Performance Evaluation of Dorsal Vein Network of Hand Imaging Using Relative Total Variation-Based Regularization for Smoothing Technique in a Miniaturized Vein Imaging System: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
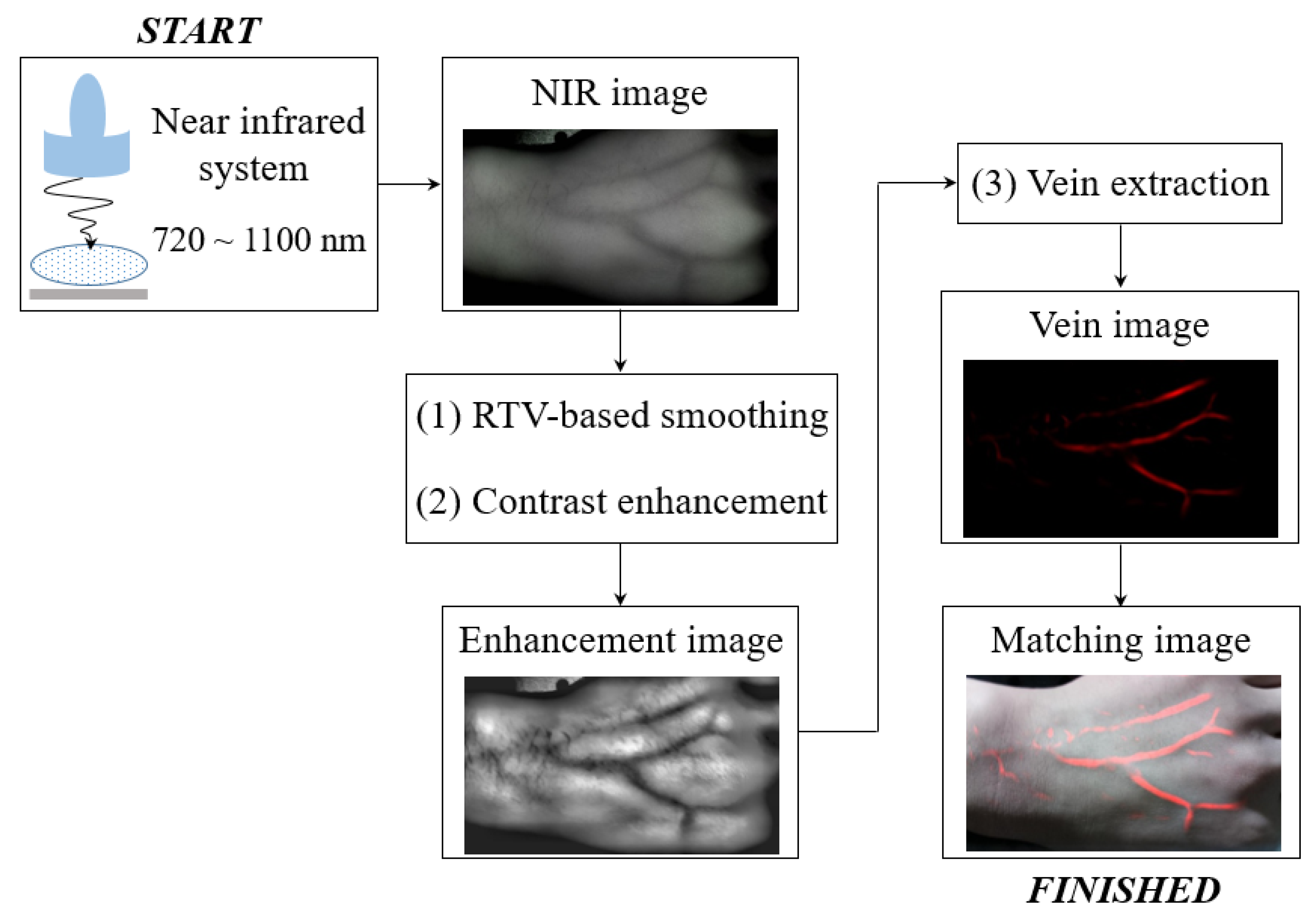
2.1. Experimental Setup of Developed Vein Imaging System
2.2. Proposed Vein Recognition Technique
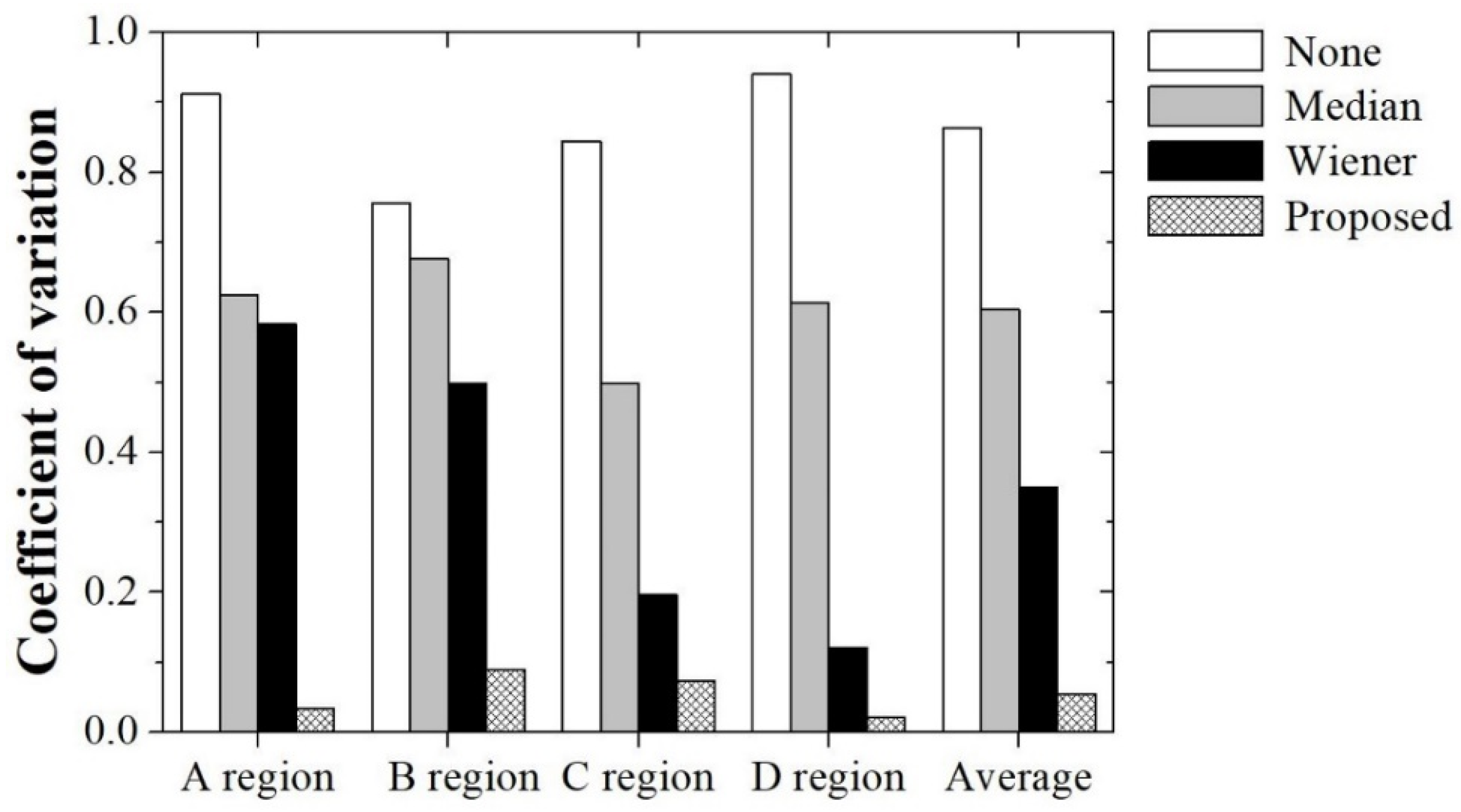
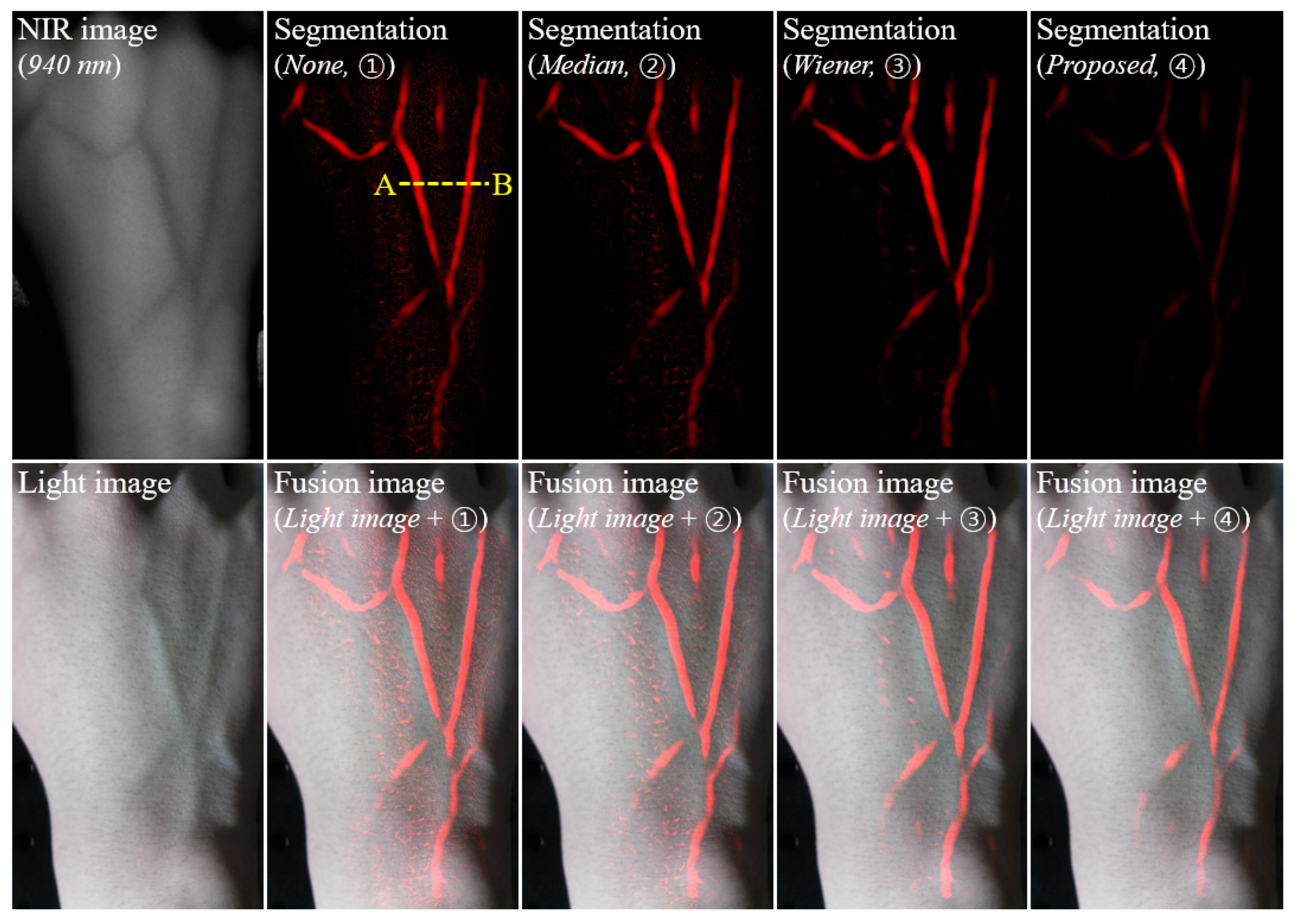
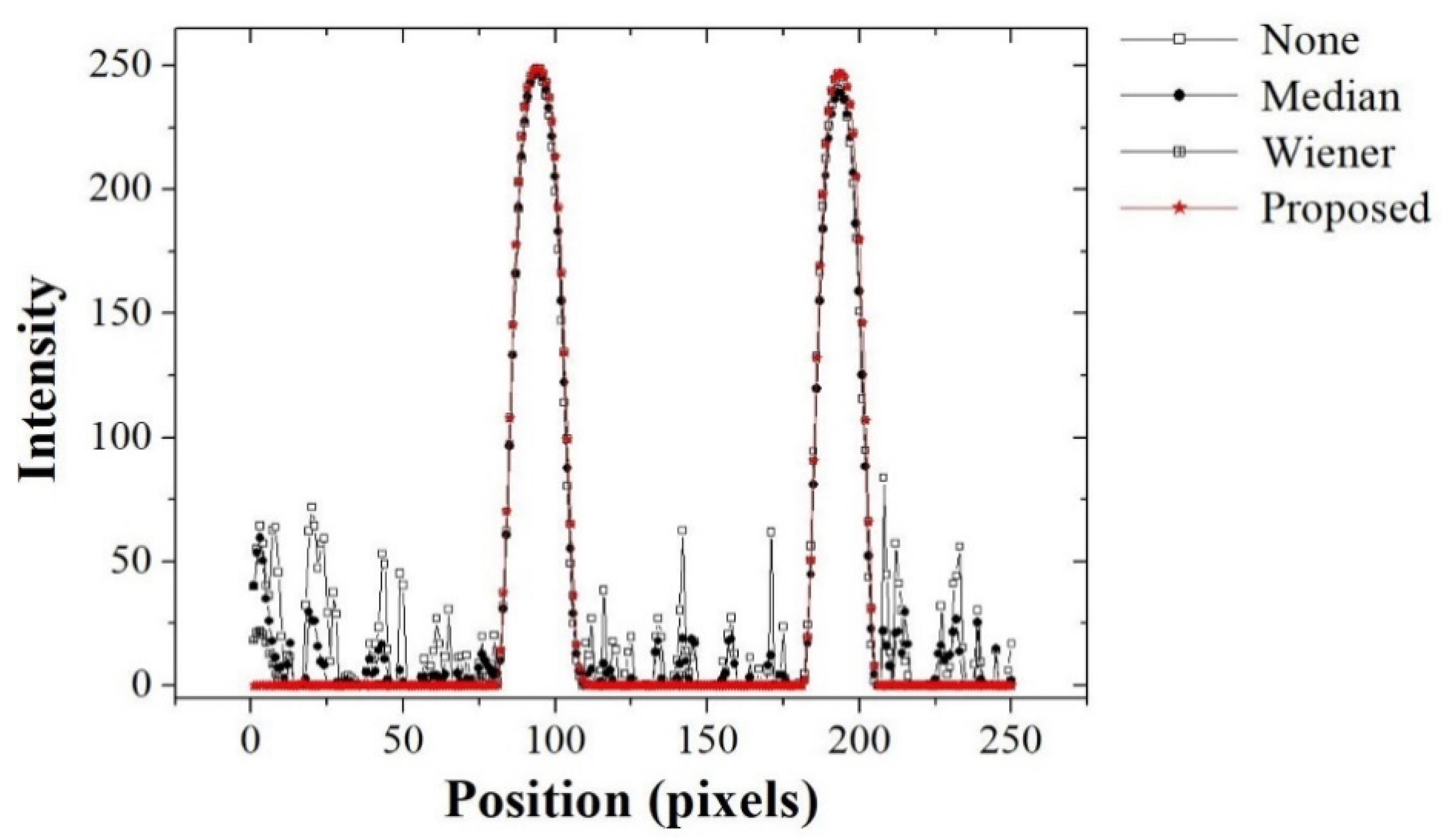
2.3. Evaluation of Recognition Accuracy
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dan, G.; Guo, Z.; Ding, H.; Zhou, Y. Enhancement of Dorsal Hand Vein Image with a Low-Cost Binocular Vein Viewer System. J. Med. Imaging Health Inform. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Sun, C.Y.; Lee, K.C.; Lin, I.H.; Wu, C.L.; Huang, H.P.; Lin, Y.Y.; Hsu, Y.F.; Yu, H.R. Near-infrared light device can improve intravenous cannulation in critically ill children. Pediatrics Neonatol. 2013, 54, 194–197. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.M.; Rhee, N.; Je, S.M.; Hong, S.H.; Lee, Y.M.; Chung, S.P.; Kim, S.H. Efficacy of vein viewer in pediatric peripheral intravenous access: A randomized controlled trial. Eur. J. Pediatrics 2012, 171, 1121–1125. [Google Scholar] [CrossRef]
- Szmuk, P.; Steiner, J.; Pop, R.B.; Farrow-Gillespie, A.; Mascha, E.J.; Sessler, D.I. The viewer vascular imaging system worsens first-attempt cannulation rate for experienced nurses in infants and children with anticipated difficult intravenous access. Anesth. Analg. 2013, 116, 1087–1092. [Google Scholar] [CrossRef]
- Gaikwad, V.; Pardeshi, S. Vein detection using infrared imaging system. Int. J. Electr. Electron. Comput. Syst. 2014, 2, 2347–2820. [Google Scholar]
- Karaaltin, M.V. Utilizing the vein viewer technology to map out a venous flap preoperatively. J. Reconstr. Microsurg. 2013, 29, 423–424. [Google Scholar] [CrossRef]
- Yusoff, S.; Ramli, A.R.; Hasjim, S.J.; Rokhani, F.Z. Review on Vein Enhancement Methods for Biometric System. Int. J. Res. Eng. Technol. 2015, 4, 833–841. [Google Scholar]
- Murata, M.; Kuroda, R.; Fujihara, Y.; Otsuka, Y.; Shibata, H.; Shibaguchi, T.; Kamata, Y.; Miura, N.; Kuriyama, N.; Sugawa, S. A High Near-Infrared Sensitivity Over 70-dB SNR CMOS Image Sensor with Lateral Overflow Integration Trench Capacitor. IEEE Trans. Electron Dev. 2020, 67, 1653–1659. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, Y.; Mo, D. Comparative Study on Noise Reduction Effect of Fiber Optic Hydrophone Based on LMS and NLMS Algorithm. Sensors 2020, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Kauba, C.; Reissing, J.; Uhl, A. Pre-processing cascades and fusion in finger vein recognition. In Proceedings of the International Conference of the Biometrics Special Interest Group (BIOSIG), Darmstadt, Germany, 10–12 September 2014. [Google Scholar]
- Fernandez, R.; Armada, M. Multisensory system for the detection and localization of peripheral subcutaneous vein. Sensors 2017, 17, 897. [Google Scholar] [CrossRef]
- Miura, N.; Nagasaka, A.; Miyatake, T. Feature extraction of finger-vein patterns based on repeated line tracking and its application to personal identification. Mach. Vis. Appl. 2004, 15, 194–203. [Google Scholar] [CrossRef]
- Miura, N.; Nagasaka, A.; Miyatake, T. Extraction of finger-vein patterns using maximum curvature points in image profiles. IEICE Trans. Inf. Syst. 2017, 90, 1185–1194. [Google Scholar] [CrossRef]
- Huang, B.; Dai, Y.; Li, R.; Tang, D.; Li, W. Finger-vein authentication based on wide line detector and pattern normalization. In Proceedings of the 20th International Conference on Pattern Recognition, Istanbul, Turkey, 23–26 August 2010. [Google Scholar]
- Kumar, A.; Zhou, Y. Human identification using finger images. IEEE Trans. Image Process. 2012, 21, 2228–2244. [Google Scholar] [CrossRef]
- Ton, B.; Veldhuis, R. A high quality finger vascular pattern dataset collected using a custom designed capturing device. In Proceedings of the International Conference on Biometrics (ICB), Madrid, Spain, 4–7 June 2013. [Google Scholar]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. ManCybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Lee, E.C.; Lee, H.C.; Park, K.R. Finger vein recognition using minutia-based alignment and local binary pattern-based feature extraction. Int. J. Imaging Syst. Technol. 2009, 19, 179–186. [Google Scholar] [CrossRef]
- Lowe, D.G. Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vis. 2004, 60, 91–110. [Google Scholar] [CrossRef]
- Bay, H.; Ess, A.; Tuytelaars, T.; Gool, L.V. Sppeded-up robust features (SURF). Comput. Vis. Image Underst. 2008, 110, 346–359. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Z.; Li, J. Micro-blood vessel detection using k-means clustering and morphological thinning. Adv. Neural Netw.-ISNN 2011 2011, 6677, 348–354. [Google Scholar] [CrossRef]
- Li, X.; Huang, D.; Wang, Y. Comparative study of deep learning methods on dorsal hand vein recognition. Chin. Conf. Biom. Recognit. CCBR 2016 2016, 9967, 296–306. [Google Scholar] [CrossRef]
- Zuiderveld, K. Contrast limited adaptive histogram equalization. In Graphic Gems IV; Elsevier: Amsterdam, The Netherlands, 1994; pp. 474–485. [Google Scholar]
- Zhao, J.; Tian, H.; Xu, W.; Li, X. A new approach to hand vein image enhancement. In Proceedings of the 2009 Second International Conference on Intelligent Computation Technology and Automation, Changsha, Hunan, China, 10–11 October 2009. [Google Scholar]
- Zhang, J.; Yang, J. Finger-vein image enhancement based on combination of gray-level grouping and circular gabor filter. In Proceedings of the 2009 International Conference on Information Engineering and Computer Science, Wuhan, China, 19–20 December 2009. [Google Scholar]
- Shaheed, K.; Liu, H.; Yang, G.; Qureshi, I.; Gou, J.; Yin, Y. A systematic review of finger vein recognition techniques. Information 2018, 9, 213. [Google Scholar] [CrossRef]
- Moccia, S.; Momi, E.D.; Hadji, S.E.; Mattos, L.S. Blood vessel segmentation algorithms-review of methods, datasets and evaluation metrics. Comput. Methods Progr. Biomed. 2018, 158, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Uhl, A.; Busch, C.; Marcel, S.; Veldhuis, R. Handbook of vascular biometrics. In Advances in Computer Vision and Pattern Recognition; Springer: Cham, Switzerland, 1994; pp. 3–61. [Google Scholar] [CrossRef]
- Xu, L.; Yan, Q.; Xia, Y. Structure extraction from texture via relative total variation. ACM Trans. Graph. 2012, 31. [Google Scholar] [CrossRef]
- Rudin, L.I.; Osher, S.; Fatemi, E. Nonlinear total variation based noise removal algorithms. Phys. D 1992, 60, 259–268. [Google Scholar] [CrossRef]
- Kang, S.H.; Yoon, M.S.; Han, D.K.; Lee, Y. Total variation noise reduction algorithm in computed tomograph image with custom-built phantom using 3D-printer. Radiat. Phys. Chem. 2020, 170, 108631. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y. Performance evaluation of total variation (TV) denoising technique for dual-energy contrast-enhanced digital mammography (CEDM) with photon counting detector (PCD): Monte Carlo simulation study. Radiat. Phys. Chem. 2019, 156, 94–100. [Google Scholar] [CrossRef]
- Han, Y.; Feng, X.C.; Baciu, G.; Wang, W.W. Nonconvex sparse regularizer based speckle noise removal. Pattern Recognit. 2013, 46, 989–1001. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, B.; Yang, D.; Zhang, M. A generalized relative total variation method for image smoothing. Multimed. Tools Appl. 2016, 75, 7909–7930. [Google Scholar] [CrossRef]
- Gong, C.; Zeng, L. Adaptive iterative reconstruction based on relative total variation for low-intensity computed tomography. Signal Process. 2019, 165, 149–162. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Xiao, M.; Xu, C. Image decomposition and completion using relative total variation and schatten quasi-norm regularization. Neurocomputing 2020. [Google Scholar] [CrossRef]
- Cadena, L.; Zotin, A.; Cadena, F.; Espinosa, N. Noise removal of the x-ray medical image using fast spatial filters and GPU. In Proceedings of SPIE 10752, Applications of Digital Image Processing XLI; SPIE: Bellingham, WA, USA, 2018; Volume 10752. [Google Scholar] [CrossRef]
- Cannistraci, C.V.; Abbas, A.; Gao, X. Median modified wiener filter for nonlinear adaptive spatial denoising of protein NMR multidimensional spectra. Sci. Rep. 2015, 5, 8017. [Google Scholar] [CrossRef]
- Kanungo, T.; Mount, D.M.; Netanyahu, N.S.; Piatko, C.D.; Silverman, R.; Wu, A.Y. An efficient k-means clustering algorithm: Analysis and implementation. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 881–892. [Google Scholar] [CrossRef]
- Dempster, A.P.; Laird, N.M.; Rubin, D.B. Maximum likelihood from incomplete data via the EM algorithm. J. R. Satistical Soc. Ser. B (Methodol.) 1977, 39, 1–38. [Google Scholar]
- Li, Y.; Zhang, J.; He, R.; Tian, L.; Wei, H. Hybrid DE-EM Algorithm for Gaussian Mixture Model-Based Wireless Channel Multipath Clustering. Int. J. Antennas Propag. 2019, 2019. [Google Scholar] [CrossRef]
- Han, D.K.; Kim, K.; Lee, Y. Development and Application of a Deep Convolutional Neural Network Noise Reduction Algorithm for Diffusion-weighted Magnetic Resonance Imaging. J. Magn. 2019, 24, 223–229. [Google Scholar] [CrossRef]
- Park, C.R.; Kang, S.H.; Lee, Y. Optimization of mask size with median modified Wiener filter algorithm for gamma images using pixelated semiconductor detector: Monte Carlo simulation study. Nucl. Instrum. Methods Phys. Res. Sect. A 2020, 980, 164472. [Google Scholar] [CrossRef]
- Cesaro, A.; Moscarella, E.; Gragnano, F.; Perrotta, R.; Diana, V.; Pariggiano, I.; Concilio, C.; Alfieri, A.; Cesaro, F.; Mercone, G.; et al. Transradial access versus transfemoral access: A comparison of outcomes and efficacy in reducing hemorrhagic events. Expert Rev. Cardiovasc. Ther. 2019, 17, 435–447. [Google Scholar] [CrossRef]
- Jang, Y.S.; Kook, Y.H.; Kim, J.L.; Jeong, H.W. Development of the cost-effective, miniaturized vein imaging system with enhanced noise reduction. J. Adv. Trends Comput. Sci. Eng. 2019, 8, 3166–3170. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Jeong, H.-W.; Lee, Y. Performance Evaluation of Dorsal Vein Network of Hand Imaging Using Relative Total Variation-Based Regularization for Smoothing Technique in a Miniaturized Vein Imaging System: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 1548. https://doi.org/10.3390/ijerph18041548
Kim K, Jeong H-W, Lee Y. Performance Evaluation of Dorsal Vein Network of Hand Imaging Using Relative Total Variation-Based Regularization for Smoothing Technique in a Miniaturized Vein Imaging System: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(4):1548. https://doi.org/10.3390/ijerph18041548
Chicago/Turabian StyleKim, Kyuseok, Hyun-Woo Jeong, and Youngjin Lee. 2021. "Performance Evaluation of Dorsal Vein Network of Hand Imaging Using Relative Total Variation-Based Regularization for Smoothing Technique in a Miniaturized Vein Imaging System: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 4: 1548. https://doi.org/10.3390/ijerph18041548
APA StyleKim, K., Jeong, H.-W., & Lee, Y. (2021). Performance Evaluation of Dorsal Vein Network of Hand Imaging Using Relative Total Variation-Based Regularization for Smoothing Technique in a Miniaturized Vein Imaging System: A Pilot Study. International Journal of Environmental Research and Public Health, 18(4), 1548. https://doi.org/10.3390/ijerph18041548





