Subcritical Transmission in the Early Stage of COVID-19 in Korea
Abstract
1. Introduction
2. Materials and Methods
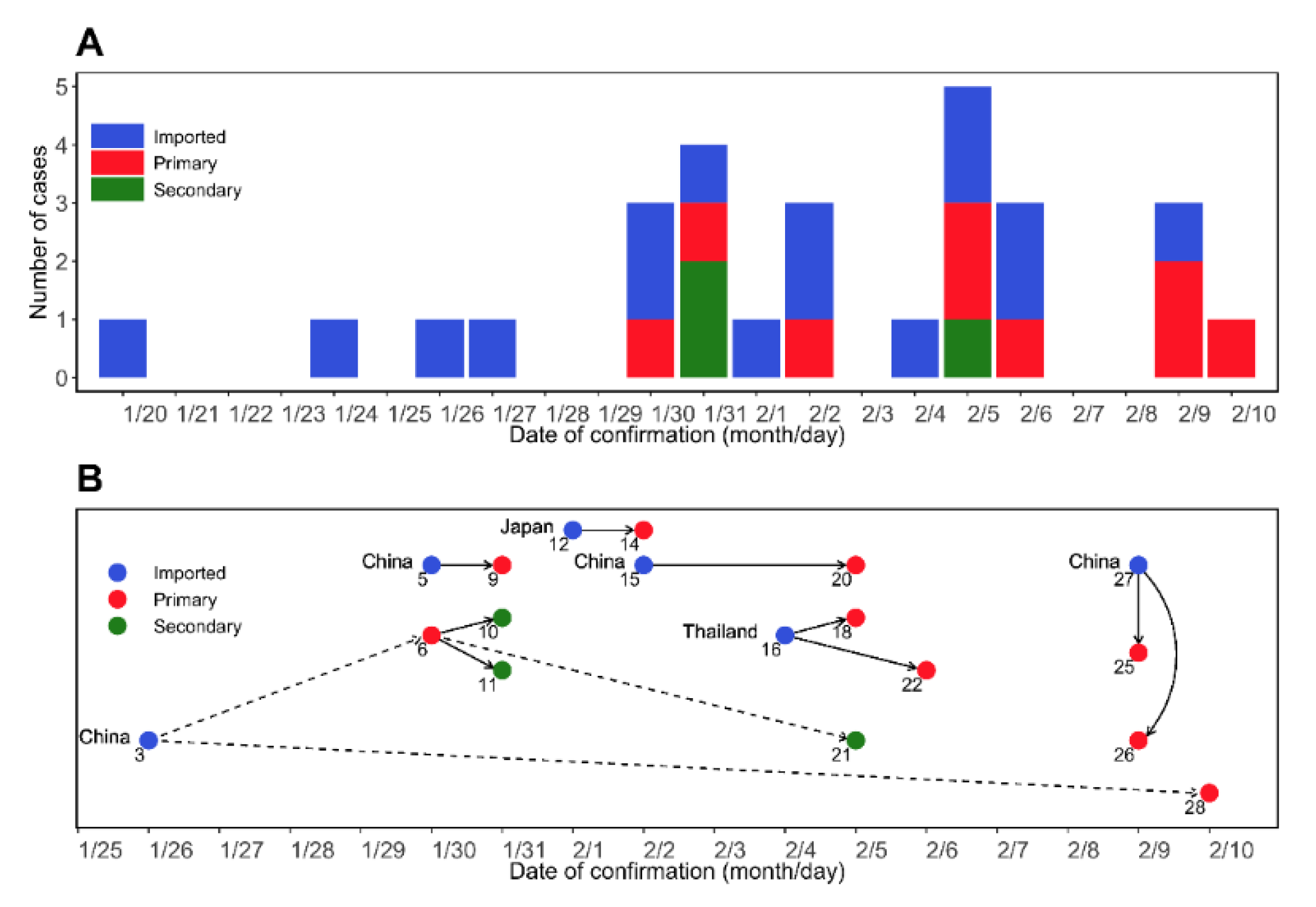
2.1. Epidemiological Data
2.2. Statistical Inference
2.3. Transmission Model
2.4. Ethical Considerations
3. Results
3.1. Estimation of Epidemiological Periods
3.2. Basic Reproduction Number
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronaviridae Study Group of the International Committee on Taxonomy of, Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 11 November 2020).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Wong, G.; Cui, J. Bat origin of a new human coronavirus: There and back again. Sci. China Life Sci. 2020, 63, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 11 November 2020).
- WHO. Weekly Epidemiological Update—12 January 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---12-january-2021 (accessed on 30 January 2021).
- Imai, N.; Dorigatti, I.; Cori, A.; Donnelly, C.; Riley, S.; Ferguson, N.M. Report 2: Estimating the Potential Total Number of Novel Coronavirus Cases in Wuhan City, China. Preprint published by the Imperial College London. 2020. Available online: https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-2-update-case-estimates-covid-19/ (accessed on 30 January 2021).
- Zhao, S.; Lin, Q.; Ran, J.; Musa, S.S.; Yang, G.; Wang, W.; Lou, Y.; Gao, D.; Yang, L.; He, D.; et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020, 92, 214–217. [Google Scholar] [CrossRef]
- Tang, B.; Wang, X.; Li, Q.; Bragazzi, N.L.; Tang, S.; Xiao, Y.; Wu, J. Estimation of the Transmission Risk of the 2019-nCoV and Its Implication for Public Health Interventions. J. Clin. Med. 2020, 9, 462. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Small, D.S. Analysis of the epidemic growth of the early 2019-nCoV outbreak using internationally confirmed cases. medRxiv 2020. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency (KDCA) Press Release. Available online: https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015 (accessed on 30 January 2021).
- Ki, M. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol. Health 2020, 42, e2020007. [Google Scholar] [CrossRef]
- Linton, N.M.; Kobayashi, T.; Yang, Y.; Hayashi, K.; Akhmetzhanov, A.R.; Jung, S.M.; Yuan, B.; Kinoshita, R.; Nishiura, H. Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data. J. Clin. Med. 2020, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, S.; Liao, Y.; Zhao, T.; Wang, X.; Zhang, X.; Jiao, H.; Li, H.; Yin, Y.; Wang, M.H.; et al. Estimating the serial interval of the novel coronavirus disease (COVID-19) based on the public surveillance data in Shenzhen, China, from 19 January to 22 February 2020. Transbound. Emerg. Dis. 2020, 67, 2818–2822. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.; Tariq, A.; Choi, W.; Lee, Y.; Chowell, G. Transmission potential and severity of COVID-19 in South Korea. Int. J. Infect. Dis. 2020, 93, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Lee, H.; Lee, M.; Eun, Y.; Park, K.; Kim, S.; Park, W.; Kwon, S.; Ahn, B.; Kim, D.; et al. Epidemiological characteristics of and containment measures for COVID-19 in Busan, Korea. Epidemiol. Health 2020, 42, e2020035. [Google Scholar] [CrossRef]
- Cori, A.; Ferguson, N.M.; Fraser, C.; Cauchemez, S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am. J. Epidemiol. 2013, 178, 1505–1512. [Google Scholar] [CrossRef]
- White, L.F.; Pagano, M. A likelihood-based method for real-time estimation of the serial interval and reproductive number of an epidemic. Stat. Med. 2008, 27, 2999–3016. [Google Scholar] [CrossRef]
- Lee, H.; Kayano, T.; Nishiura, H. Predicting congenital rubella syndrome in Japan, 2018–2019. Int. J. Infect. Dis. 2019, 82, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.S.; Liu, W.; Xiong, H.; Chen, Z. Modelling and Estimating the Transmissibility of COVID-19: Transmission Dynamics in Shaanxi Province of China as a Case Study. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect. Dis. 2020, 20, 911–919. [Google Scholar] [CrossRef]
- Nishiura, H.; Linton, N.M.; Akhmetzhanov, A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020, 93, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Russell, T.W.; Diamond, C.; Liu, Y.; Edmunds, J.; Funk, S.; Eggo, R.M. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 553–558. [Google Scholar] [CrossRef]
- Riou, J.; Althaus, C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance 2020, 25, 2000058. [Google Scholar] [CrossRef] [PubMed]
- Tindale, L.; Coombe, M.; Stockdale, J.E.; Garlock, E.; Lau, W.Y.V.; Saraswat, M.; Lee, Y.-H.B.; Zhang, L.; Chen, D.; Wallinga, J.; et al. Transmission interval estimates suggest pre-symptomatic spread of COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Yang, L.; Dai, J.; Zhao, J.; Wang, Y.; Deng, P.; Wang, J. Estimation of incubation period and serial interval of COVID-19: Analysis of 178 cases and 131 transmission chains in Hubei province, China. Epidemiol. Infect. 2020, 148, e117. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; Schreiber, S.J.; Kopp, P.E.; Getz, W.M. Superspreading and the effect of individual variation on disease emergence. Nature 2005, 438, 355–359. [Google Scholar] [CrossRef]
- Lau, E.H.; Hsiung, C.A.; Cowling, B.J.; Chen, C.H.; Ho, L.M.; Tsang, T.; Chang, C.W.; Donnelly, C.A.; Leung, G.M. A comparative epidemiologic analysis of SARS in Hong Kong, Beijing and Taiwan. BMC Infect. Dis. 2010, 10, 50. [Google Scholar] [CrossRef]
- Chowell, G.; Castillo-Chavez, C.; Fenimore, P.W.; Kribs-Zaleta, C.M.; Arriola, L.; Hyman, J.M. Model parameters and outbreak control for SARS. Emerg. Infect. Dis. 2004, 10, 1258–1263. [Google Scholar] [CrossRef]
- Riley, S.; Fraser, C.; Donnelly, C.A.; Ghani, A.C.; Abu-Raddad, L.J.; Hedley, A.J.; Leung, G.M.; Ho, L.M.; Lam, T.H.; Thach, T.Q.; et al. Transmission dynamics of the etiological agent of SARS in Hong Kong: Impact of public health interventions. Science 2003, 300, 1961–1966. [Google Scholar] [CrossRef]
- Leung, G.M.; Hedley, A.J.; Ho, L.M.; Chau, P.; Wong, I.O.; Thach, T.Q.; Ghani, A.C.; Donnelly, C.A.; Fraser, C.; Riley, S.; et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: An analysis of all 1755 patients. Ann. Intern Med. 2004, 141, 662–673. [Google Scholar] [CrossRef]
- Lipsitch, M.; Cohen, T.; Cooper, B.; Robins, J.M.; Ma, S.; James, L.; Gopalakrishna, G.; Chew, S.K.; Tan, C.C.; Samore, M.H.; et al. Transmission dynamics and control of severe acute respiratory syndrome. Science 2003, 300, 1966–1970. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Nouvellet, P.; Cori, A.; Jombart, T.; Garske, T.; Clapham, H.; Moore, S.; Mills, H.L.; Salje, H.; Collins, C.; et al. Unraveling the drivers of MERS-CoV transmission. Proc. Natl. Acad. Sci. USA 2016, 113, 9081–9086. [Google Scholar] [CrossRef] [PubMed]
- Virlogeux, V.; Fang, V.J.; Park, M.; Wu, J.T.; Cowling, B.J. Comparison of incubation period distribution of human infections with MERS-CoV in South Korea and Saudi Arabia. Sci. Rep. 2016, 6, 35839. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Jung, S.; Kim, A.; Park, J.E. MERS transmission and risk factors: A systematic review. BMC Public Health 2018, 18, 574. [Google Scholar] [CrossRef]
- Cowling, B.J.; Park, M.; Fang, V.J.; Wu, P.; Leung, G.M.; Wu, J.T. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Eurosurveillance 2015, 20, 7–13. [Google Scholar] [CrossRef]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Parker, M.; Bonsall, D.; Fraser, C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020, 368, 6491. [Google Scholar] [CrossRef]
- Ali, S.T.; Wang, L.; Lau, E.H.Y.; Xu, X.K.; Du, Z.; Wu, Y.; Leung, G.M.; Cowling, B.J. Serial interval of SARS-CoV-2 was shortened over time by nonpharmaceutical interventions. Science 2020, 369, 1106–1109. [Google Scholar] [CrossRef]
- Ganyani, T.; Kremer, C.; Chen, D.; Torneri, A.; Faes, C.; Wallinga, J.; Hens, N. Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data, March 2020. Eurosurveillance 2020, 25, 2000257. [Google Scholar] [CrossRef]
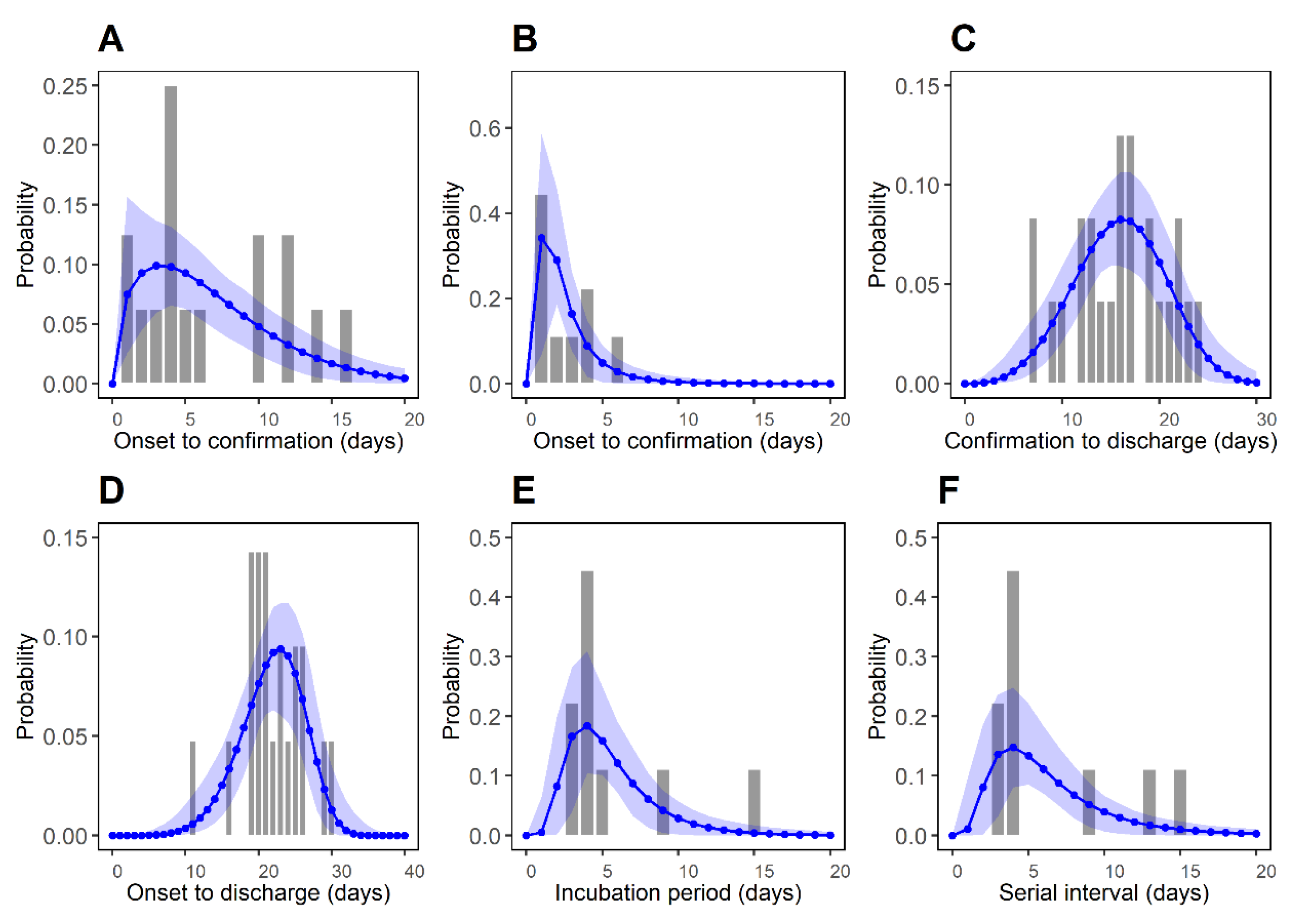
| Time Delay | Distribution | m | Mean (day) | SD* (day) | AICC | BIC |
|---|---|---|---|---|---|---|
| Symptom onset to confirmation for imported cases (P1) | Weibull | 16 | 6.76 (4.53, 9.28) | 4.74 (3.05, 8.70) | 95.11 | 95.73 |
| Symptom onset to confirmation for local cases (P2) | Log-normal | 9 | 2.57 (1.57, 4.23) | 1.99 (0.72, 4.97) | 37.51 | 35.90 |
| Confirmation to discharge (P3) | Weibull | 24 | 15.91 (14.06, 17.72) | 4.66 (3.63, 6.30) | 147.10 | 148.88 |
| Symptom onset to discharge (P4) | Weibull | 21 | 21.87 (19.97, 23.72) | 4.29 (3.42, 6.02) | 124.30 | 125.72 |
| Incubation period (P5) | Log-normal | 9 | 5.53 (3.98, 8.09) | 2.96 (1.28, 6.09) | 47.65 | 46.04 |
| Serial interval (P6) | Log-normal | 9 | 6.45 (4.32, 9.65) | 4.16 (1.67, 8.87) | 52.45 | 50.85 |
| 95% CI* | ||
|---|---|---|
| 0.0 | 1.27 | (0.48, 2.95) |
| 0.1 | 1.05 | (0.40, 2.47) |
| 0.3 | 0.78 | (0.30, 1.86) |
| 0.5 | 0.62 | (0.24, 1.49) |
| 0.7 | 0.51 | (0.20, 1.24) |
| 1.0 | 0.40 | (0.16, 0.99) |
| Virus | Epidemics | R0 | Incubation Period | Serial Interval |
|---|---|---|---|---|
| SARS-CoV | China (Beijing) 2003 | 1.88 (mean) overall [31] | 5.7 (SD 9.7) [32] | NA |
| Hong Kong 2003 | 1.70 (95% CI: 0.44, 2.29) overall [33], 2.7 (95% CI: 2.2, 3.7) in the early phase (excluding SSE) [34] 0.14–1 in the later phase (excluding SSE) [34] | 4.6 (95% CI: 3.8, 5.8) [35] | (8, 12) [36] | |
| MERS-CoV | Saudi Arabia 2013–2014 | 0.45 (95% CI: 0.33, 0.58) overall [37] | 5.0 (95% CrI: 4.0, 6.6) [38] | 6.8 (SD 4.1) [37] |
| Republic of Korea 2015 | 0.91 (95% CI: 0.36, 1.44) overall, 2.0–8.1 in early phase (including SSE) [39] | 6.9 (95% CrI: 6.3, 7.5) [38] | 12.4 (SD 2.8) [40] | |
| SARS-CoV-2 | China 2019–2020 | 2.58 (95% CrI: 2.47, 2.86) [6] | 5.2 (95% CI: 4.1, 7.0) [13] | 7.5 (95% CI: 5.3, 19) [13] |
| Republic of Korea2020 | 0.48 (95% CI: 0.25, 0.84) in early phase (before SSE) [15] | 3.9 (0–15) [15] | 6.6 (3–15) [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, Y.S.; Kim, J.-H.; Ahn, C.Y.; Lee, H. Subcritical Transmission in the Early Stage of COVID-19 in Korea. Int. J. Environ. Res. Public Health 2021, 18, 1265. https://doi.org/10.3390/ijerph18031265
Won YS, Kim J-H, Ahn CY, Lee H. Subcritical Transmission in the Early Stage of COVID-19 in Korea. International Journal of Environmental Research and Public Health. 2021; 18(3):1265. https://doi.org/10.3390/ijerph18031265
Chicago/Turabian StyleWon, Yong Sul, Jong-Hoon Kim, Chi Young Ahn, and Hyojung Lee. 2021. "Subcritical Transmission in the Early Stage of COVID-19 in Korea" International Journal of Environmental Research and Public Health 18, no. 3: 1265. https://doi.org/10.3390/ijerph18031265
APA StyleWon, Y. S., Kim, J.-H., Ahn, C. Y., & Lee, H. (2021). Subcritical Transmission in the Early Stage of COVID-19 in Korea. International Journal of Environmental Research and Public Health, 18(3), 1265. https://doi.org/10.3390/ijerph18031265







