Motor Development in Typical Developing Toddlers: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 3597. [Google Scholar] [CrossRef]
- Edwards, S.L.; Sarwark, J.F. Infant and Child Motor Development. Clin. Orthop. Relat. Res. 2005, 434, 33–39. [Google Scholar] [CrossRef]
- Osorio-Valencia, E.; Torres-Sánchez, L.; López-Carrillo, L.; Rothenberg, S.J.; Schnaas, L. Early motor development and cognitive abilities among Mexican preschoolers. Child Neuropsychol. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Noritz, G.H.; Murphy, N.A.; Panel, N.S.E.; Hagan, J.F.; Lipkin, P.H.; Macias, M.M.; Navsaria, D.; Peacock, G.; Rosenbaum, P.L.; Saal, H.M.; et al. Motor Delays: Early Identification and Evaluation. Pediatrics 2013, 131, e2016–e2027. [Google Scholar] [CrossRef] [PubMed]
- Poulain, T.; Vogel, M.; Sobek, C.; Hilbert, A.; Körner, A.; Kiess, W. Associations Between Socio-Economic Status and Child Health: Findings of a Large German Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 677. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, R.; Fujioka, K.; Kyono, Y.; Yoshida, A.; Kido, T.; Suga, S.; Abe, S.; Ashina, M.; Nishida, K.; Tanimura, K.; et al. Neurodevelopmental Outcomes at 18 Months of Corrected Age for Late Preterm Infants Born at 34 and 35 Gestational Weeks. Int. J. Environ. Res. Public Health 2021, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.C.; Campoy, C.; Fernandez, L.G.; López-Pedrosa, J.M.; Rueda, R.; Martín, M.J. Maternal Diabetes and Cognitive Performance in the Offspring: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0142583. [Google Scholar] [CrossRef]
- Tong, S.-L.; Baghurst, P.; Vimpani, G.; McMichael, A. Socioeconomic Position, Maternal IQ, Home Environment, and Cognitive Development. J. Pediatr. 2007, 151, 284–288.e1. [Google Scholar] [CrossRef]
- Effects of socio-economic status and maternal education on gross motor development of preschool children. Dev. Med. Child Neurol. 2015, 57, 55–56. [CrossRef]
- Ahmed, R. Evolutionary interactions between diabetes and development. Diabetes Res. Clin. Pr. 2011, 92, 153–167. [Google Scholar] [CrossRef]
- Verner, A.M.; Manderson, J.; Lappin, T.R.J.; McCance, D.R.; Halliday, H.L.; Sweet, D.G. Influence of maternal diabetes mellitus on fetal iron status. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F399–F401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santa-Marina, L.; Lertxundi, N.; Andiarena, A.; Irizar, A.; Sunyer, J.; Molinuevo, A.; Llop, S.; Julvez, J.; Beneito, A.; Ibarluzea, J.; et al. Maternal Ferritin Levels during Pregnancy and ADHD Symptoms in 4-Year-Old Children: Results from the INMA–INfancia y Medio Ambiente (Environment and Childhood) Prospective Birth Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 7704. [Google Scholar] [CrossRef]
- Girchenko, P.; Tuovinen, S.; Lahti-Pulkkinen, M.; Lahti, J.; Savolainen, K.; Heinonen, K.; Pyhälä, R.; Reynolds, R.M.; Hämäläinen, E.; Villa, P.M. Maternal early pregnancy obesity and related pregnancy and pre-pregnancy disorders: Associations with child developmental milestones in the prospective PREDO Study. Int. J. Obes. 2018, 42, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Biesenbach, G.; Grafinger, P.; Zazgornik, J.; Stöger, H. Perinatal complications and three-year follow up of infants of diabetic mothers with diabetic nephropathy stage IV. Ren. Fail. 2000, 22, 573–580. [Google Scholar] [CrossRef]
- Hod, M.; Levy-Shiff, R.; Lerman, M.; Schindel, B.; Ben-Rafael, Ζ.; Bar, J. Developmental Outcome of Offspring of Pregestational Diabetic Mothers. J. Pediatr. Endocrinol. Metab. 1999, 12, 867–872. [Google Scholar] [CrossRef]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Maternal preconception weight trajectories, pregnancy complications and offspring’s childhood physical and cognitive development. J. Dev. Orig. Health Dis. 2018, 9, 653–660. [Google Scholar] [CrossRef]
- Daraki, V.; Roumeliotaki, T.; Koutra, K.; Georgiou, V.; Kampouri, M.; Kyriklaki, A.; Vafeiadi, M.; Papavasiliou, S.; Kogevinas, M.; Chatzi, L. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: The Rhea mother–child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry 2017, 26, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Alamolhoda, S.; Ahmadi Doulabi, M.; Afraz, F. The impact of gestational diabetes mellitus on motor development in 12-month-old children attending to Qazvin University of Medical Sciences, Iran. Int. J. Pediatrics 2020, 8, 12575–12583. [Google Scholar] [CrossRef]
- Churchill, J.A.; Berendes, H.W.; Nemore, J. Neuropsychological deficits in children of diabetic mothers: A report from the Collaborative Study of Cerebral Palsy. Am. J. Obstet. Gynecol. 1969, 105, 257–268. [Google Scholar] [CrossRef]
- Sells, C.J.; Robinson, N.M.; Brown, Z.; Knopp, R.H. Long-term developmental follow-up of infants of diabetic mothers. J. Pediatrics 1994, 125, S9–S17. [Google Scholar] [CrossRef]
- Stenninger, E.; Flink, R.; Eriksson, B.; Sahlen, C. Long term neurological dysfunction and neonatal hypoglycaemia after diabetic pregnancy. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F174–F179. [Google Scholar] [CrossRef]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Vargo, E.J. The Effect of Vitamin D Supplementation on Glycaemic Control in Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 1716. [Google Scholar] [CrossRef]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Diabetes in Pregnancy and Childhood Cognitive Development: A Systematic Review. Pediatr. 2016, 137, e20154234. [Google Scholar] [CrossRef] [PubMed]
- Sesma, H.W.; Georgieff, M.K. The effect of adverse intrauterine and newborn environments on cognitive development: The experiences of premature delivery and diabetes during pregnancy. Dev. Psychopathol. 2003, 15, 991–1015. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Aromataris, E.; Tufanaru, C.; Stern, C.; Porritt, K.; Farrow, J.; Lockwood, C.; Stephenson, M.; Moola, S.; Lizarondo, L. The development of software to support multiple systematic review types: The Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI). Int. J. Evid.-Based Healthc. 2019, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cohen, P.; Chen, S. How Big is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Commun. Stat. Simul. Comput. 2010, 39, 860–864. [Google Scholar] [CrossRef]
- Nomura, Y.; Marks, D.J.; Grossman, B.; Yoon, M.; Loudon, H.; Stone, J.; Halperin, J.M. Exposure to gestational diabetes mellitus and low socioeconomic status: Effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch. Pediatrics Adolesc. Med. 2012, 166, 337–343. [Google Scholar]
- Qiao, L.-X.; Wang, J.; Yan, J.-H.; Xu, S.-X.; Wang, H.; Zhu, W.-Y.; Zhang, H.-Y.; Li, J.; Feng, X. Follow-up study of neurodevelopment in 2-year-old infants who had suffered from neonatal hypoglycemia. BMC Pediatr. 2019, 19, 133. [Google Scholar] [CrossRef]
- Torres-Espínola, F.J.; Berglund, S.K.; García-Valdés, L.M.; Moreno, M.T.S.; Jerez, A.; Campos, D.; Moreno-Torres, R.; Rueda, R.; Catena, A.; Pérez-García, M.; et al. Maternal Obesity, Overweight and Gestational Diabetes Affect the Offspring Neurodevelopment at 6 and 18 Months of Age—A Follow Up from the PREOBE Cohort. PLoS ONE 2015, 10, e0133010. [Google Scholar] [CrossRef]
- Ornoy, A.; Ratzon, N.; Greenbaum, C.; Peretz, E.; Soriano, D.; Dulitzky, M. Neurobehaviour of school age children born to diabetic mothers. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F94–F99. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Ratzon, N.; Greenbaum, C.; Wolf, A.; Dulitzky, M. School-age Children Born to Diabetic Mothers and to Mothers with Gestational Diabetes Exhibit a High Rate of Inattention and Fine and Gross Motor Impairment. J. Pediatr. Endocrinol. Metab. 2001, 14, 681–690. [Google Scholar] [CrossRef]
- Ornoy, A.; Wolf, A.; Ratzon, N.; Greenbaum, C.; Dulitzky, M. Neurodevelopmental outcome at early school age of children born to mothers with gestational diabetes. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 81, F10–F14. [Google Scholar] [CrossRef] [PubMed]
- Ratzon, N.; Greenbaum, C.; Dulitzky, M.; Ornoy, A. Comparison of the motor development of school-age children born to mothers with and without diabetes mellitus. Phys. Occup. Ther. Pediatr. 2000, 20, 43–57. [Google Scholar] [CrossRef]
- Ghassabian, A.; Sundaram, R.; Wylie, A.; Bell, E.M.; Bello, S.C.; Yeung, E.H. Maternal medical conditions during pregnancy and gross motor development up to age 24 months in the Upstate KIDS study. Dev. Med. Child Neurol. 2016, 58, 728–734. [Google Scholar] [CrossRef]
- Bolaños, L.; Matute, E.; Ramírez-Dueñas, M.D.L.; Zarabozo, D.; Ramirez-Duenas, M.D.L. Neuropsychological Impairment in School-Aged Children Born to Mothers With Gestational Diabetes. J. Child Neurol. 2015, 30, 1616–1624. [Google Scholar] [CrossRef]
- Bianchi, C.; De Gennaro, G.; Romano, M.; Aragona, M.; Battini, L.; Del Prato, S.; Bertolotto, A. Pre-pregnancy obesity, gestational diabetes or gestational weight gain: Which is the strongest predictor of pregnancy outcomes? Diabetes Res. Clin. Pr. 2018, 144, 286–293. [Google Scholar] [CrossRef]
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef]
- Duffany, K.O.; McVeigh, K.H.; Lipkind, H.S.; Kershaw, T.; Ickovics, J.R. Large for Gestational Age and Risk for Academic Delays and Learning Disabilities: Assessing Modification by Maternal Obesity and Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 5473. [Google Scholar] [CrossRef]
- Shefali, A.K.; Kavitha, M.; Deepa, R.; Mohan, V. Pregnancy outcomes in pre-gestational and gestational diabetic women in comparison to non-diabetic women—A prospective study in Asian Indian mothers (CURES-35). J. Assoc. Physicians India 2006, 54, 613–618. [Google Scholar] [PubMed]
- Rasmussen, L.N.; Montgomery, P. The prevalence of and factors associated with inclusion of non-English language studies in Campbell systematic reviews: A survey and meta-epidemiological study. Syst. Rev. 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Arabiat, D.; Whitehead, L.; Al Jabery, M. The 12-year prevalence and trends of childhood disabilities in Australia: Findings from the Survey of Disability, Aging and Carers. Child Care Health Dev. 2018, 44, 697–703. [Google Scholar] [CrossRef] [PubMed]


| Reference | Country | Study Design | Age at Assessment | Diabetes Group | N | Comparator Group | N | Developmental Outcome |
|---|---|---|---|---|---|---|---|---|
| Krakowiak et al. [12] | USA | Case–control | 2–6 years | Pre-existing and GDM | 45 | No diabetes | 235 | Motor scores |
| No diabetes, but had Autism Spectrum Disorder (ASD) | 266 | |||||||
| Girchenko et al. [13] | Finland | Case–control | Mean age 3.5 years | GDM and normal weight | 85 | No diabetes and normal weight | 1652 | Gross motor |
| Type 1 diabetes and normal weight | 4 | No diabetes and normal weight | 383 | |||||
| GDM and overweight | 69 | No diabetes and overweight | 212 | |||||
| Type 1 diabetes and overweight | 4 | No diabetes and obese | ||||||
| GDM and obese | 94 | |||||||
| Type 1 diabetes and obese | 1 | |||||||
| Biesenbach et al. [14] | Austria | Cohort study | 3 years | Diabetes with stage IV nephropathy | 10 | Diabetes without nephropathy | 30 | Gross motor |
| Hod et al. [15] | Israel | Cohort study | 1 year | Type 1 diabetes | 21 | No diabetes | 41 | Motor scores |
| Type 2 diabetes | 10 | |||||||
| Adane et al. [16] | Australia | Case–control | 0–6 years | Diabetes during pregnancy | 61 | No diabetes | 710 | Gross and fine motor |
| Daraki et al. [17] | Greece | Case–control | 4 years | GDM and normal weight | 35 | No diabetes and normal weight | 463 | Motor scores |
| GDM and overweight | 7 | No diabetes and overweight | 166 | |||||
| GDM and obese | 14 | No diabetes and obese | 87 | |||||
| Churchill et al. [19] | USA | Cohort study | 6 months–1 year | Class A diabetes and no acetonuria | 73 | No diabetes | 73 | Motor scores |
| Class A diabetes and acetonuria | 55 | No diabetes | 55 | |||||
| Sells et al. [20] | USA | Cohort study | 6 months–2 years | Early entry at age 6 months | 51 | No diabetes at child age 6 months | 83 | Motor scores |
| Early entry at age 12 months | 62 | No diabetes at child age 12 months | 83 | |||||
| Early entry at age 24 months | 47 | No diabetes at child age 24 months | 78 | |||||
| Late entry at age 6 months | 32 | |||||||
| Late entry at age 12 months | 31 | |||||||
| Late entry at age 24 months | 22 | |||||||
| Stenninger et al. [21] | Sweden | Cohort study | 6–12 years | Hypoglycaemic | 13 | No diabetes | 28 | Motor scores |
| Non-hypoglycaemic | 15 | |||||||
| Nomura et al. [28] | USA | Case study | 2–6 years | GDM | 214 | No diabetes | 191 | Fine motor |
| Qiao et al. [29] | China | Case–control | 2 years | Group 1 hypoglycaemic <2 h after birth | 103 | No diabetes | 144 | Gross and fine motor |
| Group 2 hypoglycaemic 2–24 h after birth | 38 | |||||||
| Group 3 hypoglycaemic >24 h after birth | 16 | |||||||
| Torres-Espinola et al. [30] | Spain | Case–control | 6–18 months | GDM at child age 6 months | 58 | Normal weight at child age 6 months | 81 | Gross motor |
| GDM at child age 18 months | 50 | Normal weight at child age 18 months | 75 | |||||
| Overweight at child age 6 months | 44 | |||||||
| Overweight at child age 18 months | 43 | |||||||
| Obese at child age 6 months | 32 | |||||||
| Obese at child age 18 months | 29 | |||||||
| Ornoy et al. [31] | Israel | Cohort study | Mean age 8 years | Pregestational diabetes | 57 | No diabetes | 57 | Gross and fine motor |
| Ornoy et al. [32] | Israel | Cohort study | Early school age | Pre-existing diabetes | 57 | No diabetes | 57 | Gross and fine motor |
| GDM | 32 | |||||||
| Ornoy et al. [33] | Israel | Cohort study | 5–12 years (young vs. old) | GDM | 32 | No diabetes | 57 | Gross and fine motor |
| Ratzon et al. [34] | Israel | Cohort study | Mean age 8 years | Pre-existing diabetes | 57 | No diabetes | 57 | Gross and fine motor |
| Ghassabian et al. [35] | USA | Case–control | 4 months–2 years | GDM | N/A | Gestational comorbidities (hypertension–eclampsia) | 4897 | Gross motor |
| Bolaños et al. [36] | Mexico | Cohort study | Mean age 8 years | GDM | 32 | No diabetes | 28 | Fine motor |
| Reference | Measurement | Adjusted Analysis | Summary of Main Results | Included in Meta-Analysis |
|---|---|---|---|---|
| Krakowiak et al. [12] | Mullen Scales of Early Learning (MSEL) | Yes | Diabetes may be associated with neurodevelopmental problems in offspring. Proportionately more mothers of children in the ASD and developmental delay groups had either type 2 diabetes or GDM. The risk having a child with developmental delay relative to type 2 diabetes was significantly increased among obese women. | Yes |
| Girchenko et al. [13] | Ages and Stages Questionnaire (ASQ) | Yes | Maternal early pregnancy overweight, obesity, and pre-eclampsia are independently associated with neurodevelopmental delay in children. GDM increased the odds of developmental delay but can be partially explained by maternal overweight/obesity and other disorders. | No |
| Biesenbach et al. [14] | Month of life when starting to walk | No | Both groups of children born to mothers with and without diabetes started to walk at the same age. | Yes |
| Hod et al. [15] | Bayley Scales of Infant Development (MDI) and Psychomotor Developmental Index (PDI) | No | Both MDI and PDI scores were significantly lower in infants of diabetes mothers compared with the control group. Moreover, infants of mothers with type 2 diabetes had lower scores on the PDI and motor quality index. | Yes |
| Adane et al. [16] | Ages and Stages Questionnaire (ASQ) | Yes | Children born to chronically obese women were more likely to be at risk developmentally. Children of mothers with diabetes during pregnancy were at slightly greater risk of developmental delay, particularly gross motor skills, compared to women without diabetes in pregnancy. | No |
| Daraki et al. [17] | The McCarthy Scales of Children’s Abilities (MSCA) | Yes | Obesity, maternal glucose tolerance in early pregnancy, and GDM were not associated with child neurodevelopment, including motor development. | No |
| Churchill et al. [19] | Bayley Scales of Infant Development (BSID); neurological posturing scales | No | The infants of diabetic mothers differed significantly from matched controls in Bayley mental and motor scores at 6 months and in posturing rating scale at 1 year. Infants of mothers who had diabetes and were acetonuria positive showed significantly greater developmental deficits than matched controls. Infants born to mothers who had diabetes and were acetone negative did not differ from their matched controls. The presence or absence of acetonuria, not the severity of diabetes, explained the differences in child development. | Yes |
| Sells et al. [20] | Bayley Scales of Infant Development (BSID) | Yes | No significant differences between groups with relation to motor development. | Yes |
| Stenninger et al. [21] | Screening for Minimal Brain Dysfunction (MBD); Assessment Battery (Movement ABC); Griffith’s Mental Developmental Test (GMDT eye and hand coordination scale) | No | No differences in neurological examination, but children in the neonatal hypoglycaemia group had significantly higher scores in the minimal brain dysfunction test. No significant differences in the Movement Assessment Battery Test (MABT). | Yes |
| Nomura et al. [28] | Developmental Neuropsychological Assessment (NEPSY) | Yes | Children exposed to both GDM and low socioeconomic status (SES) showed compromised neurobehavioural outcomes; the risk of ADHD was synergistically associated with exposure to both GDM and low SES. | Yes |
| Qiao et al. [29] | Gesell developmental test (GDT, Chinese revised version) | No | There was no difference reported in gross or fine motor skill acquisition or adaptability between the controls and any infant in Group A. | Yes |
| Torres-Espinola et al. [30] | Bayley Scales of Infant Development (BSID) | Yes | Although not significant, at age 18 months, gross motor scores were lower in the overweight, obese, and GDM groups compared to the control group. Motor skill at 18-month adjusted analysis of infants of GDM mothers had lower scores, but this disappeared in adjusted models. | Yes |
| Ornoy et al. [31] | Bruininks–Oseretsky Motor Development Test (BOMDT); | No | Children born to mothers with diabetes had significantly lower scores on the Bruininks–Oseretsky Motor Development Test (BOMDT); children born to diabetic mothers had more soft neurological signs and lower gross and fine motor movement achievements than children born to non-diabetic mothers. | Yes |
| Ornoy et al. [32] | Bruininks–Oseretsky Motor Development Test (BOMDT) | No | Children whose mothers did not have diabetes scored significantly higher on the BOMDT than children of mothers with any type of diabetes. However, differences between children of diabetic mothers and control group children lessened over time. | Yes |
| Ornoy et al. [33] | The Touwen–Prechtl neurological examination; Bender Visual Gestalt Test for the evaluation of eye–hand coordination; Goodenough Draw a Man test; Bruininks–Oseretsky Motor Development Test (BOMDT) | No, but matched by age, birth order, and socioeconomic status. | Younger children in the index group had significantly lower scores on the BOMDT, but this difference was not present in the older index group children. There were no differences between groups on the Touwen–Prechtl neurological examination. Overall, even though children born to mothers with GDM had higher rates of lower fine and gross motor skill scores in the younger age group, when compared with control group children, these differences diminished with age. | Yes |
| Ratzon et al. [34] | Bruininks–Oseretsky Test of Motor Proficiency (BOTMP) | Children born to mothers with diabetes had more fine and gross motor difficulties than children born to mothers without diabetes. There was a negative correlation between a mother’s high HbA1C and high acetonuria and the children’s BOTMP scores. Environmental variables and gross motor development positively correlated only for children born to mothers with diabetes. | Yes | |
| Ghassabian et al. [35] | World Health Organisation (WHO) major milestones/time to achieve motor milestones | Yes | Children of mothers with diabetes or GDM took longer to achieve major motor milestones measured—sitting without support, walking with assistance, and walking alone—than children of mothers without diabetes or GDM, independent of maternal obesity. Children of mothers with hypertensive diseases also took longer to achieve milestones, but this difference disappeared after adjustment for perinatal factors. | No |
| Bolaños et al. [36] | Purdue Pegboard Dexterity Test (PPDT) | No | The GDM group children had significantly lower scores in graphic and spatial abilities. Motor skills were significantly lower and there were more soft neurological signs in children whose mothers had GDM than children in the control group. | No |
| Reference | Primary Developmental Outcome | Main Findings Presented (Mean (SD), Percentage, Adjusted Model) | |
|---|---|---|---|
| Krakowiak et al. [12] | Fine motor | DM: 38.98 (28.57), Ctrl: 44.32 (0.60) | p = 0.01 *, effect size (−0.21) |
| Fine motor | DM and ASD: 27.11 (20.94), Ctrl: 27.79 (17.75), | p = NS, effect size (−0.04) | |
| Standard motor | DM: 86.15 (41.70), Ctrl: 92.06 (30.57) | p = 0.04 *, effect size (−0.16) | |
| Standard motor | DM and ASD: 75.00 (32.48), Ctrl: 74.35 (27.86), | p = NS, effect size (0.02) | |
| Girchenko et al. [13] | Fine motor | GDM: 21 (8.5%) with DD vs. 17 (6.9%) with normal development | |
| Gross motor | GDM: 15 (6.1%) with DD vs. 21 (8.5%) with normal development | ||
| Fine motor | Type 1 DM: 3 (33.3%) with DD vs. none with normal development | ||
| Gross motor | Type 1 DM: 2 (22.2%) with DD vs. none with normal development | ||
| Adjusted model for children born to mothers with GDM vs. no diabetic disorders (mild DD) | |||
| Fine motor | 0.83 (0.51–1.36) | p = NS | |
| Gross motor | 0.85 (0.48 = 1.51) | p = NS | |
| Adjusted model for children born to mothers with GDM vs. no diabetic disorders (severe DD). | |||
| Fine motor | 1.11 (0.63–1.95) | p = NS | |
| Gross motor | 1.40 (0.83–2.35) | p = NS | |
| Biesenbach et al. [14] | Gross motor | Mean age child started to walk: Nephropathy: 11 months (2), without nephropathy 11 months (1) | p < 0.05 *, effect size (0.00) |
| Hod et al. [15] | Motor quality | PGDM: 31.79 (3.96), Ctrl: 32.35 (4.23) | p = NS, effect size (−0.14) |
| Adane et al. [16] | Gross and fine motor skills | Adjusted RR (95% CI): 1.22 (0.67, 2.24) | |
| Daraki et al. [17] | Motor scale scores | GDM: Adjusted β-coefficient (95% CI): −1.50 (−5,80, 2.79). | |
| Churchill et al. [19] | Class A diabetes and acetonuria: 31.50 (4.26), Ctrl: 33.23 (4.29), | p = 0.01 *, effect size (−0.40). | |
| Class A diabetes and no acetonuria: 31.50 (4.26), Ctrl: 33.23 (4.29), | p = NS, effect size (0.16) | ||
| Sells et al. [20] | Bayley Motor Scale 6 months | Early entry: 109 (16.2), Ctr: 108 (15) | p = NS, effect size (0.06). |
| Bayley Motor Scale 6 months | Late entry: 104 (17.0), Ctrl: 108 (15) | p = NS, effect size (−0.25) | |
| Bayley Motor Scale 12 months | Early entry: 104 (15.5). Ctrl: 103 (15.8) | p = NS, effects size (0.06) | |
| Bayley Motor Scale 12 months | Late entry: 102 (17.2), Ctrl: 103 (15.8) | p = NS, effect size (−0.06). | |
| Bayley Motor Scale 24 months | Early entry: 108 (17.5), Ctrl: 110 (18.2) | p = NS, effect size (−0.11) | |
| Bayley Motor Scale 24 months | Late entry: 112 (21.5), Ctrl: 110 (18.2) | p = NS, effect size (0.10) | |
| Bayley Motor Scale 6 months | Group with major malformation: 89 (19.0), Ctrl: 109 (14.8) | p < 0.0001 **, effect size (−1.17) | |
| Bayley Motor Scale 12 months | Group with major malformation: 93 (21.0), Ctrl: 106 (14.3) | p < 0.024 *, effect size (−0.72) | |
| Stenninger et al. [21] | MBD scores | Hypoglycaemic: 2.5 (2.8), Ctrl: 0.5 (0.6) | p < 0.05 *, effect size (0.99) |
| MBD scores | Non-hypoglycaemic: 1.6 (1.9), Ctrl: 0.5 (0.6) | p < 0.05 *, effect size (0.78) | |
| Manual dexterity (fine motor) | Hypoglycaemic: 1.96 (2.4), Ctrl: 0.93 (1) | p = NS, effect size (0.56) | |
| Manual dexterity (fine motor) | Non-hypoglycaemic: 1.33 (1.7), Ctrl: 0.93 (1) | p = NS, effect size (0.29). | |
| Balls skills (gross motor) | Hypoglycaemic: 2.12 (2), Ctrl: 1.75 (1.9) | p = NS, effect size (0.19) | |
| Balls skills (gross motor) | Non-hypoglycaemic: 2.13 (2.2), Ctrl: 1.75 (1.9), | p = NS, effect size (0.18) | |
| Static and dynamic balance | Hypoglycaemic: 1.5 (2.4), Ctrl: 0.59 (1.2) | p = NS, effect size (0.48) | |
| Static and dynamic balance | Non-hypoglycaemic: 0.83 (1.3), Ctrl: 0.59 (1.2) | p = NS, (0.19) | |
| Total ABC scores | Hypoglycaemic: 5.58 (6), Ctrl: 3.27 (2.4) | p = NS, effect size (0.51) | |
| Total ABC scores | Non-hypoglycaemic: 4.3 (3.9), Ctrl: 3.27 (2.4), | p = NS, effect size (0.32) | |
| GMDT eye and hand coordination | Hypoglycaemic: 5.9 (2.4), Ctrl: 6.6 (1.6) | p = NS, effect size (−0.34) | |
| GMDT eye and hand coordination | Non-hypoglycaemic 7.2 (1.7), Ctrl: 6.6 (1.6) | p = NS, effect size (0.36) | |
| Nomura et al. [28] | Sensorimotor | GDM: 92.26 (4.17), Ctrl: 94.76 (4.13) | p = NS, effect size (−0.60) |
| Qiao et al. [29] | Gross motor | Group 1: 87.4 (10.3), Ctrl: 86.1 (9.4) | p = NS, effect size (0.13) |
| Fine motor | Group 1: 90.2 (6.2), Ctrl: 91.8 (12.9) | p = NS, effect size (−0.16) | |
| Gross motor | Group 2: 84.6 (7.8), Ctrl: 86.1 (9.4) | p = NS, effect size (−0.17) | |
| Fine motor | Group 2: 86.8 (8.2), Ctrl: 91.8 (12.9) | p = NS, effect size (−0.46) | |
| Gross motor | Group 3: 83.5 (11.4), Ctrl: 86.1 (9.4) | p = NS, effect size (−0.25) | |
| Fine motor | Group 3: 85.4 (9.6), Ctrl: 91.8 (12.9) | p = NS, effect size (−0.56) | |
| Torres-Espinola et al. [30] | Composite motor | GDM 6 months: 103.7 (12.6), Ctrl: 105.8 (11.2) | p = NS, effect size (−0.18) |
| Fine motor | GDM 6 months: 12 (2.2), Ctrl: 12.5 (2.2) | p = NS, effect size (−0.25) | |
| Gross motor | GDM 6 months: 9.2 (3.0), Ctrl: 9.4 (2.7) | p = NS, effect size (−0.07) | |
| Composite motor | GDM 18 months: 115 (7.5), Ctrl: 118.1 (10.2) | p = 0.09 **, effect size (−35) | |
| Fine motor | GDM 18 months: 12.9 (1.7), Ctrl: 13 (2.3) | p = NS, effect size (−0.05) | |
| Gross motor | GDM 18 months: 12 (1.9), Ctrl: 13 (2.1) | p = 0.041 *, effect size (−0.50) | |
| Ornoy et al. [31] | Bruininks total | (Mean, SE): DM: 129.2 (3.9), Ctrl: 138.2 (3.7) | MD = −9.0, p = 0.008. |
| Bruininks gross motor | DM: 57.2 (1.7). Ctrl: 60.8 (1.7) | MD = −3.6, p = 0.03 | |
| Bruininks fine motor | DM: 58.0 (1.9), Ctrl: 62.5 (1.7), | MD = −4.5, p = 0.01. | |
| Ornoy et al. [32] | Bruininks total | Diabetic mothers: 129 (20), Ctrl: 138 (21) | p < 0.05 *, effect size (−0.44) |
| Bruininks total | GDM: 121 (27), Ctr: 138 (21) | p < 0.05 *, effect size (−0.70) | |
| Bruininks gross motor | Diabetic mothers: 57 (11), Ctrl: 60.8 (12) | p < 0.05 *, effect size (−0.33) | |
| Bruininks gross motor | GDM: 57 (15), Ctrl: 60.8 (12) | p < 0.05 *, effect size (−0.28) | |
| Bruininks fine motor | Diabetic mothers: 58 (10), Ctrl: 62.5 (9) | p < 0.05 *, effect size (−0.47) | |
| Bruininks fine motor | GDM: 49 (11), Ctrl: 62.5 (9) | p < 0.05 *, effect size (−1.34) | |
| Ornoy et al. [33] | Bruininks total | GDM (young): 113 (28), Ctrl: 128(23) | p < 0.05 *, effect size (−0.59) |
| Bruininks total | GDM (old): 131 (26), Ctrl: 127 (18) | p = NS, effect size (0.18) | |
| Bruininks gross motor | GDM (young): 52.1 (15.5), Ctrl: 59.2 (130) | p < 0.05 *, effect size (−0.08) | |
| Bruininks gross motor | GDM (old): 61.8 (14.7), Ctr: 66.8 (10.3) | p = NS, effect size (−0.39) | |
| Bruininks fine motor | GDM (young): Bruininks 45.9 (11.6), Ctr: 53.4 (9.7) | p < 0.05 *, effect size (−0.70) | |
| Fine motor | GDM (old): 45.9 (11.6), Ctrl: 53.4 (9.7) | p < 0.05 *, effect size (−0.70). | |
| Ratzon et al. [34] | Total motor scores | DM: 129.2 (29.2), Ctrl: 138.2 (27.6) | p = 0.008 **, effect size (−0.32) |
| Gross motor | DM: 57.2 (12.7), Ctrl: 60.8 (13.2) | p = 0.03 *, effect size (−0.28) | |
| Fine motor | DM: 58.0 (14.6), Ctrl: 62.5 (12.7) | p = 0.01 *, effect size (−0.33) | |
| Ghassabian et al. [35] | Time to achieve gross motor milestone | GDM up to age 24 months: Adjusted HR (95% CI): 0.84 (0.75–0.93) | p = 0.002 ** |
| Bolaños et al. [36] | Right hand | GDM: 9.47 (1.46), Ctrl: 10.18 (1.77) | p = NS, effect size −0.44. |
| Left hand | GDM: 9.31 (1.3), Ctrl: 9.61 (1.83) | p = NS, effect size (−0.19) | |
| Both hands | GDM: 14.25 (2.06), Ctrl: 15.56 (2.21) | p = 0.023 *, effect size (−0.061) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabiat, D.; AL Jabery, M.; Kemp, V.; Jenkins, M.; Whitehead, L.C.; Adams, G. Motor Developmental Outcomes in Children Exposed to Maternal Diabetes during Pregnancy: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 1699. https://doi.org/10.3390/ijerph18041699
Arabiat D, AL Jabery M, Kemp V, Jenkins M, Whitehead LC, Adams G. Motor Developmental Outcomes in Children Exposed to Maternal Diabetes during Pregnancy: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(4):1699. https://doi.org/10.3390/ijerph18041699
Chicago/Turabian StyleArabiat, Diana, Mohammad AL Jabery, Vivien Kemp, Mark Jenkins, Lisa C. Whitehead, and Gary Adams. 2021. "Motor Developmental Outcomes in Children Exposed to Maternal Diabetes during Pregnancy: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 4: 1699. https://doi.org/10.3390/ijerph18041699
APA StyleArabiat, D., AL Jabery, M., Kemp, V., Jenkins, M., Whitehead, L. C., & Adams, G. (2021). Motor Developmental Outcomes in Children Exposed to Maternal Diabetes during Pregnancy: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(4), 1699. https://doi.org/10.3390/ijerph18041699







