Regional Strain Pattern Index—A Novel Technique to Predict CRT Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Echocardiography—General Data
- ○
- Septal flash (0/1)
- ○
- SPWMD (Septal to posterior wall motion delay, which was assessed using M-mode echocardiography from the parasternal short-axis view at the papillary muscle level)
- ○
- Four-chamber max intraventricular delay—maximal difference in the time-to-peak systolic velocity curves among the four sites (two basal, two midventricular) in the 4-chamber apical view
- ○
- Two-chamber max intraventricular delay—maximal difference in the time-to-peak systolic velocity curves among the four sites (two basal, two midventricular) in the 2-chamber apical view
- ○
- Three-chamber max intraventricular delay—maximal difference in the time-to-peak systolic velocity curves among the four sites (two basal, two midventricular) in the 3-chamber apical view
- ○
- Maximum time delay technique—maximal difference in the time-to-peak systolic velocity curves between any two of the 12 LV segments (six basal, six midventricular)
- ○
- Mechanical dyssynchrony index (Yu index)—standard deviation of the time-to-peak systolic velocity in the 12 LV segments (six basal, six midventricular)
- ○
- Strain pattern analysis
- ○
- Regional strain pattern index—RSPI
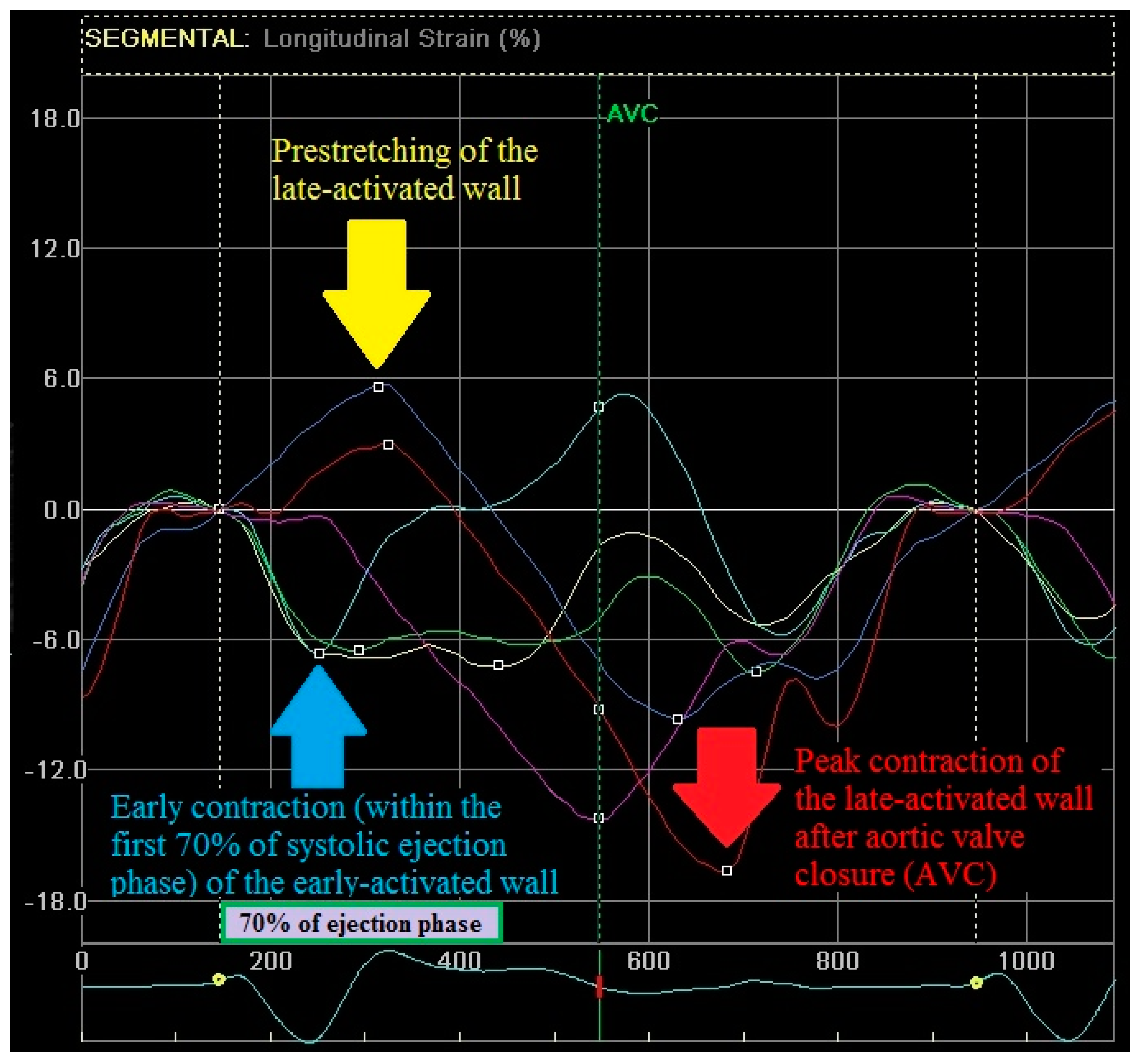
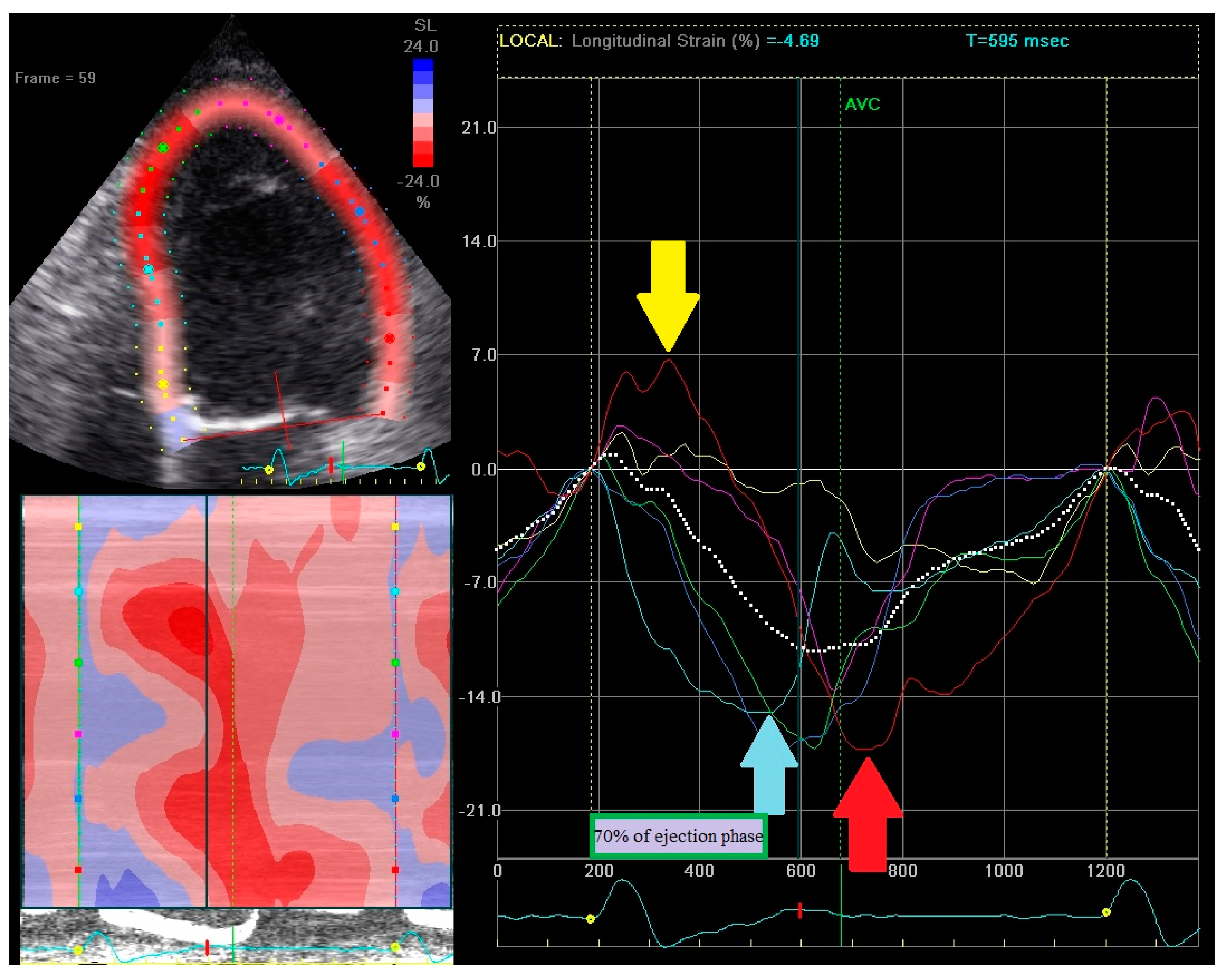
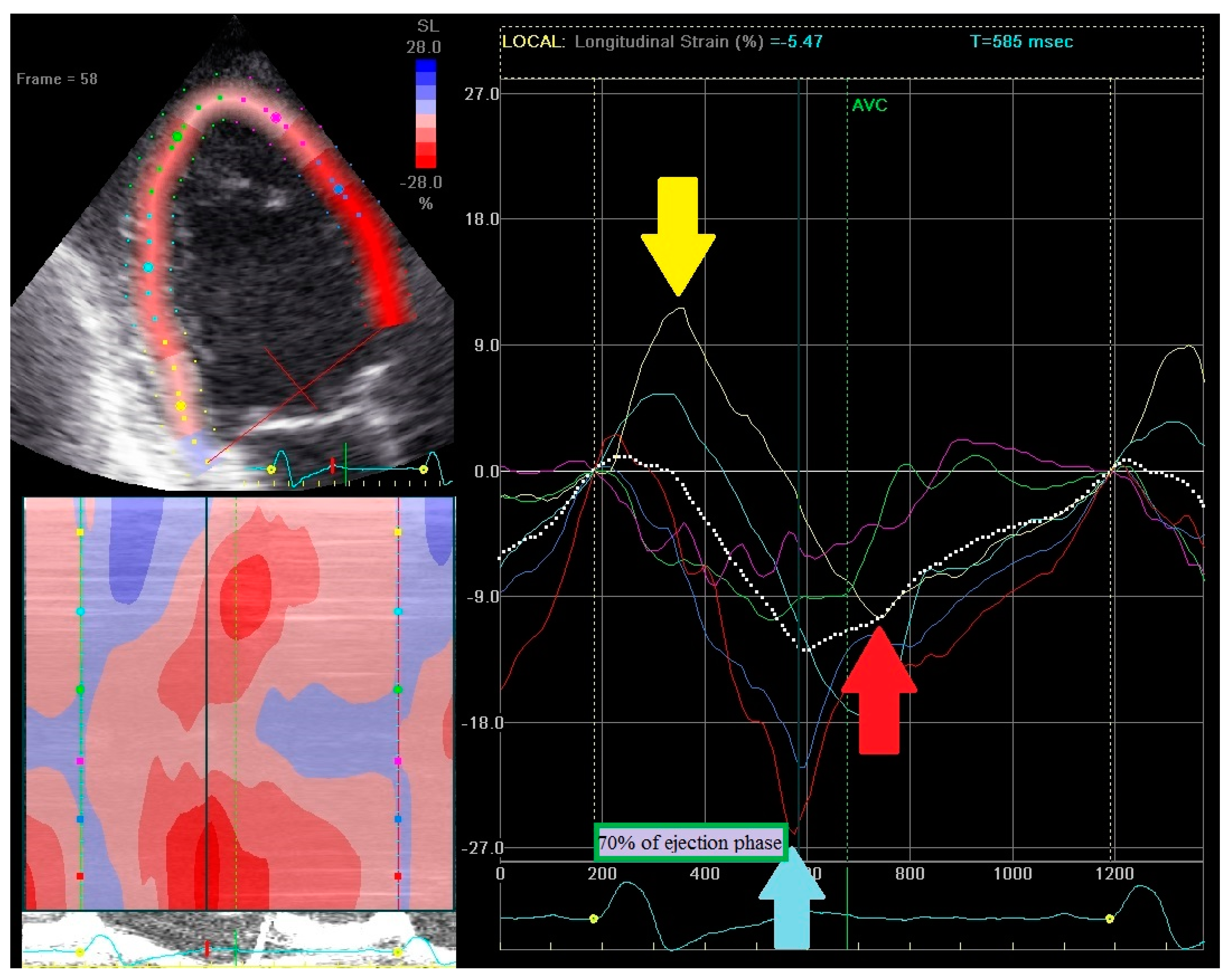
2.3. Echocardiography—Strain Pattern Analysis
- (1)
- Early contraction of at least one basal or midventricular segment in septal or anteroseptal wall and early stretching in at least one basal or midventricular segment in the opposing wall,
- (2)
- the early peak contraction does not exceed 70% of the ejection phase,
- (3)
- the early stretching wall shows a peak contraction after aortic valve closure.
2.4. Echocardiography—Regional Strain Pattern Index
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Follow-Up
3.3. Effect of CRT on the Dyssynchrony Parameters
3.4. Regional Strain Pattern Index
3.5. Prediction of the Response to CRT
4. Discussion
5. Limitations
- (1)
- It was a single center study. The study sample was small and quite heterogeneous (some patients had atrial fibrillation, while some had non-LBBB). Therefore, the predictive value of RSPI should be validated in a larger study with a more homogeneous population.
- (2)
- The quality of the echocardiographic examination is crucial for image-based measurements of dyssynchrony. Suboptimal image quality may affect the results. From 71 consecutive patients qualified to enter into the study, finally six (8.5%) patients were excluded due to poor echocardiographic window.
- (3)
- Strain pattern methodology relies on visual evaluation of the strain-derived curves. Therefore, during RSPI assessment, in some cases there may be discrepancies between observers.
- (4)
- RSPI is a single parameter and reflects intraventricular dyssynchrony, whereas dyssynchrony and the response to CRT are multimodal ones. The selection of the most appropriate candidate for CRT might require a combined approach rather than a single parameter.
- (5)
- The QRS duration criterion for entry into the study was 120 ms (according to the previous guidelines), whereas at present the cut-off value is 130 ms. However, no patient had a QRS shorter than 140 ms in our population.
- (6)
- Post-implantation CRT optimization was not taken into consideration.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; Dicarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Rossi, G.; Piacenti, M.; Startari, U.; Panchetti, L.; Morales, M.-A. The current role of cardiac resynchronization therapy in reducing mortality and hospitalization in heart failure patients: A meta-analysis from clinical trials. Heart Vessel. 2008, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur. Heart J. 2006, 27, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Risum, N. Assessment of mechanical dyssynchrony in cardiac resynchronization therapy. Dan. Med. J. 2014, 61, B4981. [Google Scholar] [PubMed]
- Parsai, C.; Bijnens, B.; Sutherland, G.R.; Baltabaeva, A.; Claus, P.; Marciniak, M.; Paul, V.; Scheffer, M.; Donal, E.; Derumeaux, G.; et al. Toward understanding response to cardiac resynchronization therapy: Left ventricular dyssynchrony is only one of multiple mechanisms. Eur. Heart J. 2009, 30, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Bleasdale, R.; Vinereanu, D.; Mumford, C.E.; Paul, V.; Fraser, A.G.; Frenneaux, M.P. Electrical and mechanical components of dyssynchrony in heart failure patients with normal QRS duration and left bundle-branch block: Impact of left and biventricular pacing. Circulation 2004, 109, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- To, A.C.Y.; Benatti, R.D.; Sato, K.; Grimm, R.A.; Thomas, J.D.; Wilkoff, B.L.; Agler, D.; Thamilarasan, M. Strain-time curve analysis by speckle tracking echocardiography in cardiac resynchronization therapy: Insight into the pathophysiology of responders vs. non-responders. Cardiovasc. Ultrasound 2016, 14, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Risum, N.; Jons, C.; Olsen, N.T.; Fritz-Hansen, T.; Bruun, N.E.; Hojgaard, M.V.; Valeur, N.; Kronborg, M.B.; Kisslo, J.; Sogaard, P. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: Rationale, initial results, and advantages. Am. Heart J. 2012, 163, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.G.F.; Deharo, J.-C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association. Europace 2013, 15, 1070–1118. [Google Scholar] [PubMed]
- Prinzen, F.W.; Hunter, W.C.; Wyman, B.T.; McVeigh, E.R. Mapping of regional myocardial strain and work during ventricular pacing: Experimental study using magnetic resonance imaging tagging. J. Am. Coll. Cardiol. 1999, 33, 1735–1742. [Google Scholar] [CrossRef]
- Yu, C.-M.; Fung, J.W.-H.; Zhang, Q.; Chan, C.K.; Chan, Y.-S.; Lin, H.; Kum, L.C.; Kong, S.-L.; Zhang, Y.; Sanderson, J.E.; et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 2004, 110, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Suffoletto, M.S.; Dohi, K.; Cannesson, M.; Saba, S.; Gorcsan, J. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 2006, 113, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.; Lin, G.; Powell, B.D.; Espinosa, R.E.; Bruce, C.J.; Miller, F.A.; Karon, B.L.; Rea, R.F.; Hayes, D.L.; Oh, J.K.; et al. Strain dyssynchrony index correlates with improvement in left ventricular volume after cardiac resynchronization therapy better than tissue velocity dyssynchrony indexes. Circ. Cardiovasc. Imaging 2008, 1, 14–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, P.; Donal, E.; Lafitte, S.; Derumeaux, G.; Habib, G.; Réant, P.; Thivolet, S.; Lellouche, N.; Grimm, R.A.; Gueret, P. Multicentre study using strain delay index for predicting response to cardiac resynchronization therapy (MUSIC study). Eur. J. Heart Fail. 2011, 13, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Leenders, G.E.; Lumens, J.; Cramer, M.J.; De Boeck, B.W.; Doevendans, P.A.; Delhaas, T.; Prinzen, F.W. Septal deformation patterns delineate mechanical dyssynchrony and regional differences in contractility: Analysis of patient data using a computer model. Circ. Heart Fail. 2012, 5, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Samy, W.; Tayeh, O.; Behairy, N.; Abd El Fattah, A. Left ventricular scar impact on left ventricular synchronization parameters and outcomes of cardiac resynchronization therapy. Int. J. Cardiol. 2016, 222, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Morishima, I.; Okumura, K.; Tsuboi, H.; Morita, Y.; Takagi, K.; Yoshida, R.; Nagai, H.; Tomomatsu, T.; Ikai, Y.; Terada, K.; et al. Impact of basal inferolateral scar burden determined by automatic analysis of 99mTc-MIBI myocardial perfusion SPECT on the long-term prognosis of cardiac resynchronization therapy. Europace 2017, 19, 573–580. [Google Scholar] [PubMed]
- Werys, K.; Petryka-Mazurkiewicz, J.; Błaszczyk, Ł.; Misko, J.; Śpiewak, M.; Małek, Ł.A.; Miłosz-Wieczorek, B.; Marczak, M.; Kubik, A.; Dąbrowska, A.; et al. Cine dyscontractility index: A novel marker of mechanical dyssynchrony that predicts response to cardiac resynchronization therapy. J. Magn. Reson. Imaging 2016, 44, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Gorcsan, J.; Anderson, C.P.; Tayal, B.; Sugahara, M.; Walmsley, J.; Starling, R.C.; Lumens, J. Systolic Stretch Characterizes the Electromechanical Substrate Responsive to Cardiac Resynchronization Therapy. JACC Cardiovasc. Imaging 2019, 12, 1741–1752. [Google Scholar] [CrossRef] [PubMed]


| Study Population (n = 49) | Responders (n = 36) | Non-Responders (n = 13) | |
|---|---|---|---|
| Age (years) | 67 ± 10 | 68 ± 10 | 63 ± 10 |
| Male Sex, n (%) | 41 (84) | 30 (83.3) | 11 (84.6) |
| NYHA Functional Class | 2.8 ± 0.5 | 2.8 ± 0.6 | 2.7 ± 0.4 |
| Baseline NYHA Class III, n (%) | 31 (63.3) | 21 (58.3) | 10 (76.9) |
| Ischemic Etiology of HF, n (%) | 28 (57.1) | 20 (55.6) | 8 (61.5) |
| QRS (ms) | 173 ± 19 | 173 ± 21 | 174 ± 16 |
| LBBB, n (%) | 35 (71.4) | 27 (75) | 8 (61.5) |
| AF at Implantation, n (%) | 7 (14.3) | 6 (16.7) | 1 (7.7) |
| CIED Before CRT (= up-grade to CRT), n (%) * | 20 (40.8) | 11 (30.6) | 9 (69.2) |
| LVESV (mL) | 218 ± 109 | 217 ± 107 | 223 ± 119 |
| LVEF (%) | 25 ± 6 | 24 ± 6 | 27 ± 7 |
| Responders | Non-Responders | |||||
|---|---|---|---|---|---|---|
| Baseline | After CRT | p Value | Baseline | After CRT | p Value | |
| Echocardiographic Parameters | ||||||
| LVEF (%) | 24 ± 6 | 34 ± 7 | <0.001 | 27 ± 7 | 27 ± 5 | 0.433 |
| LVESV (mL) | 217 ± 107 | 147 ± 87 | <0.001 | 223 ± 119 | 218 ± 110 | 0.6 |
| LVEDV (mL) | 277 ± 127 | 218 ± 107 | <0.001 | 298 ± 139 | 294 ± 142 | 0.35 |
| Dyssynchrony Indexes | ||||||
| LVDFT/RR (%) | 40.5 ± 9 | 49.3 ± 6.6 | <0.001 | 43.5 ± 9.5 | 43.8 ± 8.2 | 0.753 |
| Interventricular Mechanical Delay (IVMD) (ms) | 36.7 ± 36 | 13.2 ± 19.5 | <0.001 | 36.9 ± 31.7 | 14.6 ± 22 | 0.025 |
| Septal Flash, n (%) | 11 (32.4) | 2 (5.9) | 0.016 | 3 (27.3) | 3 (27.3) | 0.617 |
| SPWMD (ms) | 82.3 ± 176.5 | −44.3 ± 101.2 | 0.002 | 85.4 ± 116 | 20 ± 194.4 | 0.424 |
| 4-chamber Max Intraventricular Delay (ms) | 99.2 ± 80.5 | 100.6 ± 84.8 | 0.812 | 123.8 ± 101.5 | 108.3 ± 137.4 | 0.456 |
| Maximum Time Delay Technique (ms) | 168.3 ± 107.7 | 137.8 ± 74.8 | 0.164 | 204.6 ± 93 | 162.5 ± 113.7 | 0.196 |
| Maximal Opposing Wall Delay (ms) | 144.7 ± 94.3 | 123.3 ± 76.5 | 0.21 | 177.7 ± 92 | 139.2 ± 117.2 | 0.21 |
| Yu Index (ms) | 60.1 ± 40.6 | 46.9 ± 24.3 | 0.109 | 71.6 ± 34.4 | 53.8 ± 31.2 | 0.158 |
| Classical Pattern (0/1), n (%) | 11/25 (30.6/69.4) | 32/4 (88.9/11.1) | <0.001 (McNemar’s test) | 6/7 (46.2/53.8) | 11/2 (84.6/15.4) | 0.13 (McNemar’s test) |
| RSPI | 5.86 ± 2.9 | 2.69 ± 2.3 | <0.001 | 4.08 ± 2.4 | 2.31 ± 2.2 | 0.083 |
| Clinical Response | ||||||
| NYHA Class | 2.8 ± 0.6 | 1.9 ± 0.7 | <0.0001 | 2.7 ± 0.4 | 2 ± 0.7 | 0.005 |
| Univariate Logistic Regression Analysis (Responder: ∆LVESV ≥ 15%) | |
|---|---|
| Odds Ratio [OR]; 95% Confidence Interval. (‘p’ Value) | |
| Atrioventricular Dyssynchrony | |
| LVDFT/RR (Left Ventricular Diastolic Filling Time/RR time) (%) | 0.963; 95% CI = 0.89–1.038 (p = 0.32) |
| LVDFT/RR < 40% (0/1) | 0.5; 95% CI = 0.12–1.98 (p = 0.31) |
| Interventricular Dyssynchrony | |
| IVMD (Interventricular Mechanical Delay) (ms) | 0.999; 95% CI = 0.98–1.02 (p = 0.99) |
| IVMD ≥ 40 ms (0/1) | 1.07; 95% CI = 0.29–3.96 (p = 0.92) |
| Intraventricular Dyssynchrony | |
| Septal flash (0/1) | 1.56; 95% CI = 0.34–7.17 (p = 0.56) |
| SPWMD (Septal to Posterior Wall Motion Delay) (ms) | 0.999; 95% CI = 0.995–1.004 (p = 0.95) |
| 4-chamber max intraventricular delay (ms) | 0.99; 95% CI = 0.989–1.004 (p = 0.39) |
| Maximum Time Delay Technique (ms) | 0.997; 95% CI = 0.99–1.003 (p = 0.29) |
| Maximal Opposing Wall Delay (ms) | 0.996; 95% CI = 0.99–1.003 (p = 0.29) |
| Yu Index (ms) | 0.993; 95% CI = 0.977–1.009 (p = 0.37) |
| Strain Pattern Analysis and RSPI | |
| General Classical Pattern (0/1) | 1.95; 95% CI = 0.51–7.4 (p = 0.32) |
| RSPI (General Score) | 1.26; 95% CI = 0.98–1.61 (p = 0.068) |
| RSPI ≥ 4 points | 1.84; 95% CI = 0.42–8.06 (p = 0.41) |
| RSPI ≥ 7 points | 12; 95% CI = 1.33–108.17 (p = 0.027) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orszulak, M.; Filipecki, A.; Wróbel, W.; Berger-Kucza, A.; Orszulak, W.; Urbańczyk-Swić, D.; Kwaśniewski, W.; Płońska-Gościniak, E.; Mizia-Stec, K. Regional Strain Pattern Index—A Novel Technique to Predict CRT Response. Int. J. Environ. Res. Public Health 2021, 18, 926. https://doi.org/10.3390/ijerph18030926
Orszulak M, Filipecki A, Wróbel W, Berger-Kucza A, Orszulak W, Urbańczyk-Swić D, Kwaśniewski W, Płońska-Gościniak E, Mizia-Stec K. Regional Strain Pattern Index—A Novel Technique to Predict CRT Response. International Journal of Environmental Research and Public Health. 2021; 18(3):926. https://doi.org/10.3390/ijerph18030926
Chicago/Turabian StyleOrszulak, Michał, Artur Filipecki, Wojciech Wróbel, Adrianna Berger-Kucza, Witold Orszulak, Dagmara Urbańczyk-Swić, Wojciech Kwaśniewski, Edyta Płońska-Gościniak, and Katarzyna Mizia-Stec. 2021. "Regional Strain Pattern Index—A Novel Technique to Predict CRT Response" International Journal of Environmental Research and Public Health 18, no. 3: 926. https://doi.org/10.3390/ijerph18030926
APA StyleOrszulak, M., Filipecki, A., Wróbel, W., Berger-Kucza, A., Orszulak, W., Urbańczyk-Swić, D., Kwaśniewski, W., Płońska-Gościniak, E., & Mizia-Stec, K. (2021). Regional Strain Pattern Index—A Novel Technique to Predict CRT Response. International Journal of Environmental Research and Public Health, 18(3), 926. https://doi.org/10.3390/ijerph18030926






