Probabilistic Risk Analysis to Assess Dietary Exposure to Aluminum in the Taiwanese Population
Abstract
1. Introduction
2. Material and Methods
2.1. Aluminum Concentration in Food
2.2. Food Consumption Data
2.3. Dietary Exposure Assessment (EWI and %PTWI)
2.4. Monte Carlo Simulation
3. Results
3.1. Aluminum Concentration in Different Food Items
3.2. Food Consumption Data across Age-Sex Subgroups
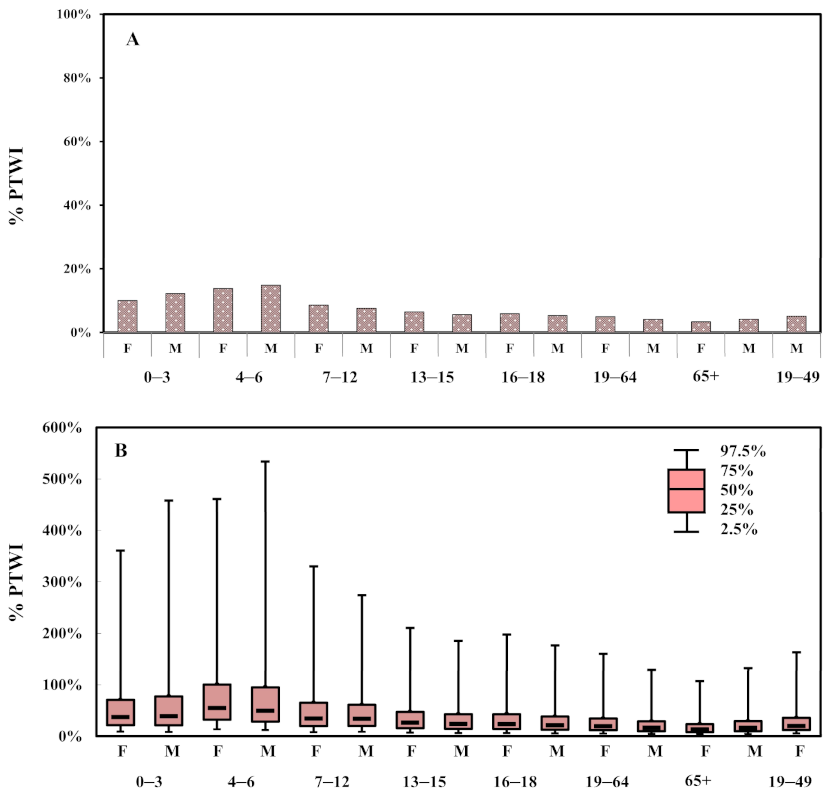
3.3. Risk Assessment of Dietary Exposure to Aluminum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahdy, K.A.; Farrag, A.R.H. Amelioration of aluminum toxicity with black seed supplement on rats. Toxicl. Environ. Chem. 2009, 91, 567–576. [Google Scholar] [CrossRef]
- Walton, J.R. Evidence that ingested aluminum additives contained in processed foods and alum-treated drinking water-treated drinking water are major risk factor for Alzheimer’s disease. Curr. Inorg. Chem. 2012, 2, 19–39. [Google Scholar] [CrossRef]
- Martinez, C.S.; Piagette, J.T.; Escobar, A.G.; Martín, Á.; Palacios, R.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Alonso, M.J.; Miguel, M.; et al. Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: A concerted action of NAD(P)H oxidase and COX-2. Toxicology 2017, 390, 10–21. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Ayaz, A. Is aluminum exposure a risk factor for neurological disorders? J. Res. Med. Sci. 2018, 23, 51. [Google Scholar]
- Crépeaux, G.; Eidi, H.; David, M.O.; Baba-Amer, Y.; Tzavara, E.; Giros, B.; Authier, F.J.; Exley, C.; Shaw, C.A.; Cadusseau, J.; et al. Non-linear dose-response of aluminum hydroxide adjuvant particles: Selective low dose neurotoxicity. Toxicology 2017, 375, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. The identification of aluminum in human brain tissue using lumogallion and fluorescence microscopy. J. Alzheimers. Dis. 2016, 54, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, C.; Zhu, Y.; Sun, H.; Li, Y.; Zhang, Z. Effects of aluminum exposure on bone mineral density, mineral, and trace elements in rats. Biol. Trace. Elem. Res. 2011, 143, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Justin-Thenmozhi, A.; Bharathi, M.D.; Kiruthika, R.; Manivasagam, T.; Borah, A.; Essa, M.M. Attenuation of aluminum chloride-induced neuroinflammation and caspase activation through the AKT-GSK-3 beta pathway by hesperidin in wistar rats. Neurotox. Res. 2018, 34, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.; Safar, M.M.; Abdelsalam, R.M.; Zaki, H.F. Telmisartan protects against aluminum-induced Alzheimer-like pathological changes in rats. Neurotox. Res. 2020, 37, 275–285. [Google Scholar] [CrossRef]
- McLachlan, D.R.C.; Bergeron, C.; Alexandrov, P.N.; Walsh, W.J.; Pogue, A.L.; Percy, M.E.; Kruck, T.P.A.; Fang, Z.D.; Sharfman, N.M.; Jaber, V.; et al. Aluminum in neurological and neurodegenerative disease. Mol. Neurobiol. 2019, 56, 1531–1538. [Google Scholar] [CrossRef]
- Hill, J.M.; Percy, M.E.; Lukiw, W.J. Early insight into the potential contribution of aluminum to neurodegeneration—A tribute to the research work of Robert D. Terry, Igor Klatzo, Henryk M. Wisniewski and Donald R.C. Mclachlan. J. Inorg. Biochem. 2020, 203, 110860. [Google Scholar] [CrossRef] [PubMed]
- The Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Thirty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. 1989. Available online: https://apps.who.int/iris/bitstream/handle/10665/39252/WHO_TRS_776.pdf?sequence=1&isAllowed=y (accessed on 21 November 2020).
- The Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Sixty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/43592/WHO_TRS_940_eng.pdf?sequence=1&isAllowed=y (accessed on 21 November 2020).
- The Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives. 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/44788/WHO_TRS_966_eng.pdf?sequence=1&isAllowed=y (accessed on 21 November 2020).
- Poirier, J.; Semple, H.; Davies, J.; Lapointe, R.; Dziwenka, M.; Hiltz, M.; Mujibi, D. Double-blind, vehicle-controlled randomized twelve-month neurodevelopmental toxicity study of common aluminum salts in the rat. Neuroscience 2011, 193, 338–362. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Li, K.; Ma, J.; Liu, F.; Dai, J.J.; Li, H.B. Aluminum content of some processed foods, raw materials and food additives in China by inductively coupled plasma-mass spectrometry. Food Addit. Contam. B 2011, 4, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, S.; Tian, M.; Wang, L.; Chen, B.; Wu, M.; He, G.S. Dietary exposure to aluminum form wheat flour and puffed products of residents in Shanghai, China. Food. Addit. Contam. A 2015, 32, 2018–2026. [Google Scholar]
- Fekete, V.; Vandevijvere, S.; Bolle, F.; van Loco, J. Estimation of dietary aluminum exposure of the Belgian adult population: Evaluation of contribution of food and kitchenware. Food. Chem. Toxicol. 2013, 55, 602–608. [Google Scholar] [CrossRef]
- Wong, W.W.K.; Chung, S.W.C.; Kwong, K.P.; Ho, Y.Y.; Xiao, Y. Dietary exposure to aluminum of the Hong Kong population. Food Addit. Contam. Part A Chem. 2010, 27, 457–463. [Google Scholar] [CrossRef]
- González-Weller, D.; Gutiérrez, A.J.; Rubio, C.; Revert, C.; Hardission, A. Dietary intake of aluminum in a Spanish population (Canary Islands). J. Agric. Food Chem. 2010, 58, 10452–10457. [Google Scholar] [CrossRef]
- Yang, M.; iang, L.; Huang, H.; Zeng, S.; Qiu, F.; Yu, M.; Li, X.R.; Wei, S. Dietary exposure to aluminum and health risk assessment in the residents of Shenzhen, China. PLoS ONE 2014, 9, e89715. [Google Scholar]
- Sato, K.; Suzuki, I.; Kubota, H.; Furusho, N.; Inoue, T.; Yasukouchi, Y.; Akiyama, H. Estimation of daily aluminum intake in Japan based on food consumption inspection results: Impact of food additives. Food Sci. Nutr. 2014, 2, 389–397. [Google Scholar] [CrossRef]
- Ma, N.; Liu, Z.P.; Yang, D.J.; Liang, J.; Zhu, J.H.; Xu, H.B.; Li, F.Q.; Li, N. Risk assessment of dietary exposure to aluminum in the Chinese population. Food Addit. Contam. Part A Chem. 2016, 33, 1557–1562. [Google Scholar] [CrossRef]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Cilloni, S.; Malavolti, M.; Violi, F.; Vescovi, L.; Bargellini, A.; Vinceti, M. Aluminum and tin: Food contamination and dietary intake in an Italian population. J. Trace Elem. Med. Biol. 2019, 52, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.S.; Liu, Y.T.; Liou, P.J.; Li, H.P.; Chen, C.C. Investigation of aluminum content of imported candies and snack foods in Taiwan. J. Food Drug Anal. 2016, 24, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S. Risk Assessment of Dietary Intake of Aluminum-Rich Foods in Taiwan. Master’s Thesis, China Medical University, Taichung, China, 2014. [Google Scholar]
- Jiang, Q.; Wang, J.; Li, M.; Liang, X.; Dai, G.; Hu, Z.; Wen, J.; Huang, Q.; Zhang, Y. Dietary exposure to aluminum of urban residents from cities in South China. Food Addit. Contam. Part A Chem. 2013, 30, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Taiwan National Food Consumption Database (TNFCD). 2017. Available online: http://tnfcds.cmu.edu.tw/index.php?action=index (accessed on 21 November 2020).
- You, S.H.; Wang, S.L.; Pan, W.H.; Chan, W.C.; Fan, A.M.; Lin, P. Risk assessment of methylmercury based on internal exposure and fish and seafood consumption estimates in Taiwanese children. Int. J. Hyg. Environ. Health. 2018, 221, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, X.; Wu, S.; Hua, H.; Wang, Q.; Zhang, Z. Dietary exposure to aluminum in the popular Chinese fried bread youtiao. Food Addit. Contam. Part A Chem. 2017, 34, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Corkins, M.R.; Committee on Nutrition. Aluminum effects in infants and children. Pediatrics 2019, 144, e20193148. [Google Scholar] [CrossRef] [PubMed]
- Tietz, T.; Lenzner, A.; Kolbaum, A.E.; Zellmer, S.; Riebeling, C.; Gürtler, R.; Jung, C.; Kappenstein, O.; Tentschert, J.; Giulbudagian, M.; et al. Aggregated aluminium exposure: Risk assessment for the general population. Arch. Toxicol. 2019, 93, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.H.; Andersen, J.H.; Petersen, A.; Christensen, T. Dietary exposure assessment of Danish consumers to dithiocarbamate residues in food: A comparison of the deterministic and probabilistic approach. Food Addit. Contam A. 2008, 25, 714–721. [Google Scholar] [CrossRef]
- Morgano, M.A.; Arisseto-Bragotto, A.P.; de Paiva, E.L.; Milani, R.F. Aluminum in infant formulas commercialized in Brazil: Occurrence and exposure assessment. J. Food Compos. Anal. 2019, 82, 1–6. [Google Scholar]
- Chau, C.F.; Wu, S.H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends. Food Sci. Technol. 2006, 17, 313–323. [Google Scholar] [CrossRef]
- Chen, C.Y. Elemental analysis of the Taiwanese health food Angelica keiskei by INAA. J. Radioanal. Nucl. Chem. 2002, 252, 551–558. [Google Scholar] [CrossRef]
- Durgnat, J.M.; Heuser, J.; Andrey, D.; Prttin, C. Quality and safety assessment of ginseng extracts by determination of the contents of pesticides and metals. Food Addit. Contam. Part A Chem. 2005, 22, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Frankova, A.; Drabek, O.; Szakova, J.; Ash, C.; Kokoska, L. Aluminum and other elements in selected herbal tea plant species and their infusions. Food Chem. 2013, 139, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Liu, C.Y. Chelating resin sorption followed by supercritical fluid extraction and liquid chromatographic determination of aluminum in liquid samples. Analytica. Chimica. Acta. 2003, 494, 125–132. [Google Scholar] [CrossRef]
- Chang, C.T.; You, C.F.; Aggarwal, S.K.; Chung, C.H.; Chao, H.C.; Liu, H.C. Boron and strontium isotope ratios and major/trace elements concentrations in tea leaves at four major tea growing gardens in Taiwan. Environ. Geochem. Health. 2016, 38, 737–748. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Das, S.; da Silva, E.B.; Gao, P.; Gress, J.; Liu, Y.; Ma, L.Q. Metal concentrations in traditional and herbal teas and their potential risks to human health. Sci. Total Environ. 2018, 633, 649–657. [Google Scholar] [CrossRef]
- Troisi, J.; Richards, S.; Symes, S.; Ferretti, V.; di Maio, A.; Amoresano, A.; Daniele, B.; Aliberti, F.; Guida, M.; Trifuoggi, M. A comparative assessment of metals and phthalates in commercial tea infusions: A starting point to evaluate their tolerance limits. Food. Chem. 2019, 288, 193–200. [Google Scholar] [CrossRef]
- World Health Organization. Aluminum in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Daouk, S.E.L.; Pineau, A.; Taha, M.; Ezzeddine, R.; Hijazi, A.; Iskandarani, M.A.I. Aluminum exposure from food in the population of Lebanon. Toxicol. Rep. 2020, 7, 1025–1031. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of aluminium sulphates (E 520–523) and sodium aluminium phosphate (E 541) as food additives. EFSA J. 2018, 16, e05372. [Google Scholar]
- Keavency, K.; Murphy, A.; Jaiswal, A.K.; Jaiswal, S. Aluminum content of selected foods and beverages available in Irish market. JFCN 2020, 6, 150–158. [Google Scholar]
- Micha, R.; Coates, J.; Leclercq, C.; Charrondiere, U.R.; Mozaffarian, D. Global dietary surveillance: Data gaps and challenges. Food. Nutr. Bull. 2018, 39, 175–205. [Google Scholar] [CrossRef] [PubMed]
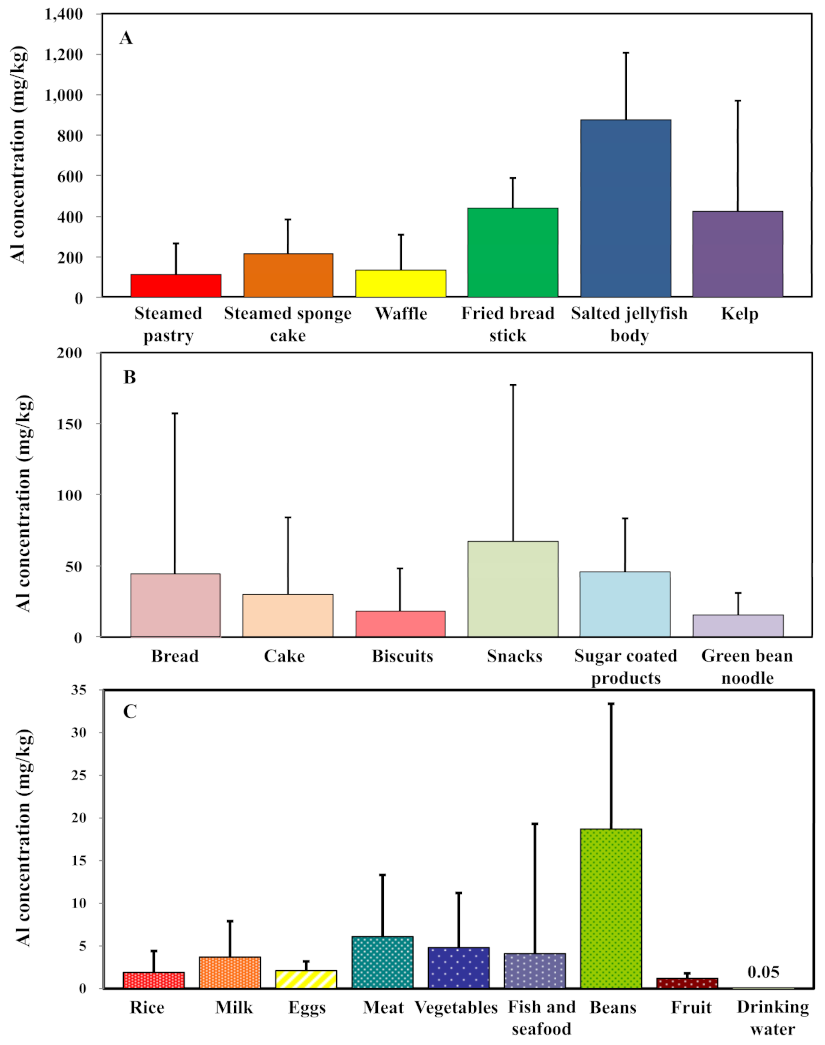

| Food Items | Sample Size | Range | P50 | P95 | Mean | SD |
|---|---|---|---|---|---|---|
| 1. Steamed pastry a | 17 | ND–497.2 | 53.4 | 399.7 | 114 | 152.6 |
| 2. Steamed sponge cake a | 15 | ND–628.1 | 230.3 | 456 | 217.3 | 169 |
| 3. Bread a | 13 | ND–415.6 | 7.4 | 191.3 | 44.5 | 112.6 |
| 4. Cake a* | 12 | ND–178.7 | 4.5 | 130.6 | 30.1 | 53.7 |
| 5. Waffle * | 25 | ND–535.2 | 27.3 | 485.7 | 132 | 177.4 |
| 6. Biscuits a | 14 | ND–112.3 | 4.8 | 63.9 | 18.3 | 30 |
| 7. Fried bread stick a | 14 | ND–608.1 | 462.1 | 607.3 | 440.9 | 150.1 |
| 8. Snacks a | 13 | ND–414.6 | 31.4 | 232.2 | 67.5 | 110 |
| 9. Salted jellyfish body a | 10 | ND–1389.6 | 785.8 | 1337.2 | 876.6 | 328.2 |
| 10. Kelp a | 10 | ND–1239.5 | 12.4 | 1163.2 | 424.5 | 547.1 |
| 11. Sugar coated products b | 53 | ND–94.7 | 39.5 | 88.7 | 45.7 | 37.7 |
| 12. Green bean noodle a | 5 | ND–36.2 | 12 | 34.1 | 15.2 | 15.5 |
| 13. Rice c | 55 | 0.1–18.3 | 1.2 | - | 1.9 | 2.5 |
| 14. Milk c | 23 | 0.2–15.2 | 1.9 | - | 3.7 | 4.2 |
| 15. Eggs c | 23 | 0.4–4.2 | 1.8 | - | 2.1 | 1.1 |
| 16. Meat c | 23 | 0.3–26.8 | 4.0 | - | 6.1 | 7.2 |
| 17. Vegetables c | 284 | 0.0–61.3 | 2.6 | - | 4.8 | 6.4 |
| 18. Fish and seafood c | 74 | 0.0–122.7 | 1.2 | - | 4.1 | 15.2 |
| 19. Beans c | 23 | 3.2–48.5 | 15.4 | - | 18.7 | 14.7 |
| 20. Fruit c | 4 | 0.6–2.0 | 1.2 | - | 1.2 | 0.6 |
| 21. Drinking water d | 184 | ND–0.118 | 0.052 | - | - | - |
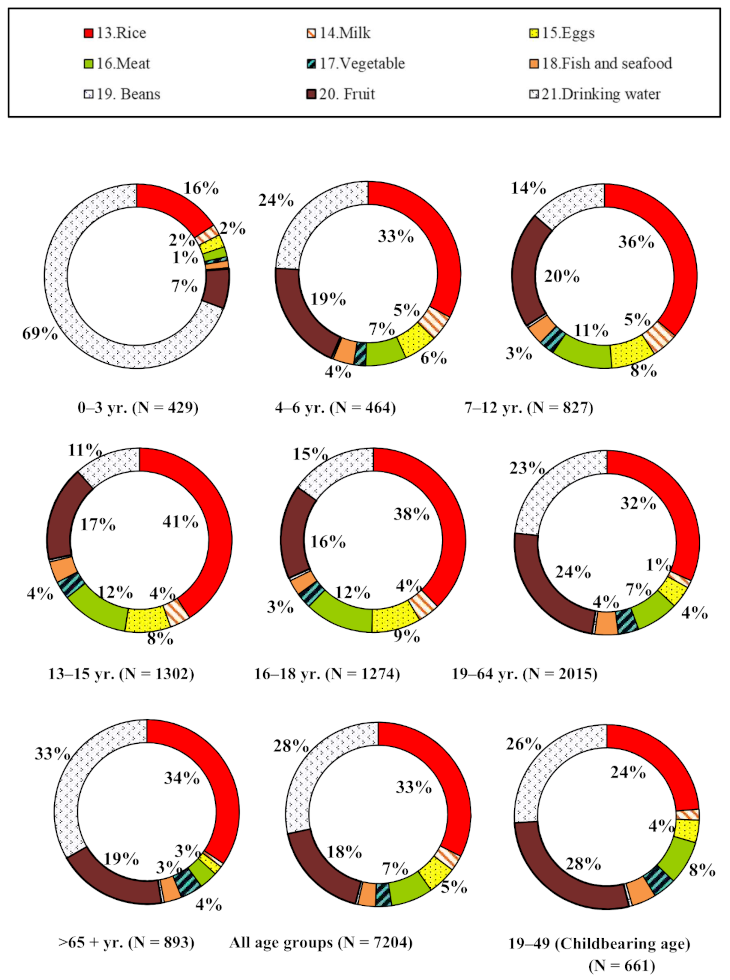
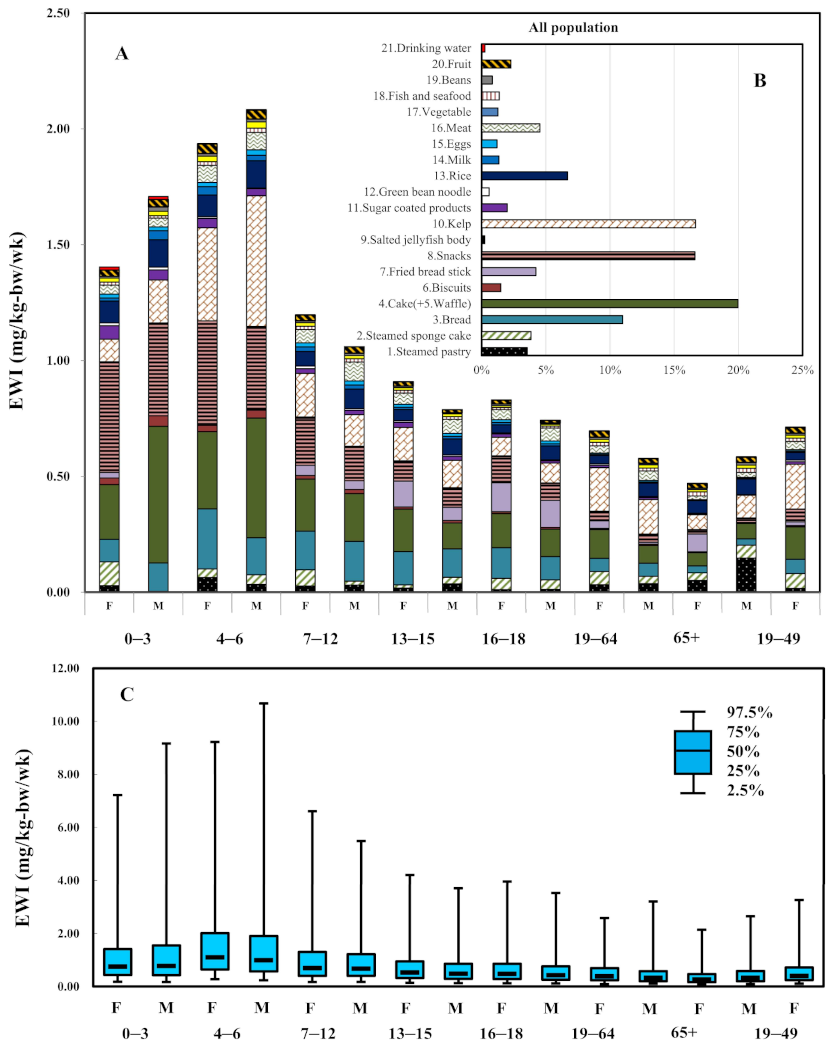
| Food Items | 0–3 | 4–6 | 7–12 | 13–15 | 16–18 | 19–64 | 65+ | Average across Age-Sex Subgroups | Percentage (%) | 19–49 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |||
| 1.Steamed pastry | 0.028 | 0.003 | 0.064 | 0.034 | 0.027 | 0.031 | 0.018 | 0.036 | 0.011 | 0.012 | 0.033 | 0.037 | 0.051 | 0.148 | 0.038 | 3.56% | 0.016 |
| 2.Steamed sponge cake | 0.103 | 0.000 | 0.036 | 0.041 | 0.071 | 0.017 | 0.014 | 0.029 | 0.049 | 0.041 | 0.057 | 0.031 | 0.033 | 0.055 | 0.041 | 3.84% | 0.064 |
| 3.Bread | 0.097 | 0.123 | 0.259 | 0.160 | 0.166 | 0.172 | 0.144 | 0.122 | 0.132 | 0.101 | 0.057 | 0.057 | 0.029 | 0.027 | 0.118 | 10.99% | 0.063 |
| 4.Cake(+5.Waffle) | 0.236 | 0.590 | 0.333 | 0.517 | 0.225 | 0.207 | 0.183 | 0.112 | 0.147 | 0.119 | 0.124 | 0.077 | 0.057 | 0.067 | 0.214 | 19.97% | 0.139 |
| 6.Biscuits | 0.028 | 0.046 | 0.028 | 0.034 | 0.016 | 0.017 | 0.009 | 0.011 | 0.009 | 0.007 | 0.005 | 0.006 | 0.004 | 0.004 | 0.016 | 1.50% | 0.005 |
| 7.Fried bread stick | 0.024 | 0.000 | 0.000 | 0.001 | 0.044 | 0.038 | 0.111 | 0.057 | 0.125 | 0.117 | 0.032 | 0.008 | 0.077 | 0.000 | 0.045 | 4.23% | 0.016 |
| 8.Snacks | 0.477 | 0.400 | 0.451 | 0.360 | 0.203 | 0.144 | 0.084 | 0.081 | 0.113 | 0.073 | 0.040 | 0.033 | 0.016 | 0.017 | 0.178 | 16.62% | 0.054 |
| 9.Salted jellyfish body | 0.000 | 0.000 | 0.000 | 0.000 | 0.006 | 0.005 | 0.004 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.24% | 0.001 |
| 10.Kelp | 0.097 | 0.185 | 0.402 | 0.565 | 0.188 | 0.136 | 0.144 | 0.118 | 0.080 | 0.086 | 0.187 | 0.149 | 0.063 | 0.098 | 0.178 | 16.66% | 0.193 |
| 11.Sugar coated products | 0.059 | 0.044 | 0.040 | 0.031 | 0.020 | 0.019 | 0.022 | 0.017 | 0.014 | 0.010 | 0.010 | 0.009 | 0.002 | 0.002 | 0.021 | 1.99% | 0.011 |
| 12.Green bean noodle | 0.012 | 0.012 | 0.007 | 0.002 | 0.012 | 0.007 | 0.006 | 0.006 | 0.005 | 0.004 | 0.006 | 0.004 | 0.005 | 0.001 | 0.006 | 0.59% | 0.007 |
| 13.Rice | 0.094 | 0.118 | 0.094 | 0.119 | 0.062 | 0.085 | 0.049 | 0.068 | 0.035 | 0.060 | 0.037 | 0.059 | 0.055 | 0.068 | 0.072 | 6.69% | 0.034 |
| 14.Milk | 0.013 | 0.039 | 0.036 | 0.023 | 0.020 | 0.018 | 0.009 | 0.012 | 0.010 | 0.009 | 0.004 | 0.004 | 0.002 | 0.003 | 0.014 | 1.34% | 0.005 |
| 15.Eggs | 0.017 | 0.016 | 0.018 | 0.024 | 0.018 | 0.018 | 0.013 | 0.013 | 0.012 | 0.013 | 0.006 | 0.007 | 0.003 | 0.004 | 0.013 | 1.20% | 0.006 |
| 16.Meat | 0.039 | 0.038 | 0.074 | 0.075 | 0.058 | 0.081 | 0.049 | 0.060 | 0.043 | 0.055 | 0.031 | 0.041 | 0.015 | 0.021 | 0.049 | 4.55% | 0.035 |
| 17.Vegetable | 0.013 | 0.010 | 0.016 | 0.018 | 0.014 | 0.015 | 0.012 | 0.012 | 0.009 | 0.009 | 0.016 | 0.013 | 0.018 | 0.017 | 0.014 | 1.28% | 0.015 |
| 18.Fish and seafood | 0.017 | 0.019 | 0.024 | 0.027 | 0.016 | 0.014 | 0.011 | 0.012 | 0.007 | 0.008 | 0.013 | 0.014 | 0.009 | 0.014 | 0.015 | 1.37% | 0.013 |
| 19.Beans | 0.008 | 0.018 | 0.011 | 0.012 | 0.008 | 0.010 | 0.007 | 0.005 | 0.008 | 0.005 | 0.009 | 0.006 | 0.007 | 0.011 | 0.009 | 0.84% | 0.008 |
| 20.Fruit | 0.027 | 0.033 | 0.041 | 0.038 | 0.025 | 0.028 | 0.019 | 0.013 | 0.017 | 0.011 | 0.027 | 0.020 | 0.019 | 0.024 | 0.024 | 2.28% | 0.025 |
| 21.Drinking water | 0.013 | 0.013 | 0.002 | 0.002 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.003 | 0.26% | 0.001 |
| Total | 1.404 | 1.708 | 1.937 | 2.083 | 1.198 | 1.060 | 0.909 | 0.788 | 0.830 | 0.742 | 0.697 | 0.578 | 0.471 | 0.585 | 1.071 | 0.713 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.-H.; Chen, S.-C.; Lin, C.-H.; Chen, Y.-C. Probabilistic Risk Analysis to Assess Dietary Exposure to Aluminum in the Taiwanese Population. Int. J. Environ. Res. Public Health 2021, 18, 1099. https://doi.org/10.3390/ijerph18031099
You S-H, Chen S-C, Lin C-H, Chen Y-C. Probabilistic Risk Analysis to Assess Dietary Exposure to Aluminum in the Taiwanese Population. International Journal of Environmental Research and Public Health. 2021; 18(3):1099. https://doi.org/10.3390/ijerph18031099
Chicago/Turabian StyleYou, Shu-Han, Szu-Chieh Chen, Chin-Hsin Lin, and Yen-Chu Chen. 2021. "Probabilistic Risk Analysis to Assess Dietary Exposure to Aluminum in the Taiwanese Population" International Journal of Environmental Research and Public Health 18, no. 3: 1099. https://doi.org/10.3390/ijerph18031099
APA StyleYou, S.-H., Chen, S.-C., Lin, C.-H., & Chen, Y.-C. (2021). Probabilistic Risk Analysis to Assess Dietary Exposure to Aluminum in the Taiwanese Population. International Journal of Environmental Research and Public Health, 18(3), 1099. https://doi.org/10.3390/ijerph18031099






