Temporal and Spatial Variation of Radon Concentrations in Environmental Water from Okinawa Island, Southwestern Part of Japan
Abstract
1. Introduction
2. Materials and Methods
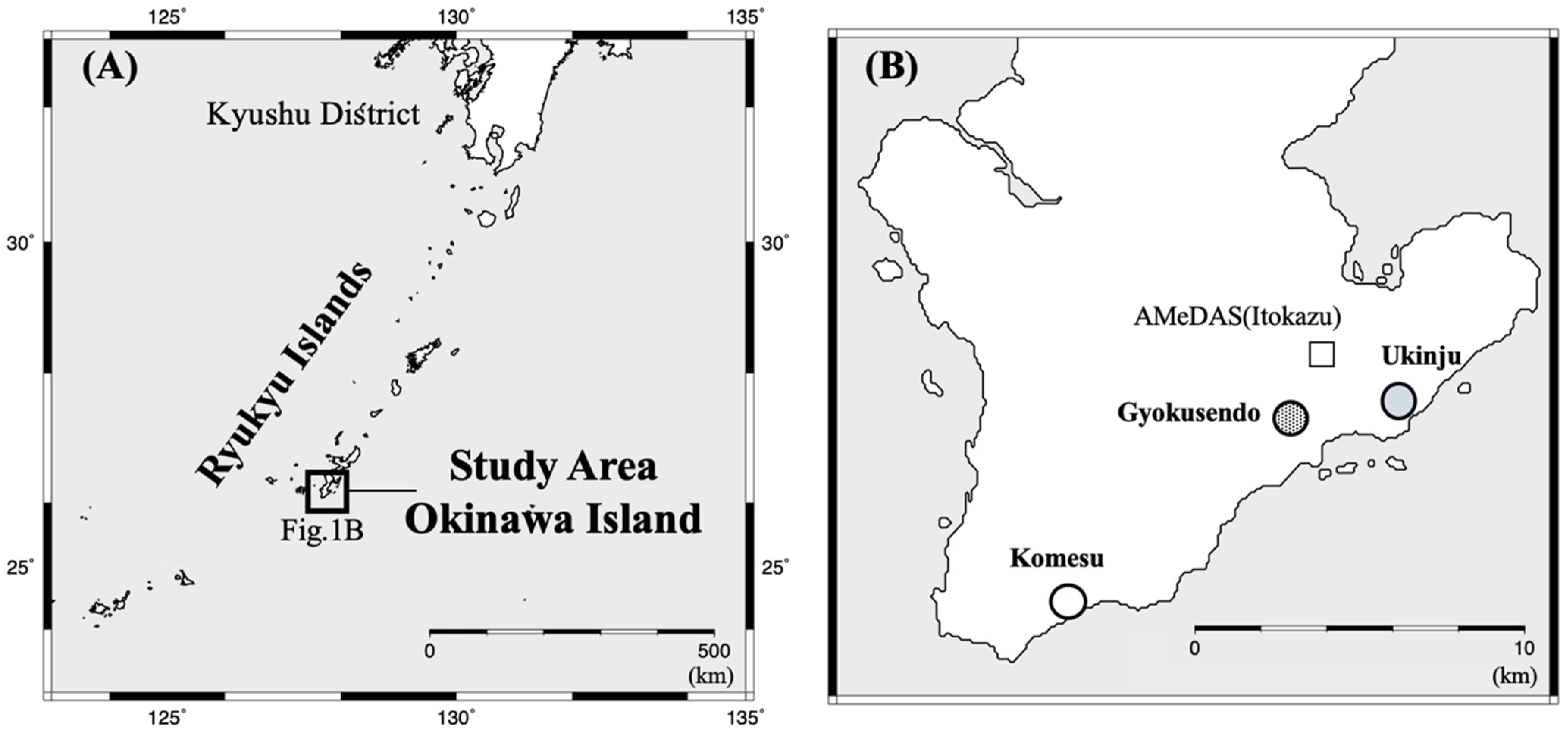
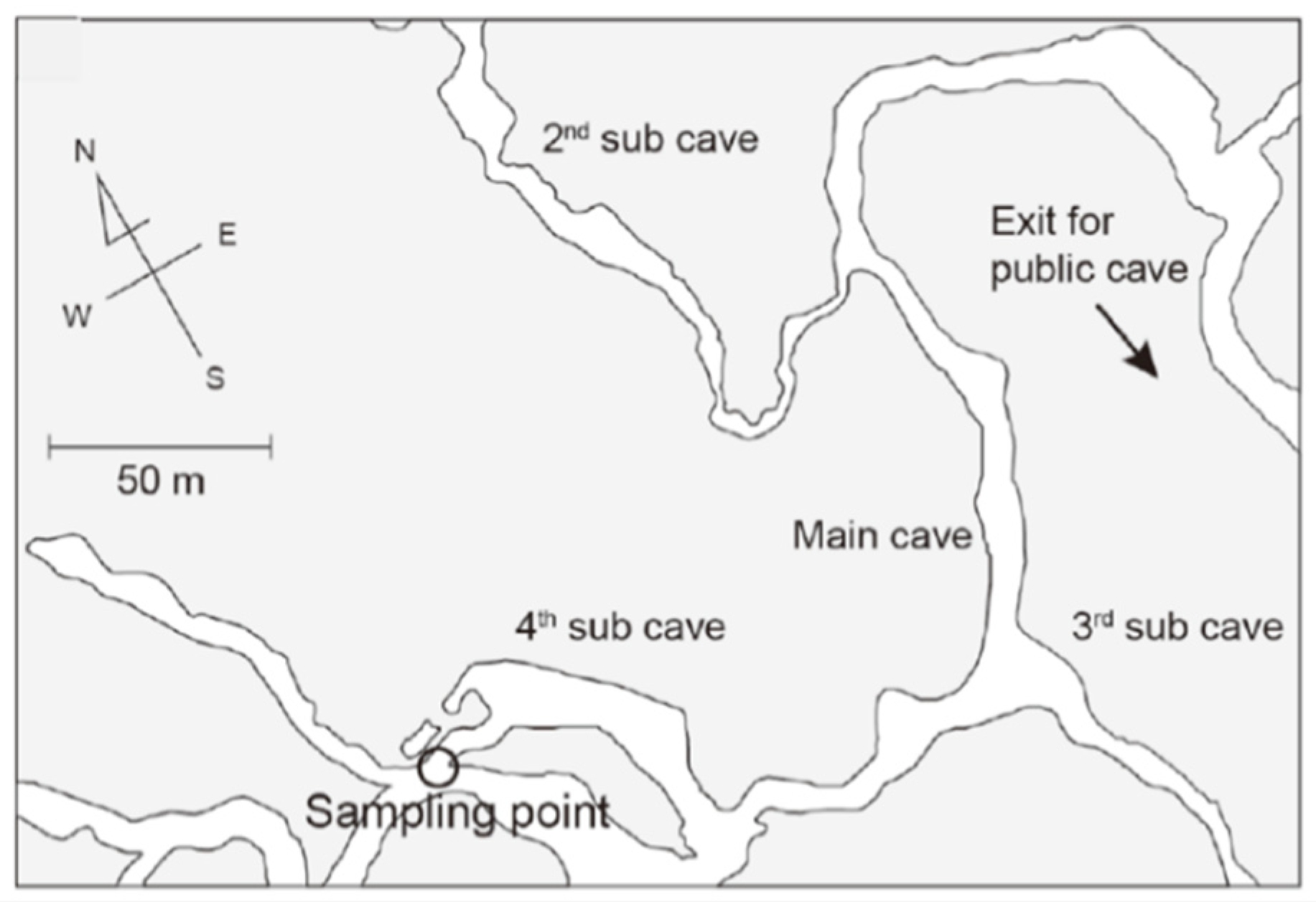
2.1. Overview of the Study Site
2.2. Sample Collection
2.3. Analysis of Radon Concentration in Water
3. Results and Discussion
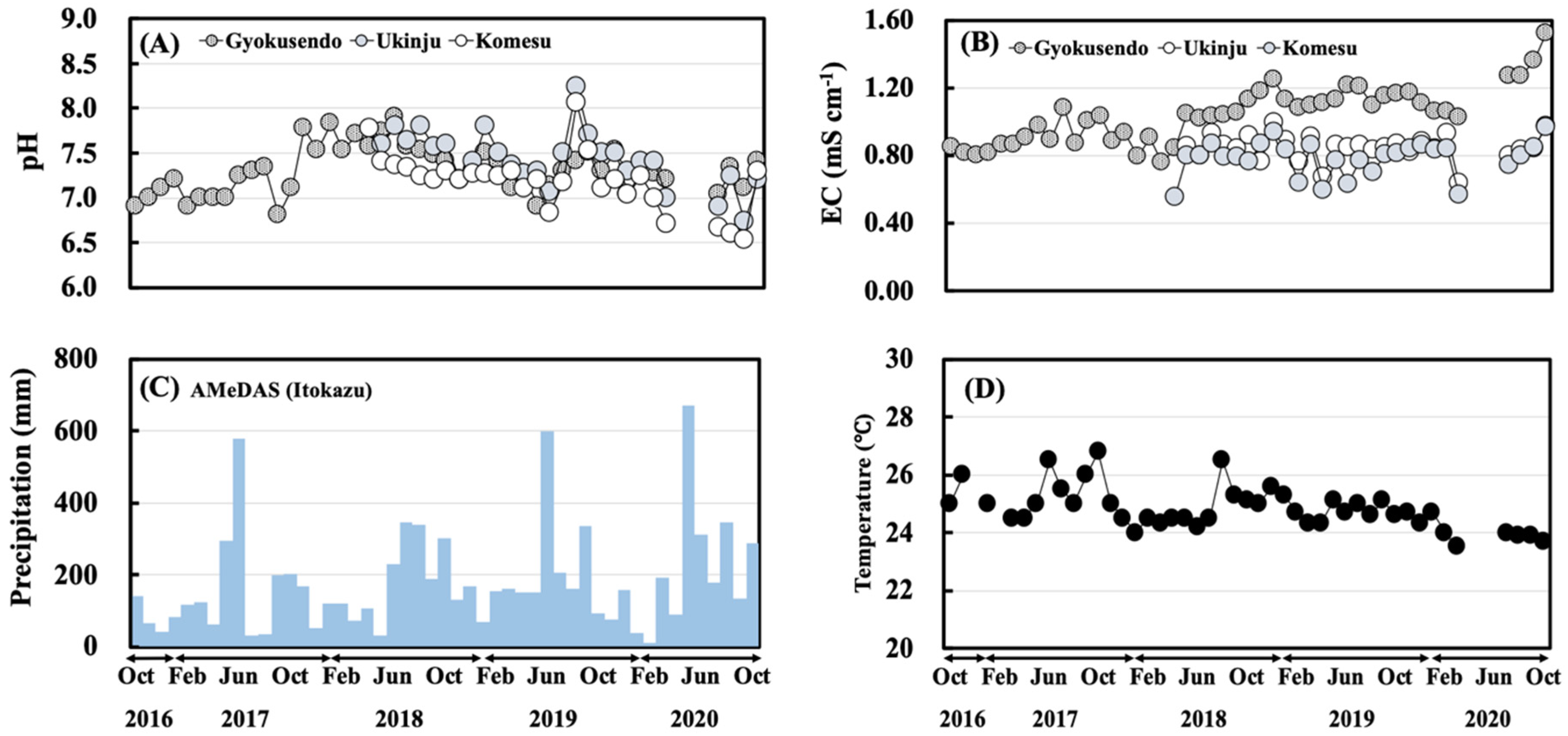
3.1. Physical Parameters and Meteorological Data
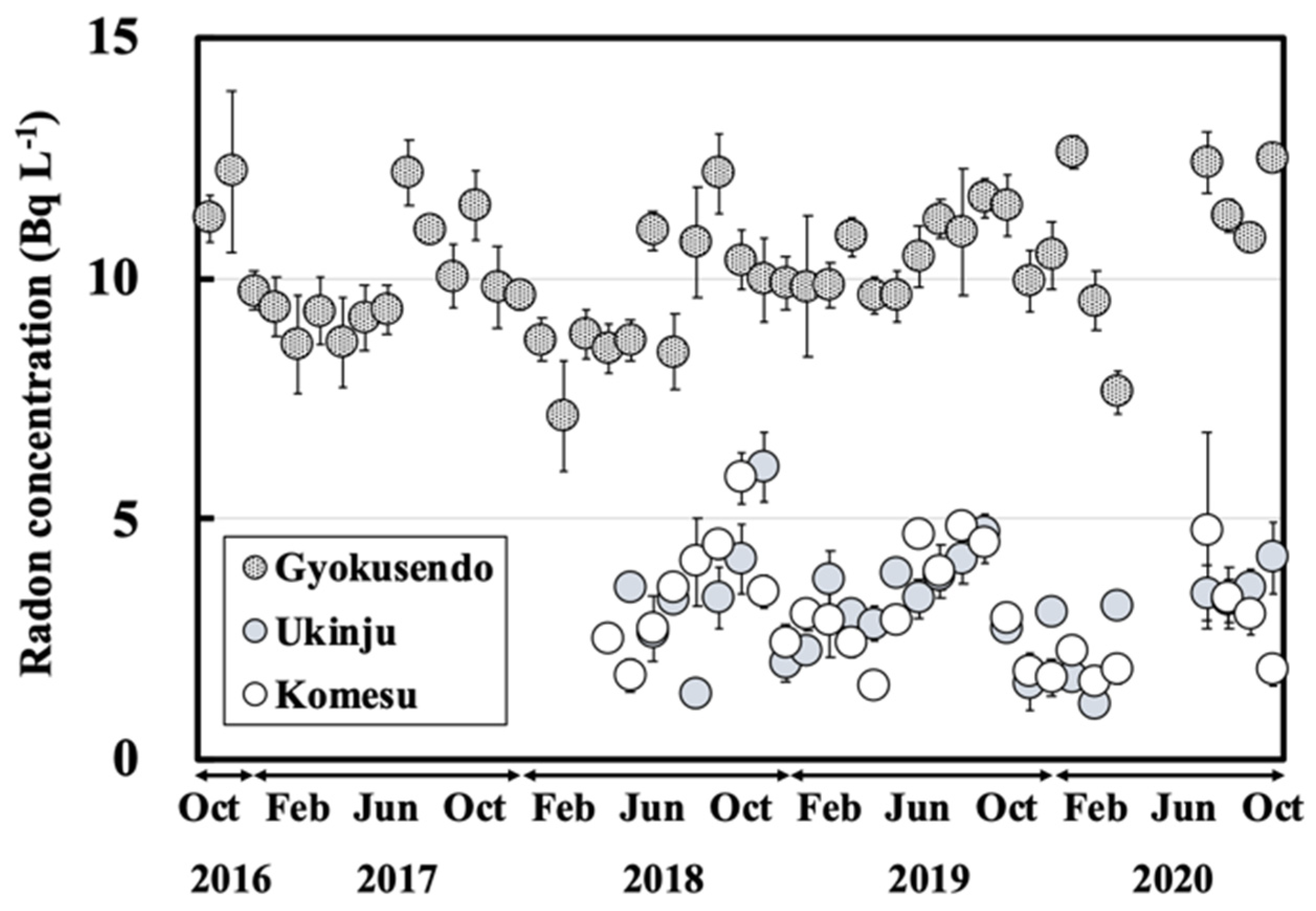
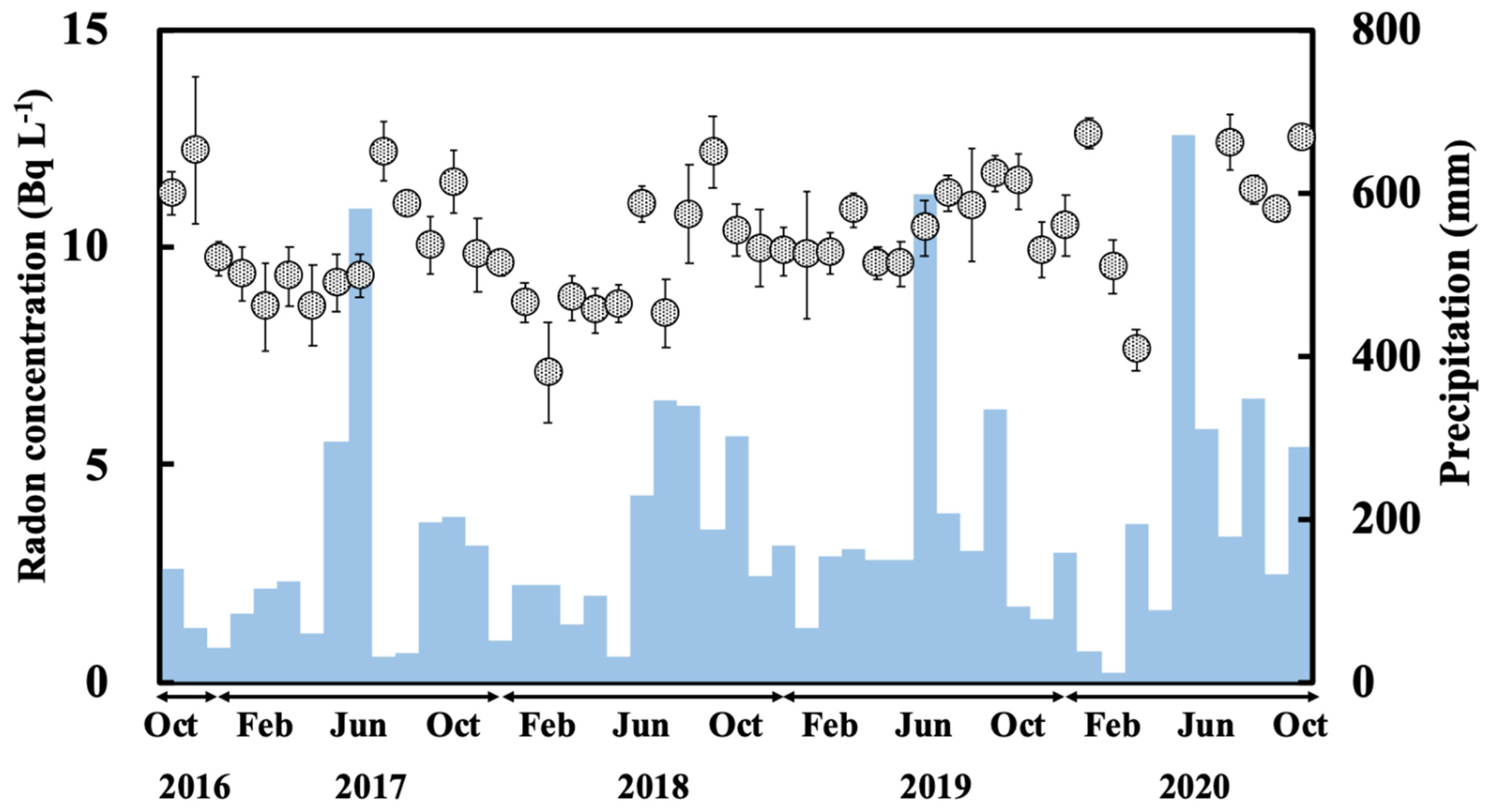
3.2. Temporal Variation of Radon Concentrations for the Water Samples
3.3. Relationship between Radon Concentration for Dripping Water and Monthly Precipitation
3.4. Estimation of Residence Time Using Radon Decay in the Dripping Water
4. Conclusions
- (1)
- The radon concentrations in the water samples from the Gyokusendo cave, Ukinju spring, and Komesu spring were 10 ± 1.3 Bq L−1, 3.2 ± 1.0 Bq L−1, and 3.1 ± 1.1 Bq L−1, respectively. Radon concentrations for the water samples showed a gradually increasing trend from summer to autumn and decreased in the winter. This was particularly noticeable in the radon concentrations measured in the dripping water sample from the Gyokusendo cave.
- (2)
- From the variation in the radon concentrations for the dripping water and precipitation, we estimated that the radon concentration changes in the dripping water lags precipitation changes by approximately 2–3 months. These results indicate that precipitation takes 60 to 90 d to percolate into the soil and accumulate in the Ryukyu limestone overlying the Gyokusendo cave. The water then enters the cave as dripping water from the cave ceiling.
- (3)
- From a simple radon behavior model, groundwater in the Gyokusendo cave system was estimated to percolate through the Ryukyu limestone in 7 to 10 d, and the residence time of groundwater in the soil above the Gyokusendo cave was estimated at approximately 50 to 80 d.
- (4)
- The residence times of groundwater in the Ryukyu limestone at the Ukinju spring and Komesu spring sites were calculated to be 12 to 21 d and 12 to 19 d, respectively. However, this estimation was lower than that of previous studies (14–34 years). Therefore, it will be necessary for future studies to discuss the residence time after understanding factors such as the frequency of collection, and the number of sites, and depth of sampling sites. Additionally, to verify the residence time and mixing of groundwater in the area, radioactive isotopes with longer half-lives, such as tritium (half-life: 12.3 y) should be analyzed in the future.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Handbook on Indoor Radon: A Public Health Perspective; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Nazaroff, W.W. Radon transport from soil to air. Rev. Geophys. 1992, 30, 137–160. [Google Scholar] [CrossRef]
- Hirao, S.; Yamazawa, H.; Moriizumi, J.; Yoshioka, K.; Iida, T. Development and verification of long-range atmospheric radon-222 transport model. J. Nucl. Sci. Technol. 2008, 45, 166–172. [Google Scholar] [CrossRef][Green Version]
- Csondor, K.; Erőss, A.; Horváth, Á.; Szieberth, D. Radon as a natural tracer for underwater cave exploration. J. Environ. Radioact. 2017, 173, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, E.; Von Guten, H.R. Radon in groundwater: A tool to assess infiltration from surface waters to aquifers. Water Resour. Res. 1989, 25, 1795–1803. [Google Scholar] [CrossRef]
- Ellins, K.K.; Roman-mas, A.; Lee, R. Using 222Rn to examine groundwater/surface discharge interaction in the Rio Grande de Manati, Puerto Rico. J. Hydrol. 1990, 115, 319–341. [Google Scholar] [CrossRef]
- Hamada, H. Estimation of groundwater flow rate using the decay of 222Rn in a well. J. Environ. Radioact. 2000, 47, 1–13. [Google Scholar] [CrossRef]
- Savoy, L.; Subeck, H.; Hunkeler, D. Radon and CO2 as natural tracers to investigate the recharge dynamics of karst aquifers. J. Hydrol. 2011, 406, 148–157. [Google Scholar] [CrossRef]
- Hamada, H.; Komae, T. Analysis of recharge by paddy field irrigation using 222Rn concentration in groundwater as an indicator. J. Hydrol. 1998, 205, 92–100. [Google Scholar] [CrossRef]
- Igarashi, G.; Saeki, S.; Takahata, N.; Sumikawa, K.; Tasaka, S.; Sasaki, Y.; Takahashi, M.; Sano, Y. Ground-water radon anomaly before the Kobe earthquake in Japan. Science 1995, 269, 60–61. [Google Scholar] [CrossRef]
- Yasuoka, Y.; Shinogi, M. Anomaly in atmospheric radon concentration: A possible precursor of the 1995 Kobe, Japan, earthquake. Health Phys. 1997, 72, 759–761. [Google Scholar] [CrossRef]
- Koike, K.; Yoshinaga, T.; Asaue, H. Characterizing long-term radon concentration changes in a geothermal area for correlation with volcanic earthquakes and reservoir temperatures: A case study from Mt. Aso, southwestern Japan. J. Volcanol. Geotherm. Res. 2014, 275, 85–102. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kim, G. A radon-thoron isotope pair as a reliable earthquake precursor. Sci. Rep. 2015, 5, 13084. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Fan, K.; Kuochen, H.; Han, Y.; Chu, H.; Lee, Y. Anomalous decrease in groundwater radon before the Taiwan M6.8 Chengkung earthquake. J. Environ. Radioact. 2006, 88, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tanahara, A.; Taira, H.; Takemura, M. Radon distribution and the ventilation of a limestone cave on Okinawa. Geochem. J. 1997, 31, 49–56. [Google Scholar] [CrossRef]
- Tanahara, A.; Iha, H.; Taira, H. Factors controlling the changes in 222Rn concentration in Gyokusendo cave on Okinawa Island. J. Speleol. Soc. Jpn. 1997, 22, 98–105. (In Japanese) [Google Scholar]
- Iha, H.; Tanahara, A.; Taira, H. A critical analysis of changing radon concentration patterns on Gyokusendo cave in Okinawa Island. J. Speleol. Soc. Jpn. 1999, 24, 51–56. (In Japanese) [Google Scholar]
- Ford, D.C.; Williams, P.W. Karst Hydrogeology and Geomorphology, 1st ed.; Springer: Dordrecht, The Netherlands, 1989; pp. 99–120. ISBN 978–0470849972. [Google Scholar]
- Japan Meteorological Agency. Automated Meteorological Data Acquistion System (AMeDAS). Available online: https://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 27 November 2020).
- Shiroma, Y.; Shiroma, M.; Kina, S.; Hosoda, M.; Yasuoka, Y.; Akata, N.; Furukawa, M. Source of atmospheric radon in the Gyokusendo, a limestone cave in Okinawa, Japan. Jpn. J. Health Phys. 2016, 51, 218–226. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Iryu, Y.; Matsuda, H.; Machiyama, H.; Piller, E.W.; Quinn, M.T.; Mutti, M. Introductory perspective on the COREF project. Island Arc 2006, 15, 393–406. (In Japanese) [Google Scholar] [CrossRef]
- Furukawa, H. The southern part of Okinawa Island. In Geological Feature of the Ryukyu Arc, 1st ed.; Kizaki, K., Ed.; The Okinawa Times Co., Ltd.: Okinawa, Japan, 1985; pp. 107–112. (In Japanese) [Google Scholar]
- Kaneko, N.; Ujiie, H. Ryukyu Group. In Geology of the Itoman and Kudaka Jima District, 1st ed.; Kaneko, N., Ed.; Geological Survey of Japan, AIST: Ibaraki, Japan, 2006; pp. 16–25. (In Japanese) [Google Scholar]
- Hamazaki, T. Parent materials and soils of Nansei-shoto in Japan. Pedologist 1979, 23, 43–57. (In Japanese) [Google Scholar] [CrossRef]
- Tokashiki, Y. The characteristic properties of the Shimajiri Mahji and Jahgaru soils in Okinawa prefecture. Pedologist 1993, 37, 99–112. [Google Scholar] [CrossRef]
- Ishihara, Y.; Yoshimura, K.; Ooka, S.; Sasaki, H. Topography, geology of Sakitarido ruins and ruins formation process. In Excavation Report of the Sakitari-Do Cave Site, Nanjo City, Okinawa Pref., 1st ed.; Yamazaki, S., Ed.; Okinawa Prefectural Museum and Art Museum: Okinawa, Japan, 2018; pp. 221–252. (In Japanese) [Google Scholar]
- Tanaka, R.; Araki, S.; Yasuoka, Y.; Mukai, T.; Ohnuma, S.; Ishikawa, T.; Fukuhori, N.; Sanada, T. A simplified method for improved determination of radon concentration in environmental water samples. Radioisotopes 2013, 62, 423–438. [Google Scholar] [CrossRef]
- Yasuoka, Y.; Ishii, T.; Kataoka, Y.; Kubo, T.; Suda, H.; Tokonami, S.; Ishikawa, T.; Shinogi, M. Determination of radon concentration in water using liquid scintillation counter. Radioisotopes 2004, 53, 123–131. [Google Scholar] [CrossRef]
- Yasuoka, Y.; Ishikawa, T.; Fukuhori, N.; Tokonami, S. Comparison of simplified liquid scintillation counter (Triathler) with conventional liquid scintillation counter in the measurement of radon concentration in water. J. Hot Spring Sci. 2009, 59, 11–21. [Google Scholar]
- Knoll, G.F. Radiation Detection and Measurement, 4th ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 94–99. ISBN 978–0-470–13148-0. [Google Scholar]
- Kraguten, J. Tutorial review. Calculating standard deviations and confidence intervals with a universally applicable spread sheet technique. Analyst 1994, 119, 2161–2165. [Google Scholar] [CrossRef]
- Shumway, R.H.; Stoffer, D.S. Time Series Analysis and Its Applications with R Examples, 4th ed.; Springer: New York, NY, USA, 2017; pp. 1–74. ISBN 978–3-319–52452-8. [Google Scholar]
- Tenner, A.B. Physical and chemical controls on the distribution of radium-226 and radon-222 in groundwater near Great Salt Lake, Utah. In The Natural Radiation Environment, 1st ed.; Actams, J.A.S., Lowder, W.M., Eds.; University of Chicago Press: Chicago, IL, USA, 1964; pp. 253–276. ISBN 978–0080441375. [Google Scholar]
- Furukawa, M.; Kina, S.; Shiroma, M.; Shiroma, Y.; Masuda, N.; Motomura, D.; Hiraoka, H.; Fujioka, S.; Kawakami, T.; Yasuda, Y.; et al. Terrestrial gamma radiation dose rate in Ryukyu Islands, subtropical region of Japan. Radiat. Prot. Dosim. 2015, 167, 223–227. [Google Scholar] [CrossRef]
- Noguchi, M. Liquid scintillation counting technique(X), special applications (2), measurements of radon activity. Radioisotopes 1975, 24, 745–748. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Annex B: Exposures from natural radiation sources. In Sources and Effects of Ionizing Radiation UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes; United Nations: New York, NY, USA, 2000; Volume 1, pp. 97–108. ISBN 92–1-142238–8. [Google Scholar]
- Shiroma, Y.; Hosoda, M.; Ishikawa, T.; Sahoo, S.K.; Tokonami, S.; Furukawa, M. Estimation of radon emanation coefficient for representative soils in Okinawa Japan. Radiat. Prot. Dosim. 2015, 167, 147–150. [Google Scholar] [CrossRef]
- Nakaya, S.; Yasumoto, J.; Ha, M.P.; Aoki, H.; Kohara, F.; Masuda, H.; Masuoka, K. Hydrochemical behavior of an underground dammed limestone aquifer in the subtropics. Hydrol. Process. 2018, 32, 3529–3546. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakasone, S.; Ishimine, A.; Shiroma, S.; Masuda, N.; Nakamura, K.; Shiroma, Y.; Ooka, S.; Tanaka, M.; Kato, A.; Hosoda, M.; et al. Temporal and Spatial Variation of Radon Concentrations in Environmental Water from Okinawa Island, Southwestern Part of Japan. Int. J. Environ. Res. Public Health 2021, 18, 998. https://doi.org/10.3390/ijerph18030998
Nakasone S, Ishimine A, Shiroma S, Masuda N, Nakamura K, Shiroma Y, Ooka S, Tanaka M, Kato A, Hosoda M, et al. Temporal and Spatial Variation of Radon Concentrations in Environmental Water from Okinawa Island, Southwestern Part of Japan. International Journal of Environmental Research and Public Health. 2021; 18(3):998. https://doi.org/10.3390/ijerph18030998
Chicago/Turabian StyleNakasone, Shunya, Akinobu Ishimine, Shuhei Shiroma, Natsumi Masuda, Kaori Nakamura, Yoshitaka Shiroma, Sohei Ooka, Masahiro Tanaka, Akemi Kato, Masahiro Hosoda, and et al. 2021. "Temporal and Spatial Variation of Radon Concentrations in Environmental Water from Okinawa Island, Southwestern Part of Japan" International Journal of Environmental Research and Public Health 18, no. 3: 998. https://doi.org/10.3390/ijerph18030998
APA StyleNakasone, S., Ishimine, A., Shiroma, S., Masuda, N., Nakamura, K., Shiroma, Y., Ooka, S., Tanaka, M., Kato, A., Hosoda, M., Akata, N., Yasuoka, Y., & Furukawa, M. (2021). Temporal and Spatial Variation of Radon Concentrations in Environmental Water from Okinawa Island, Southwestern Part of Japan. International Journal of Environmental Research and Public Health, 18(3), 998. https://doi.org/10.3390/ijerph18030998






