Multimodal Imaging Analysis Reveals Frontal-Associated Networks in Relation to Individual Resilience Strength
Abstract
1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Resilience Score Measurement
2.3. Image Acquisitions
2.4. Imaging Quality Control
2.5. Image Preprocessing
2.5.1. Structural MRI (sMRI)
2.5.2. Resting-State Functional MRI (rfMRI)
2.5.3. Diffusion MRI (dMRI)
2.6. Joint ICA Analysis
3. Statistics for Correlation
3.1. Multiple Regression Model for Predicting RSA Scores
3.2. Leave-One-Out Cross-Validation
4. Results
4.1. Neuropsychological Test Scores: BDI-II and RSA
4.2. Independent Components (ICs)
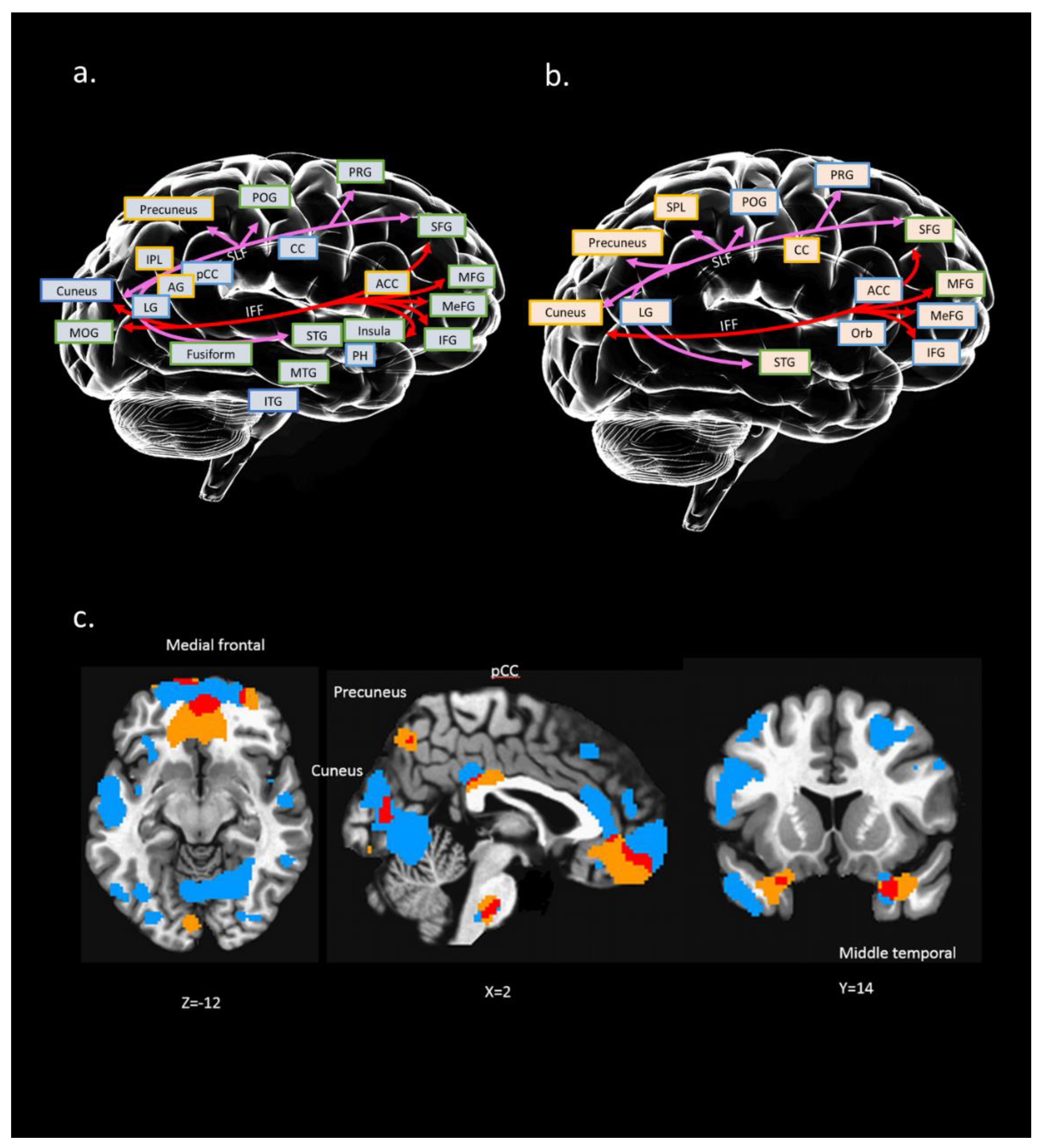
4.3. IC#23 Different Spatial Maps Associated with Psychological Resilience (RSA_p)
4.4. Interaction of Multi Modalities Among rfMRI, sMRI, and dMRI of IC#23 and Its Association with RSA_p
4.5. Multiple Regression Model of IC#23 for Predicting RSA_p Scores
4.6. Leave-One-Out Cross-Validation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luthar, S.S.; Cicchetti, D.; Becker, B. The construct of resilience: A critical evaluation and guidelines for future work. Child Dev. 2000, 71, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Feder, A.; Nestler, E.J.; Charney, D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009, 10, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, X.; Hu, S.; Liu, J. Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. Neuroimage 2015, 123, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Southwick, S.M.; Bonanno, G.A.; Masten, A.S.; Panter-Brick, C.; Yehuda, R. Resilience definitions, theory, and challenges: Interdisciplinary perspectives. Eur. J. Psychotraumatol. 2014, 5, 25338. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Kotozaki, Y.; Sugiura, M.; Nouchi, R.; Takeuchi, H.; Hanawa, S.; Nakagawa, S.; Miyauchi, C.M.; Araki, T.; Sakuma, A.; et al. Resilience after 3/11: Structural brain changes 1 year after the Japanese earthquake. Mol. Psychiatry 2015, 20, 552–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, F.R.; Pfingst, K.; Carnevali, L.; Sgoifo, A.; Nalivaiko, E. In the search for integrative biomarker of resilience to psychological stress. Neurosci. Biobehav. Rev. 2017, 74, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.F.; Hsieh, S. Neurocognitive mechanism of human resilience: A conceptual framework and empirical review. Int. J. Env. Res. Public Health 2019, 16, 5123. [Google Scholar] [CrossRef]
- Kong, F.; Ma, X.; You, X.; Xiang, Y. The resilient brain: Psychological resilience mediates the effect of amplitude of low-frequency fluctuations in orbitofrontal cortex on subjective well-being in young healthy adults. Soc. Cogn. Affect. Neurosci. 2018, 13, 755–763. [Google Scholar] [CrossRef]
- Parsons, S.; Kruijt, A.W.; Fox, E. A Cognitive Model of Psychological Resilience. J. Exp. Psychopathol. 2016, 7, 296–310. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Wolfers, T.; Buitelaar, J.K.; Beckmann, C.F.; Franke, B.; Marquand, A.F. From estimating activation locality to predicting disorder: A review of pattern recognition for neuroimaging-based psychiatric diagnostics. Neurosci. Biobehav. Rev. 2015, 57, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Feder, A.; Charney, D.; Collins, K. Neurobiology of resilience. Resil. Ment. Health 2011, 15, 1–29. [Google Scholar] [CrossRef]
- Doukas, A.M.; D’Andrea, W.M.; Gregory, W.E.; Joachim, B.; Lee, K.A.; Robinson, G.; Freed, S.J.; Khedari-DePierro, V.; Pfeffer, K.A.; Todman, M.; et al. Hurts So Good: Pain as an Emotion Regulation Strategy. Emotion 2019. [Google Scholar] [CrossRef] [PubMed]
- Tulay, E.E.; Metin, B.; Tarhan, N.; Arıkan, M.K. Multimodal Neuroimaging: Basic Concepts and Classification of Neuropsychiatric Diseases. Clin. EEG Neurosci. 2019, 50, 20–33. [Google Scholar] [CrossRef]
- Di, X.; Gohel, S.; Thielcke, A.; Wehrl, H.F.; Biswal, B.B. Do all roads lead to Rome? A comparison of brain networks derived from inter-subject volumetric and metabolic covariance and moment-to-moment hemodynamic correlations in old individuals. Brain Struct. Funct. 2017. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Sui, J. Multimodal Fusion of Brain Imaging Data: A Key to Finding the Missing Link(s) in Complex Mental Illness. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 230–244. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Liu, J.; Adali, T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 2009, 45, S163–S172. [Google Scholar] [CrossRef]
- Sui, J.; Adali, T.; Yu, Q.; Chen, J.; Calhoun, V.D. A review of multivariate methods for multimodal fusion of brain imaging data. J. Neurosci. Methods 2012, 204, 68–81. [Google Scholar] [CrossRef]
- Calhoun, V.; Adah, T.; Liu, J. A feature-based approach to combine functional MRI, structural MRI and EEG brain imaging data. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Proceedings, New York, NY, USA, 30 August–3 September 2006; pp. 3672–3675. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.J.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Friborg, O.; Hjemdal, O.; Rosenvinge, J.H.; Martinussen, M.; Aslaksen, P.M.; Flaten, M.A. Resilience as a moderator of pain and stress. J. Psychosom. Res. 2006, 61, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Friborg, O.; Barlaug, D.; Martinussen, M.; Rosenvinge, J.H.; Hjemdal, O. Resilience in relation to personality and intelligence. Int. J. Methods Psychiatr. Res. 2005, 14, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Sills, L.; Cohan, S.L.; Stein, M.B. Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behav. Res. 2006, 44, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Eley, D.S.; Robert Cloninger, C.; Walters, L.; Laurence, C.; Synnott, R.; Wilkinson, D. The relationship between resilience and personality traits in doctors: Implications for enhancing well being. PeerJ 2013. [Google Scholar] [CrossRef]
- Cloninger, C.R. Healthy personality development and well-being. World Psychiatry 2012, 11, 103. [Google Scholar] [CrossRef]
- Mancini, A.D.; Bonanno, G.A. Predictors and parameters of resilience to loss: Toward an individual differences model. J. Pers. 2009, 77, 1805–1832. [Google Scholar] [CrossRef]
- Van der Werff, S.J.A.; van den Berg, S.M.; Pannekoek, J.N.; Elzinga, B.M.; van der Wee, N.J.A. Neuroimaging resilience to stress: A review. Front. Behav. Neurosci. 2013, 7, 1–14. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Staib, L.H.; Southwick, S.M.; McGlashan, T.; Charney, D.S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry 1999, 156, 1787–1795. [Google Scholar] [CrossRef]
- Bremner, J.D.; Vythilingam, M.; Vermetten, E.; Southwick, S.M.; McGlashan, T.; Staib, L.H.; Soufer, R.; Charney, D.S. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol. Psychiatry 2003, 53, 879–889. [Google Scholar] [CrossRef]
- Bremner, J.D.; Vermetten, E.; Schmahl, C.; Vaccarino, V.; Vythilingam, M.; Afzal, N.; Grillon, C.; Charney, D.S. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol. Med. 2005, 35, 791. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Staib, L.H.; Kaloupek, D.; Southwick, S.M.; Soufer, R.; Charney, D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol. Psychiatry 1999, 45, 806–816. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, J.C.; Toga, A.W.; Evans, A.; Fox, P.; Lancaster, J. A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 1995, 2, 89–101. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S. Non-Linear Registration Aka Spatial Normalisation; FMRIB Technial Report TR07JA2; ScienceOpen Inc.: Berlin, Germany, 2007. [Google Scholar]
- Riddle, W.R.; Li, R.; Fitzpatrick, J.M.; DonLevy, S.C.; Dawant, B.M.; Price, R.R. Characterizing changes in MR images with color-coded Jacobians. Magn. Reson. Imaging 2004, 2, 769–777. [Google Scholar] [CrossRef]
- Geerligs, L.; Tsvetanov, K.A. The use of resting state data in an integrative approach to studying neurocognitive ageing–commentary on Campbell and Schacter (2016). Lang. Cogn. Neurosci. 2017, 32, 684–691. [Google Scholar] [CrossRef]
- Johnstone, T.; Ores Walsh, K.S.; Greischar, L.L.; Alexander, A.L.; Fox, A.S.; Davidson, R.J.; Oakes, T.R. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum. Brain Mapp. 2006, 27, 779–788. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.E.J.; Johansen-Berg, H.; Woolrich, M.W.; Smith, S.M.; Wheeler-Kingshott, C.A.M.; Boulby, P.A.; Barker, G.J.; Sillery, E.L.; Sheehan, K.; Ciccarelli, O.; et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003, 6, 750–757. [Google Scholar] [CrossRef]
- Smith, S.M.; Johansen-Berg, H.; Jenkinson, M.; Rueckert, D.; Nichols, T.E.; Klein, J.C.; Robson, M.D.; Jones, D.K.; Behrens, T.E.J. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat. Protoc. 2007, 2, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.G.; Reid, R.I.; Gunter, J.L.; Senjem, M.L.; Przybelski, S.A.; Zuk, S.M.; Whitwell, J.L.; Vemuri, P.; Josephs, K.A.; Kantarci, K.; et al. Improved DTI registration allows voxel-based analysis that outperforms Tract-Based Spatial Statistics. Neuroimage 2014, 94, 65–78. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Adali, T. Unmixing fMRI with independent component analysis. IEEE Eng. Med. Biol. Mag. 2006, 25, 79–90. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Adali, T.; Giuliani, N.R.; Pekar, J.J.; Kiehl, K.A.; Pearlson, G.D. Method for multimodal analysis of independent source differences in schizophrenia: Combining gray matter structural and auditory oddball functional data. Hum. Brain Mapp. 2006, 27, 47–62. [Google Scholar] [CrossRef]
- Sui, J.; Adali, T.; Pearlson, G.D.; Clark, V.P.; Calhoun, V.D. A method for accurate group difference detection by constraining the mixing coefficients in an ICA framework. Hum. Brain Mapp. 2009, 30, 2953–2970. [Google Scholar] [CrossRef]
- Yang, M.H.; Yao, Z.F.; Hsieh, S. Multimodal neuroimaging analysis reveals age-associated common and discrete cognitive control constructs. Hum. Brain Mapp. 2019, 40, 2639–2661. [Google Scholar] [CrossRef]
- Li, Y.O.; Adali, T.; Calhoun, V.D. A feature-selective independent component analysis method for functional MRI. Int. J. Biomed. Imaging 2007. [Google Scholar] [CrossRef]
- Bell, A.J.; Sejnowski, T.J. The “independent components” of natural scenes are edge filters. Vis. Res. 1997, 37, 3327–3338. [Google Scholar] [CrossRef]
- Itahashi, T.; Yamada, T.; Watanabe, H.; Nakamura, M.; Ohta, H.; Kanai, C.; Iwanami, A.; Kato, N.; Hashimoto, R.I. Alterations of local spontaneous brain activity and connectivity in adults with high-functioning autism spectrum disorder. Mol. Autism 2015, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Zhang, J.; Wakana, S.; Jiang, H.; Li, X.; Reich, D.S.; Calabresi, P.A.; Pekar, J.J.; van Zijl, P.C.M.; Mori, S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Morey, R.D.; Rouder, J.N.; Jamil, T. Package ‘BayesFactor’. R Packag. Version 0.9.12-2 2015. Available online: https://cran.r-project.org/web/packages/BayesFactor/index.html (accessed on 27 January 2021).
- Wagenmakers, E.J.; Love, J.; Marsman, M.; Jamil, T.; Ly, A.; Verhagen, J.; Selker, R.; Gronau, Q.F.; Dropmann, D.; Boutin, B.; et al. Bayesian inference for psychology. Part II: Example applications with JASP. Psychon. Bull. Rev. 2018, 25, 58–76. [Google Scholar] [CrossRef]
- Rosenberg, M.D.; Finn, E.S.; Scheinost, D.; Papademetris, X.; Shen, X.; Constable, R.T.; Chun, M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 2016, 19, 165–171. [Google Scholar] [CrossRef]
- Hsu, W.T.; Rosenberg, M.D.; Scheinost, D.; Constable, R.T.; Chun, M.M. Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc. Cogn. Affect. Neurosci. 2018, 13, 224–232. [Google Scholar] [CrossRef]
- Cleophas, T.J.; Zwinderman, A.H. Modern Bayesian Statistics in Clinical Research; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Salmerón Gómez, R.; García Pérez, J.; López Martín, M.D.M.; García, C.G. Collinearity diagnostic applied in ridge estimation through the variance inflation factor. J. Appl. Stat. 2016, 43, 1831–1849. [Google Scholar] [CrossRef]
- Maier, S.F.; Amat, J.; Baratta, M.V.; Paul, E.; Watkins, L.R. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin. Neurosci. 2006, 8, 397–406. [Google Scholar] [CrossRef]
- Shackman, A.J.; Salomons, T.V.; Slagter, H.A.; Fox, A.S.; Winter, J.J.; Davidson, R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011, 12, 154–167. [Google Scholar] [CrossRef]
- Carnevali, L.; Koenig, J.; Sgoifo, A.; Ottaviani, C. Autonomic and brain morphological predictors of stress resilience. Front. Neurosci. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Dedovic, K.; D.’Aguiar, C.; Pruessner, J.C. What stress does to your brain: A review of neuroimaging studies. Can. J. Psychiatry 2009, 54, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Belleville, S.; Mellah, S.; Cloutier, S.; Dang-Vu, T.T.; Duchesne, S.; Maltezos, S.; Phillips, N.; Hudon, C. Neural correlates of resilience to the effects of hippocampal atrophy on memory. Neuroimage Clin. 2021, 29, 102526. [Google Scholar] [CrossRef] [PubMed]
- Bolsinger, J.; Seifritz, E.; Kleim, B.; Manoliu, A. Neuroimaging Correlates of Resilience to Traumatic Events—A Comprehensive Review. Front. Psychiatry 2018, 9, 693. [Google Scholar] [CrossRef]
- Sui, J.; Qi, S.; van Erp, T.G.M.; Bustillo, J.; Jiang, R.; Lin, D.; Turner, J.A.; Damaraju, E.; Mayer, A.R.; Cui, Y.; et al. Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- New, A.S.; Fan, J.; Murrough, J.W.; Liu, X.; Liebman, R.E.; Guise, K.G.; Tang, C.Y.; Charney, D.S. A Functional Magnetic Resonance Imaging Study of Deliberate Emotion Regulation in Resilience and Posttraumatic Stress Disorder. Biol. Psychiatry 2009, 66, 656–664. [Google Scholar] [CrossRef]
- Martin, R.E.; Ochsner, K.N. The neuroscience of emotion regulation development: Implications for education. Curr. Opin. Behav. Sci. 2016, 10, 142–148. [Google Scholar] [CrossRef]
- Levy, D.J.; Glimcher, P.W. The root of all value: A neural common currency for choice. Curr. Opin. Neurobiol. 2012, 22, 1027–1038. [Google Scholar] [CrossRef]
- Schiller, D.; Levy, I.; Niv, Y.; LeDoux, J.E.; Phelps, E.A. From fear to safety and back: Reversal of fear in the human brain. J. Neurosci. 2008, 28, 11517–11525. [Google Scholar] [CrossRef]
- Raczka, K.A.; Mechias, M.L.; Gartmann, N.; Reif, A.; Deckert, J.; Pessiglione, M.; Kalisch, R. Empirical support for an involvement of the mesostriatal dopamine system in human fear extinction. Transl. Psychiatry 2011, 1, e12. [Google Scholar] [CrossRef]
- Gottfried, J.A.; O’Doherty, J.; Dolan, R.J. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 2003, 301, 1104–1110. [Google Scholar] [CrossRef]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Leknes, S.; Tracey, I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008, 9, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, T.; Heinzle, J.; Park, S.Q.; Haynes, J.D. The neural code of reward anticipation in human orbitofrontal cortex. Proc. Natl. Acad. Sci. USA 2010, 107, 6010–6015. [Google Scholar] [CrossRef] [PubMed]
- Sescousse, G.; Redouté, J.; Dreher, J.C. The architecture of reward value coding in the human orbitofrontal cortex. J. Neurosci. 2010, 30, 13095–13104. [Google Scholar] [CrossRef] [PubMed]
- Grabenhorst, F.; Rolls, E.T. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn. Sci. 2011, 15, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Kringelbach, M.L. Neuroscience of affect: Brain mechanisms of pleasure and displeasure. Curr. Opin. Neurobiol. 2013, 23, 294–303. [Google Scholar] [CrossRef]
- Simeon, D.; Knutelska, M.; Yehuda, R.; Putnam, F.; Schmeidler, J.; Smith, L.M. Hypothalamic-Pituitary-Adrenal Axis Function in Dissociative Disorders, Post-Traumatic Stress Disorder, and Healthy Volunteers. Biol. Psychiatry 2007, 61, 966–973. [Google Scholar] [CrossRef]
- Kim, P.; Evans, G.W.; Angstadt, M.; Ho, S.S.; Sripada, C.S.; Swain, J.E.; Liberzon, I.; Phan, K.L. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. USA 2013, 110, 18442–18447. [Google Scholar] [CrossRef]
- Geschwind, N.; Peeters, F.; Jacobs, N.; Delespaul, P.; Derom, C.; Thiery, E.; Van Os, J.; Wichers, M. Meeting risk with resilience: High daily life reward experience preserves mental health. Acta Psychiatr. Scand. 2010, 122, 129–138. [Google Scholar] [CrossRef]
- Banks, S.J.; Eddy, K.T.; Angstadt, M.; Nathan, P.J.; Luan Phan, K. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007, 2, 303–312. [Google Scholar] [CrossRef]
- Pan, J.; Zhan, L.; Hu, C.L.; Yang, J.; Wang, C.; Gu, L.; Zhong, S.; Huang, Y.; Wu, Q.; Xie, X.; et al. Emotion regulation and complex brain networks: Association between expressive suppression and efficiency in the fronto-parietal network and default-mode network. Front. Hum. Neurosci. 2018, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Golkar, A.; Lonsdorf, T.B.; Olsson, A.; Lindstrom, K.M.; Berrebi, J.; Fransson, P.; Schalling, M.; Ingvar, M.; Öhman, A. Distinct Contributions of the Dorsolateral Prefrontal and Orbitofrontal Cortex during Emotion Regulation. PLoS ONE 2012, 7, e48107. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, P.; Castellanos, F.X. Top-down dysregulation—from ADHD to emotional instability. Front. Behav. Neurosci. 2016, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Santangelo, A.M.; Roberts, A.C. Beyond the medial regions of prefrontal cortex in the regulation of fear and anxiety. Front. Syst. Neurosci. 2016, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.N.; Salovey, P.; Straus, R. Emotional intelligence, personality, and the perceived quality of social relationships. Pers. Individ. Dif. 2003, 35, 641–658. [Google Scholar] [CrossRef]
- Tugade, M.M.; Fredrickson, B.L. Resilient Individuals Use Positive Emotions to Bounce Back From Negative Emotional Experiences. J. Pers. Soc. Psychol. 2004, 86, 320–333. [Google Scholar] [CrossRef]
- Puglisi, G.; Howells, H.; Sciortino, T.; Leonetti, A.; Rossi, M.; Conti Nibali, M.; Gabriel Gay, L.; Fornia, L.; Bellacicca, A.; Viganò, L.; et al. Frontal pathways in cognitive control: Direct evidence from intraoperative stimulation and diffusion tractography. Brain 2019, 142, 2451–2465. [Google Scholar] [CrossRef]
- Rowe, J.B.; Stephan, K.E.; Friston, K.; Frackowiak, R.S.J.; Passingham, R.E. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb. Cortex 2005, 15, 85–95. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Sui, J.; Pearlson, G.; Calhoun, V.D. A hybrid machine learning method for fusing fMRI and genetic data: Combining both improves classification of schizophrenia. Front. Hum. Neurosci. 2010, 4, 192. [Google Scholar] [CrossRef]
- Liu, J.; Pearlson, G.; Windemuth, A.; Ruano, G.; Perrone-Bizzozero, N.I.; Calhoun, V. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum. Brain Mapp. 2009, 30, 241–255. [Google Scholar] [CrossRef]
- Lin, D.; Cao, H.; Wang, Y.P.; Calhoun, V.D. Classification of schizophrenia patients with combined analysis of snp and fmri data based on sparse representation. IEEE Int. Conf. Bioinform. Biomed. 2011, 394–397. [Google Scholar] [CrossRef]
- Connor, K.M.; Davidson, J.R.T. Development of a new Resilience scale: The Connor-Davidson Resilience scale (CD-RISC). Depress. Anxiety 2003, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Wheeler-Kingshott, C.A.M.; Cercignani, M. About “axial” and “radial” diffusivities. Magn. Reson. Med. 2009, 61, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Winklewski, P.J.; Sabisz, A.; Naumczyk, P.; Jodzio, K.; Szurowska, E.; Szarmach, A. Understanding the physiopathology behind axial and radial diffusivity changes-what do we Know? Front. Neurol. 2018, 9, 92. [Google Scholar] [CrossRef]
- Wu, Y.C.; Field, A.S.; Duncan, I.D.; Samsonov, A.A.; Kondo, Y.; Tudorascu, D.; Alexander, A.L. High b-value and diffusion tensor imaging in a canine model of dysmyelination and brain maturation. Neuroimage 2011, 58, 829–837. [Google Scholar] [CrossRef][Green Version]
- Bartzokis, G.; Lu, P.H.; Heydari, P.; Couvrette, A.; Lee, G.J.; Kalashyan, G.; Freeman, F.; Grinstead, J.W.; Villablanca, P.; Finn, J.P.; et al. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol. Psychiatry 2012, 72, 1026–1034. [Google Scholar] [CrossRef]
- Kemper, T.L. Neuroanatomical and Neuropathological Changes During Aging and Dementia. In Clinical Neurology of Aging; Oxford University Press: New York, NY, USA, 1994; ISBN 0195071670. [Google Scholar]
- Madden, D.J.; Bennett, I.J.; Song, A.W. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol. Rev. 2009, 19, 415. [Google Scholar] [CrossRef]
- Minati, L.; Edginton, T.; Grazia Bruzzone, M.; Giaccone, G. Reviews: Current concepts in alzheimer’s disease: A multidisciplinary review. Am. J. Alzheimers. Dis. Other Demen. 2009, 24, 95–121. [Google Scholar] [CrossRef]
- Song, S.K.; Sun, S.W.; Ramsbottom, M.J.; Chang, C.; Russell, J.; Cross, A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002, 17, 1429–1436. [Google Scholar] [CrossRef]
- Song, S.K.; Yoshino, J.; Le, T.Q.; Lin, S.J.; Sun, S.W.; Cross, A.H.; Armstrong, R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005, 26, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Pfefferbaum, A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur. J. Radiol. 2003, 45, 244–255. [Google Scholar] [CrossRef]
- Chopra, S.; Shaw, M.; Shaw, T.; Sachdev, P.S.; Anstey, K.J.; Cherbuin, N. More highly myelinated white matter tracts are associated with faster processing speed in healthy adults. Neuroimage 2018, 171, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Kurth, S.; Doucette, M.R.; Wiseheart, M.; Deoni, S.C.L.; Dean, D.C.; O’Muircheartaigh, J.; Blackwell, K.A.; Munakata, Y.; LeBourgeois, M.K. Myelination is associated with processing speed in early childhood: Preliminary insights. PLoS ONE 2015, 10, e0139897. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Lee, G.J.; Raven, E.P.; Tingus, K.; Khoo, T.; Thompson, P.M.; Bartzokis, G. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J. Clin. Exp. Neuropsychol. 2011, 33, 1059–1068. [Google Scholar] [CrossRef]


| ICs | r | BF10 | 95%CI Upper | 95%CI Lower |
|---|---|---|---|---|
| IC #2 | −0.008 | 0.118 | −0.185 | 0.169 |
| IC #4 | 0.090 | 0.185 | −0.097 | 0.276 |
| IC #5 | 0.068 | 0.152 | −0.118 | 0.258 |
| IC #7 | −0.088 | 0.186 | −0.270 | 0.092 |
| IC #8 | 0.045 | 0.132 | −0.146 | 0.234 |
| IC #9 | −0.046 | 0.133 | −0.232 | 0.139 |
| IC #10 | −0.054 | 0.141 | −0.233 | 0.124 |
| IC #11 | 0.095 | 0.200 | −0.091 | 0.280 |
| IC #12 | −0.117 | 0.257 | −0.302 | 0.069 |
| IC #13 | −0.044 | 0.132 | −0.229 | 0.139 |
| IC #14 | −0.065 | 0.148 | −0.252 | 0.120 |
| IC #15 | −0.015 | 0.119 | −0.201 | 0.175 |
| IC #16 | 0.154 | 0.443 | −0.031 | 0.342 |
| IC #17 | −0.037 | 0.127 | −0.224 | 0.150 |
| IC #18 | 0.102 | 0.214 | −0.084 | 0.290 |
| IC #19 | −0.069 | 0.153 | −0.255 | 0.119 |
| IC #20 | 0.018 | 0.120 | −0.208 | 0.169 |
| IC #21 | −0.025 | 0.122 | −0.214 | 0.164 |
| IC #22 | −0.030 | 0.124 | −0.217 | 0.157 |
| IC #23 | 0.269 | 7.554 | 0.090 | 0.451 |
| Coefficients | Std Error | t-Value | p | |
|---|---|---|---|---|
| (Intercept) | −0.00 | 0.09 | −0.00 | 0.99 |
| ALFF_positive | −0.20 | 0.11 | −1.76 | 0.08 |
| GM_negative | 0.16 | 0.10 | 1.55 | 0.12 |
| GM_positive | −0.03 | 0.10 | −0.34 | 0.74 |
| RD_negative | 0.18 | 0.09 | 1.90 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, S.; Yao, Z.-F.; Yang, M.-H. Multimodal Imaging Analysis Reveals Frontal-Associated Networks in Relation to Individual Resilience Strength. Int. J. Environ. Res. Public Health 2021, 18, 1123. https://doi.org/10.3390/ijerph18031123
Hsieh S, Yao Z-F, Yang M-H. Multimodal Imaging Analysis Reveals Frontal-Associated Networks in Relation to Individual Resilience Strength. International Journal of Environmental Research and Public Health. 2021; 18(3):1123. https://doi.org/10.3390/ijerph18031123
Chicago/Turabian StyleHsieh, Shulan, Zai-Fu Yao, and Meng-Heng Yang. 2021. "Multimodal Imaging Analysis Reveals Frontal-Associated Networks in Relation to Individual Resilience Strength" International Journal of Environmental Research and Public Health 18, no. 3: 1123. https://doi.org/10.3390/ijerph18031123
APA StyleHsieh, S., Yao, Z.-F., & Yang, M.-H. (2021). Multimodal Imaging Analysis Reveals Frontal-Associated Networks in Relation to Individual Resilience Strength. International Journal of Environmental Research and Public Health, 18(3), 1123. https://doi.org/10.3390/ijerph18031123






