Reflections on the Importance of Cost of Illness Analysis in Rare Diseases: A Proposal
Abstract
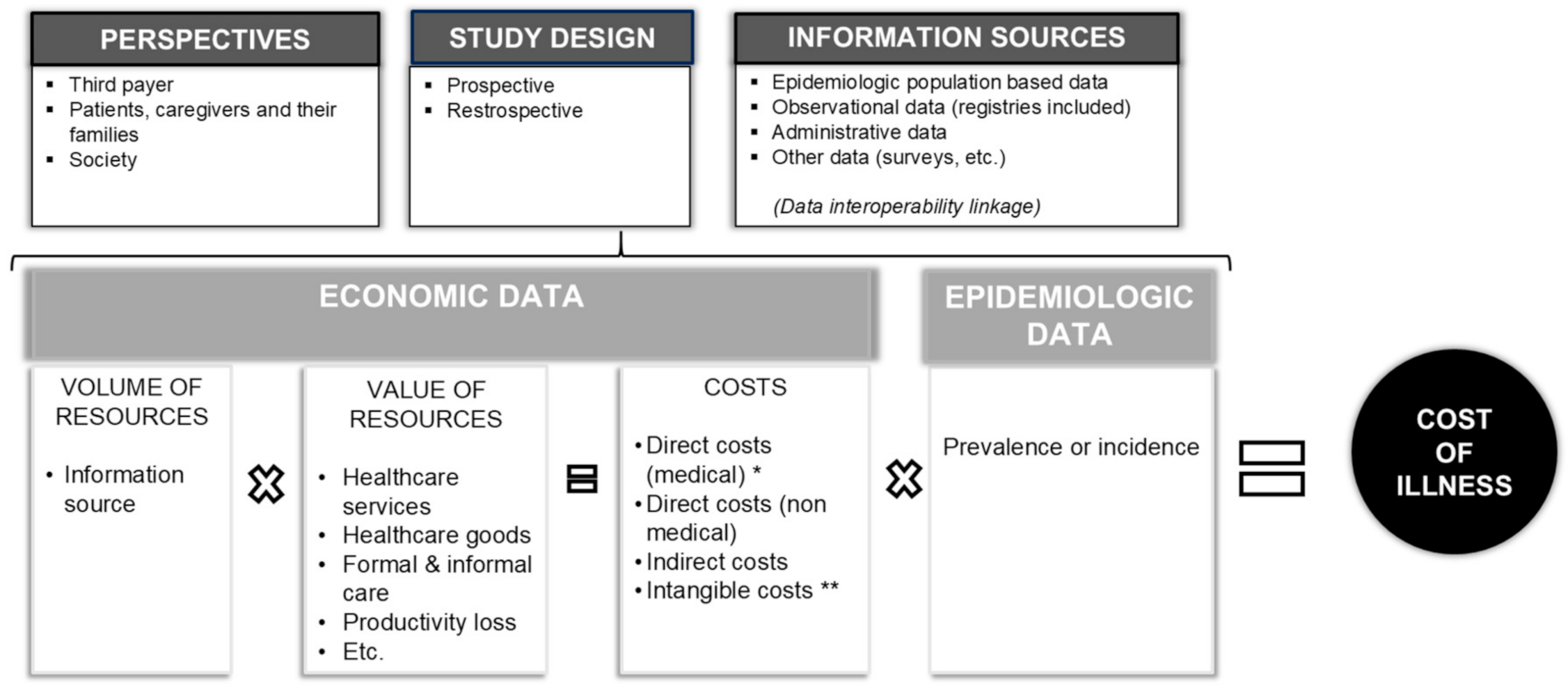
1. Introduction
2. Methods
- name of RD;
- publication year;
- country;
- study population (target and size);
- perspective (i.e., societal, third payer, patients, and families);
- study methodology;
- data source;
- annual average direct health care cost per patient (including all types of healthcare costs directly related to the studied disease from diagnosis and treatment to continuing care and rehabilitation);
- annual average direct formal non-healthcare cost per patient (including costs of transportation);
- annual average direct informal non-healthcare cost per patient (including informal care by non-professional caregivers such as family members or friends, etc.);
- annual average indirect cost per patient (productivity losses);
- total annual average cost per patient;
- costs were reported in terms of absolute values and percentage distribution referring to the total costs.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drummond, M.; Evans, B.; LeLorier, J.; Karakiewicz, P.; Martin, D.; Tugwell, P.; MacLeod, S. Evidence and values: Requirements for public reimbursement of drugs for rare diseases—A case study in oncology. Can. J. Clin. Pharmacol. 2009, 16, e273–e281. [Google Scholar] [PubMed]
- Czech, M.; Baran-Kooiker, A.; Atikeler, K.; Demirtshyan, M.; Gaitova, K.; Holownia-Voloskova, M.; Turcu-Stiolica, A.; Kooiker, C.; Piniazhko, O.; Konstandyan, N.; et al. A review of rare disease policies and orphan drug reimbursement systems in 12 Eurasian countries. Front. Public Health 2020, 7, 416. [Google Scholar] [CrossRef] [PubMed]
- Morel, T.; Arickx, F.; Befrits, G.; Siviero, P.; van der Meijden, C.; Xoxi, E.; Simoens, S. Reconciling uncertainty of costs and outcomes with the need for access to orphan medicinal products: A comparative study of managed entry agreements across seven European countries. Orphanet J. Rare Dis. 2013, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S. Public health and prevention in Europe: Is it cost-effective? J. Pharm. Health Serv. Res. 2011, 2, 151–155. [Google Scholar] [CrossRef]
- Schlander, M.; Dintsios, C.M.; Gandjour, A. Budgetary Impact and Cost Drivers of Drugs for Rare and Ultrarare Diseases. Value Health 2018, 21, 525–531. [Google Scholar] [CrossRef]
- Linertová, R.; Serrano-Aguilar, P.; Posada-de-la-Paz, M.; Hens-Pérez, M.; Kanavos, P.; Taruscio, D.; Schieppati, A.; Stefanov, R.; Péntek, M.; Delgado, C.; et al. Delphi approach to select rare diseases for a European representative survey. The BURQOL-RD study. Health Policy 2012, 108, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cavazza, M.; Kodra, Y.; Armeni, P.; De Santis, M.; López-Bastida, J.; Linertová, R.; Oliva-Moreno, J.; Serrano-Aguilar, P.; Posada-de-la-Paz, M.; Taruscio, D.; et al. Social/economic costs and quality of life in patients with haemophilia in Europe. Eur. J. Health Econ. 2016, 17, 53–65. [Google Scholar] [CrossRef]
- O’Hara, J.; Hughes, D.; Camp, C.; Burke, T.; Carroll, L.; Diego, D.G. The cost of severe haemophilia in Europe: The CHESS study. Orphanet J. Rare Dis. 2017, 31, 12. [Google Scholar] [CrossRef]
- Chen, C.X.; Baker, J.R.; Nichol, M.B. Economic Burden of Illness among Persons with Hemophilia B from HUGS Vb: Examining the Association of Severity and Treatment Regimens with Costs and Annual Bleed Rates. Value Health 2017, 20, 1074–1082. [Google Scholar] [CrossRef]
- Café, A.; Carvalho, M.; Crato, M.; Faria, M.; Kjollerstrom, P.; Oliveira, C.; Pinto, P.R.; Salvado, R.; Dos Santos, A.A.; Silva, C. Haemophilia A: Health and economic burden of a rare disease in Portugal. Orphanet J. Rare Dis. 2019, 4, 211. [Google Scholar] [CrossRef] [PubMed]
- Henrard, S.; Devleesschauwer, B.; Beutels, P.; Callens, M.; De Smet, F.; Hermans, C.; Speybroeck, N. The health and economic burden of haemophilia in Belgium: A rare, expensive and challenging disease. Orphanet J. Rare Dis. 2014, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Chevreul, K.; Gandré, C.; Brigham, K.B.; López-Bastida, J.; Linertová, R.; Oliva-Moreno, J.; Serrano-Aguilar, P.; Posada-de-la-Paz, M.; Taruscio, D.; Schieppati, A.; et al. Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. Eur. J. Health Econ. 2016, 17, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Capkun-Niggli, G.; Zhang, X.; Rosemary, J. The Economic Burden of Fragile X Syndrome: Healthcare Resource Utilization in the United States. Am. Health Drug Benefits 2013, 6, 73–83, PMID: 24991348; PMCID: PMC4031705. [Google Scholar]
- Heimeshoff, M.; Hollmeyer, H.; Schreyögg, J.; Tiemann, O.; Staab, D. Cost of Illness of Cystic Fibrosis in Germany. Pharm. Econ. 2012, 30, 763–777. [Google Scholar] [CrossRef]
- Frey, S.; Stargardt, T.; Schneider, U.; Schreyögg, J. The Economic Burden of Cystic Fibrosis in Germany from a Payer Perspective. Pharm. Econ. 2019, 37, 1029–1039. [Google Scholar] [CrossRef]
- Chevreul, K.; Berg Brigham, K.; Michel, M.; Rault, G.; BURQOL-RD Research Network. Costs and health-related quality of life of patients with cystic fibrosis and their carers in France. J. Cyst. Fibros. 2015, 4, 384–391. [Google Scholar] [CrossRef]
- Kopciuch, D.; Zaprutko, T.; Paczkowska, A.; Nowakowska, E. Costs of treatment of adult patients with cystic fibrosis in Poland and internationally. Public Health 2017, 148, 49–55. [Google Scholar] [CrossRef]
- Minden, K.; Niewerth, M.; Listing, J.; Möbius, D.; Thon, A.; Ganser, G.; Ermisch-Omran, B.; Zink, A. The economic burden of juvenile idiopathic arthritis-results from the German paediatric rheumatologic database. Clin. Exp. Rheumatol. 2009, 27, 863–869. [Google Scholar]
- Yucel, I.K.; Seyahi, E.; Kasapcopur, O.; Arisoy, N. Economic impact of juvenile idiopathic arthritis and familial Mediterranean fever. Rheumatol. Int. 2012, 32, 1955–1962. [Google Scholar] [CrossRef]
- Angelis, A.; Kanavos, P.; López-Bastida, J.; Linertová, R.; Serrano-Aguilar, P.; BURQOL-RD Research Network. Socioeconomic costs and health-related quality of life in juvenile idiopathic arthritis: A cost-of-illness study in the United Kingdom. BMC Musculoskelet. Disord. 2016, 17, 321. [Google Scholar] [CrossRef] [PubMed]
- Minden, K.; Niewerth, M.; Listing, J.; Biedermann, T.; Schöntube, M.; Zink, A. Burden and cost of illness in patients with juvenile idiopathic arthritis. Ann. Rheum. Dis. 2004, 63, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Recht, M. Current Options and New Developments in the Treatment of Haemophilia. Drugs 2011, 71, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.R.; Key, N.S.; Escobar, M.A.; Hemophilia, A.; Hemophilia, B. Williams Hematology, 8th ed.; Kaushansky, K., Lichtman, M., Beutler, E., Eds.; McGraw Hill: New York, NY, USA, 2010; ISBN -10. [Google Scholar]
- Aledort, L.; Mannucci, P.M.; Schramm, W.; Tarantino, M. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019, 17, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.L.; Di Matteo, S.; Mancuso, M.E.; Santagostino, E. Cost-utility analysis of prophylaxis versus treatment on demand in severe hemophilia A. Clinicoecon. Outcomes Res. 2011, 3, 55–61. [Google Scholar] [CrossRef]
- Thorat, T.; Neumann, P.J.; Chambers, J.D. Hemophilia Burden of Disease: A Systematic Review of the Cost-Utility Literature for Hemophilia. J. Manag. Care Spec. Pharm. 2018, 24, 632–642. [Google Scholar] [CrossRef]
- Saldarriaga, W.; Tassone, F.; González-Teshima, L.Y.; Forero-Forero, J.V.; Ayala-Zapata, S.; Hagerman, R. Fragile X Syndrome. Colomb. Med. 2014, 45, 190–198. [Google Scholar] [CrossRef]
- Orphanet Report Series—Prevalence of Rare Diseases: Bibliographic Data 2020 1. Available online: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf (accessed on 26 September 2020).
- Bailey, D.B.; Raspa, M.; Bishop, E.; Holiday, D. No change in the age of diagnosis for fragile x syndrome: Findings from a national parent survey. Pediatrics 2009, 124, 527–533. [Google Scholar] [CrossRef]
- Ouyang, L.; Grosse, S.; Raspa, M.; Bailey, D. Employment impact and financial burden for families of children with fragile X syndrome: Findings from the National Fragile X Survey. J. Intel. Disab. Res. 2010, 54, 918–928. [Google Scholar] [CrossRef]
- Goss, C.H.; Burns, J.L. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 2007, 62, 360–367. [Google Scholar] [CrossRef]
- De Boer, K.; Vandemheen, K.L.; Tullis, E.; Doucette, S.; Fergusson, D.; Freitag, A.; Paterson, N.; Jackson, M.; Lougheed, M.D.; Kumar, V.; et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.S.; Rasouliyan, L.; VanDevanter, D.R.; Pasta, D.J.; Regelmann, W.E.; Morgan, W.J.; Konstan, M.W. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr. Pulmonol. 2013, 48, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Fink, A. Background and Epidemiology. Pediatr. Clin. N. Am. 2016, 63, 567–584. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.P.; Orenstein, D.M.; Milla, C.E. Pricing for orphan drugs: Will the market bear what society cannot? JAMA 2013, 310, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Ferkol, T.; Quinton, P. Precision medicine: At what price? Am. J. Respir. Care Med. 2015, 192, 658–659. [Google Scholar] [CrossRef]
- Orestein, D.M.; O’Sullivan, B.P.; Quinton, P.M. Cystic fibrosis: Breakthrough drugs at break-the-bank prices. Glob. Adv. Health Med. 2015, 4, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.L.; Rosenberg, A.M.; Vatanparast, H. Vitamin D and juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2018, 16, 34. [Google Scholar] [CrossRef]
- Gökçe, I.; Demĭrkaya, E. New Treatment Strategies in the Treatment of Juvenile Idiopathic Arthritis. Arch. Reumathol. 2011, 26, 71–85. [Google Scholar] [CrossRef]
- EMA—Committee for Medicinal Products for Human Use (CHMP), Guideline on Clinical Investigation of Medicinal Products for the Treatment of Rheumatoid Arthritis, 14 December 2017, CPMP/EWP/556/95 Rev 2. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-rheumatoid-arthritis_en.pdf (accessed on 26 September 2020).
- Angelis, A.; Tordrup, D.; Kanavos, P. Socio-economic burden of rare diseases: A systematic review of cost of illness evidence. Health Policy 2015, 119, 964–979. [Google Scholar] [CrossRef]
- Genetic Alliance UK Hidden Costs Report. 2016. Available online: https://www.geneticalliance.org.uk/media/2502/hiddencosts-full-report_21916-v2-1.pdf (accessed on 26 September 2020).
- Italian Law December 23, 1996, n. 648. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:1996-12-23;648!vig= (accessed on 26 September 2020).
- Agence Nationale de Sécurité du Médicament et des Produits de Santé, Notice to Applicants for Marketing for Temporary Authorisation for Use (ATU), July 2015. Available online: https://ansm.sante.fr/var/ansm_site/storage/original/application/cadfbcf9594614d59c8915670853a28b.pdf (accessed on 26 September 2020).
- Jo, C. Cost-of-illness studies: Concepts, scopes, and methods. Clin. Mol. Hepatol. 2014, 20, 327–337. [Google Scholar] [CrossRef]
- Xoxi, E.; Tomino, C.; de Nigro, L.; Pani, L. The Italian post-marketing registries. Pharm. Program. 2012, 5, 57–60. [Google Scholar] [CrossRef]
- Montilla, S.; Xoxi, E.; Russo, P.; Cicchetti, A.; Pani, L. Monitoring registries at Italian Medicines Agency: Fostering access, guaranteeing sustainability. Int. J. Technol. Assess. Health Care 2015, 31, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bastida, J.; Oliva-Moreno, J.; Linertova, R.; Pedro Serrano-Aguilar, P. Social/economic costs and health-related quality of life in patients with rare diseases in Europe. Eur. J. Health Econ. 2016, 17, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Eurordis—Rare Diseases in Europe, Social Economic Burden and Health-Related Quality of Life in Patients with Rare Diseases in Europe. Available online: https://www.eurordis.org/content/burqol-rd-project (accessed on 26 September 2020).
- EUnetHTA—European Network for Health Technology Assessment. Available online: https://www.eunethta.eu/ (accessed on 26 September 2020).
- EUFP7 Program—Advancing and Strengthening the Methodological Tools and Practices Relating to the Application and Implementation of Health Technology Assessment (HTA). Available online: http://www.advance-hta.eu/ (accessed on 26 September 2020).
- Impact HTA Program—Improved Methods and Actionable Tools for Enhancing HTA. Available online: https://www.impact-hta.eu/ (accessed on 26 September 2020).
| Name of Rare Diseases | Authors and Pubblication Year | Country | Study Population (Target and Size) | Perspective | Study Methodology | Data Sources | Annual Average Direct Formal Health Care Cost per Patient | Annual Average Direct Formal non Healthcare Cost per Patient | Annual Average Direct Informal non Healthcare Cost per Patient | Annual Average Indirect Cost per Patient | Total Annual Average Cost per Patient | Reference Number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haemofilia | Cavazza M et al., 2016 | Bulgaria, France, Hungaria, Italy, Germany, Spain, Sweden, UK | 339 adult and child patients with hemophilia A and B | Societal perspective | A cross-sectional study. Cost analysis based on a bottom-up approach | Patients’ survey | Items: Rehabilitation, medical tests and examinations, visits to health professionals and home medical care) emergency visits; drugs, healthcare transport, and health materials. Value: €44,842.37 (78%) | Items: Professional care, social services, and non-healthacare transport. Value: €1896.37 (3%) | Items: Informal care. Value: €3119.43 (5%) | Items: Early retirement, sick leave. Value: €7518.33 (13%) | Value: €57,376.51 (100%) | [8] |
| O’Hara J et al., 2017 | France, Germany, Italy, Spain and UK. | 551 adult patients with severe hemophilia A and B without inhibitors | Societal perspective | A retrospective, non-interventional study | Clinicians and patients’ survey | Items: Ambulatory (Haematologist visit, nurse per visit, other specialist visits, blood tests, other tests, drugs) and hospitalization (target joint procedure, bleed event: Ward stay (per day)), Bleed event: ICU stay (per day) costs. Value for clotting factor replacement therapy (CFRT)): €189,285.00 (95%). Value for other medical costs: €4181.00 (2%) | Items: Wage (patient/caregiver), petrol (per mile). ** Value: €6075.00 (3%) | Value: €199,541.00 (100%) | [9] | |||
| Chen CX et al. 2017 | USA | 112 patients children and 50 adults with hemophilia B | Societal perspective | Prospective study with longitudinal cohort data | Clinicians and patients’ survey, and administrative data | Items: Inpatient services (all-cause hospitalizations, emergency room (ER) visits), outpatient services (comprehensive, nursing, clinician, physicaltherapist, and socialwork/psychology), laboratory tests, and outpatient procedures), and medication costs (including clotting or bypass treatments). Value for all patients: $ 133,894.00 (95%) Value for mild patients: $ 51,435.00 (92%) Value for severe patients: $ 190,312.00 (87%) | Items: Lost wages due to days of work absenteeism among those employed and unpaid hemophilia-related caregiver time reported; hemophilia-related part-time employment or unemployment reported. Value for all patient: $ 6346.00 (5%) Value for mild patients: 4416.00 $ (8%) Value for severe patients 8421 $ (13%) | Value for all patients: $ 140,240.00 (100%) Value for mild patients: $ 85,852.00 (100%)Value for severe patients: $ 198,733.00 (100%) | [10] | |||
| Café A et al., 2019 | Portugal | 127 adult and child patients with hempphilia A and B | Societal perspective | A mix of retrospective and probabilistic model | Experts panel and patients’ survey, administrative data and national literature data | Items: Hemophilia related hospitalization; outpatient care (physicians, nurses, physiotherapists, etc., visits, laboratory and imaging exams, concomitant medications (for pain), hemophilia treatment. Value: €50,255.47 (88%) | Items:Transportation to medical appointments. Value: €3692.53 (6.5%) | Items: Unemployment rate, labor absenteeism; early retirement. Value: €2927.00 (5.5%) | Value: €56,875.00 (100%) | [11] | ||
| Henrard S et al., 2017 | Belgium | A simulation of new-born males with hemophilia A and B in 2011 and male births in 2011 in Belgium with a hemophilia A and B incidence from 1/5500 to 1/4500 new-born males in Belgium | Societal perspective | Prospective study | Administrative data and friction-cost method | Items: Hemophilia medications, hospitalization, general practitioner (GP), specialist, physiotherapist, and dentist. Value: €180,517.11 (97%) | Items:Transport costs to and from the doctor’s office and hospital. Value:€3692.530 (2%) | Items: Absence from work due to these appointments and hospitalisations; absence from work due to invalidity or premature death. Value: €1880.50 (1%) | Lifetime value: €97.4 million (95% CrI: €47.1–158.1 million) | [12] | ||
| Fragile X Syndrome | Chevreul K et al., 2016 | France | 147 adult and child patients | Societal | Cross sectional study | Patients recruited through the French FXS patient associations | Items: Rehabilitation, medical tests and examinations, visits to health professionals and home medical care) emergency visits; drugs, healthcare transport, and health materials. Value: €2687.00 (10%) | Items: Professional care, social services, and non-healthacre transport. Value: €10,511.00 (40%) | Items: Informal care. Value: €12,586.00 (48%) | Items: Early retirement, sick leave. Value only for adult patients: €31,240.00 | Value: €25,784.00 (100%) * | [13] |
| Sacco Pet et al., 2013 | USA | 721 patients with Medicaid (all age) | Third payer perspective | Retrospective observation cohort study | Patients recruited from Medicaid databases | Items: Emergency department visits, hospitalizations, outpatient visits, medical procedures. Value range: $ 4548.00–$ 9702.00 | Items: Informal careValue: €3119.43 | [14] | ||||
| Cystic Fibrosis | Heimeshoff M et al., 2012 | Germany | 158 adult and child patients with severe CF | Societal perspective | Prospective study | Administrative data, register of CF pstients, clinicians, and healthcare professional survey | Items: Drugs, laboratory tests, staff cost per patient, and centre’s overhead. Value: €38,869.00 (93.7%) | Items: Transport. Value: €10,800 (0.3%) | Items: Early retirement a/o disability pensions provided by the social insurance system. Value: €2492.00 (6%) | Value: €41,468.00 (100%) | [15] | |
| Frey S et al., 2019 | Germany | 2241 patients with mild, moderate, and severe CF | Third payer perspective | Retrospective observation cohort study | Administrative claims data | Items: Outpatient treatment, drugs, care by non-physicians (e.g., physiotherapy), devices and medical equipment, inpatient treatments, rehabilitation and nursing care (at home). Value for patients with mild CF: €8920.00 (99.05%) Value for patient with moderate CF: €50,121.00 (99.5%) Value for patient with severe CF: €95,768.00 (99%) | Items: Services (e.g., transportation). Value for all patient with mild CF: €87.00 (0.95%) Value for patient with moderate CF: €269.00 (0.5%) Value for patient with severe CF: €994.00 (1%) | Value for patients with mild CF: €17,551.00 (100%) Value for patients with moderate CF: €50,390.00 Value for patients with severe CF: €96,762.00 | [16] | |||
| Chevreul K et al., 2015 | France | 240 adult and child patients | Societal perspective | Retrospective cross-sectional study | Patients’ survey | Items: Rehabilitation, medical tests and examinations, visits to health professionals, and home medical care) emergency visits; drugs, healthcare transport, and health materials. Value: €16,851.00 (46%) | Items: Professional care, social services, and non-healthacre transport. Value: €4512.00 (12%) | Items: Informal care. Value: €4827.00 (13%) | Items: Early retirement, sick leave. Value: €10,408.00 (28%) | Value: €36,598.00 (100%) | [17] | |
| Kopciuch D et al., 2017 | Poland | 46 adult patients | Societal perspective | Retrospective study | Patients’ survey and administrative data | Direct healthcare costs: Hospitalization, outpatient visits, pharmacotherapy, diagnostic tests. Value: €13,740.33 (70%), | Items: Transportation. Value: €57.80 (0.3%) | Items: Presenteeism. Value: €5782.94 (29.7%) | Value: €19,581.08 (100%) | [18] | ||
| Juvenile IdiopathicArthritis | Minden K et al., 2009 | Germany | 369 child patients | Societal perspective and patient’s perspective | An incidence based, retrospective study | Patients’ survey, medical records, and administrative data | Items: Pediatric rheumatology service use, ophthalmologist service use, Other JIA-related physician service use, non-physician service use, day-surgery, medication, devices and aids, acute hospital facilities, surgery, rehabilitation, comprehensive alternative (non-prescription) medicine. Value: €4172.00 (89%) | Items: Transportation, extra telephone, home alterations, domestic help and care. Value: €223.00 (5%) | Items: Loss of productivity. Value: €270.00 (6%) | Value: €4663.00 (100%) | [19] | |
| Yucel IK et al., 2012 | Turkey | 100 child patients | Societal perspective | A cross-sectional study | Patients and caregivers surveys | Items: Outpatient visits, biochemical tests, radiologicaltests, physiotherapy, hospitalization fees, surgery, drugs, devices, physiotherapy. Value:€3725.00 (94%) | Items: Transportation, lodging expenses. Value: €188.00 (5%). | Items: Work days lost among parents. Value: €81.00 (2%) | Value: €3994.00 (100%) | [20] | ||
| Angelis AP et al., 2016 | UK | 23 child and adult patients | Societal perspective | A cross-sectional study | Patients and caregivers surveys | Items: Medication, tests, outpatient and primary health care visits, acute hospitalization, devices, healthcare transportation. Value: €14,508.00 (46%) | Items: Professional carer, non-healthcare transportation. Value: €722.00 (2%) | Items: Informal care. Value: €7621.00 (24%) | Items: Productivity loss, early retirement, and sick leave. Value: €8715.00 (28%) | Value: €31,546.00 (100%) | [21] | |
| Minden K. et al., 2004 | Germany | 215 child patients | Societal perspective and patient’s perspective | An incidence based, retrospective study | Patients’ survey, medical records, and administrative data | Items: Inpatient care (acute hospital facilities, surgery, non-acute hospital facilities (rehabilitation)); outpatient care (JIA-related rheumatology service use; other JIA-related physician service use; non-physician service use; surgery; medication; devices and aids. Value: €1821.00 (52%) | Items: Patient expenditures. (3 months) Value: €73.00 (2%) | Items: Loss of productivity. Value: €1571.00 (45%) | Value €3465.00 (100%) | [22] |
| Hemophilia | Fragile X Syndrome | Cystic Fibrosis | Juvenile IdiopathicArthritis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cavazza et al. [8] | O’Hara et al. [9] | Chen et al. [10] | Caféet al. [11] | Henrard et al. [12] | Chevreul et al. [13] | Sacco et al. [14] | Heimeshoff et al. [15] | Frey et al. [16] | Chevreul et al. [17] | Kopciuchet al. [18] | Mindenet al. [19] | Yucel et al. [20] | Angelis et al. [21] | Minden et al. [22] | ||
| Direct formal healthcare cost | Medical tests and exams | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Visits | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Hospitalization | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Rehabilitation | X | X | X | X | X | X | X | X | X | X | ||||||
| ER access | X | X | X | X | X | |||||||||||
| Home healthcare | X | X | X | |||||||||||||
| Healthcare transportation | X | X | X | X | X | |||||||||||
| Drugs and healthcare materials | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Direct formal non-healthcare cost | Professional care | X | X | X | X | X | ||||||||||
| Home alterations | X | |||||||||||||||
| Social services | X | X | X | |||||||||||||
| Non healthcare transportation | X | X | X | X | X | X | X | X | X | X | ||||||
| Lodging expenses | X | X | ||||||||||||||
| Direct informal non-healthcare costs | Informal care | X | X | X | X | X | ||||||||||
| Indirect costs | Sick leave | X | X | X | X | X | X | X | X | |||||||
| Abstenteism | X | X | X | X | X | X | ||||||||||
| Unemployment | X | X | X | |||||||||||||
| Early retirement | X | X | X | X | X | X | X | |||||||||
| PROs | A COI analysis will help decision makers gain information on the current and/or prospective economic burden of a disease. |
| If a societal perspective is adopted, the COI analysis will allow for the identification of those societal actors bearing most of the burden (which are often excluded or neglected by other economic evaluation methods). | |
| Over time, and with the availability of new therapies, updating the COI analysis will offer the opportunity to assess how much the burden has shifted from patients/caregivers to other actors (e.g., a third party payer). | |
| CONs | COI is not a comparative analysis, so it should not be used to assess the opportunity of introducing a new therapy. However, when the economic burden of a disease is heavily concentrated on patients, caregivers, and society, with a small amount born by the healthcare system, this could be a signal that a (new) therapy is needed. |
| Data sources are often scarce or outdated. This is a major problem with the use of COI: The more epidemiological data are sound and up to date, the more COI will be informative. | |
| COI describes the economic burden at a specific point in time: It needs frequent updates to keep its informative value. This requires the availability of human and economic resources that are generally not invested by national authorities. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armeni, P.; Cavazza, M.; Xoxi, E.; Taruscio, D.; Kodra, Y. Reflections on the Importance of Cost of Illness Analysis in Rare Diseases: A Proposal. Int. J. Environ. Res. Public Health 2021, 18, 1101. https://doi.org/10.3390/ijerph18031101
Armeni P, Cavazza M, Xoxi E, Taruscio D, Kodra Y. Reflections on the Importance of Cost of Illness Analysis in Rare Diseases: A Proposal. International Journal of Environmental Research and Public Health. 2021; 18(3):1101. https://doi.org/10.3390/ijerph18031101
Chicago/Turabian StyleArmeni, Patrizio, Marianna Cavazza, Entela Xoxi, Domenica Taruscio, and Yllka Kodra. 2021. "Reflections on the Importance of Cost of Illness Analysis in Rare Diseases: A Proposal" International Journal of Environmental Research and Public Health 18, no. 3: 1101. https://doi.org/10.3390/ijerph18031101
APA StyleArmeni, P., Cavazza, M., Xoxi, E., Taruscio, D., & Kodra, Y. (2021). Reflections on the Importance of Cost of Illness Analysis in Rare Diseases: A Proposal. International Journal of Environmental Research and Public Health, 18(3), 1101. https://doi.org/10.3390/ijerph18031101





