Multifactorial Predictors of Late Epileptic Seizures Related to Stroke: Evaluation of the Current Possibilities of Stratification Based on Existing Prognostic Models—A Comprehensive Review
Abstract
1. Introduction
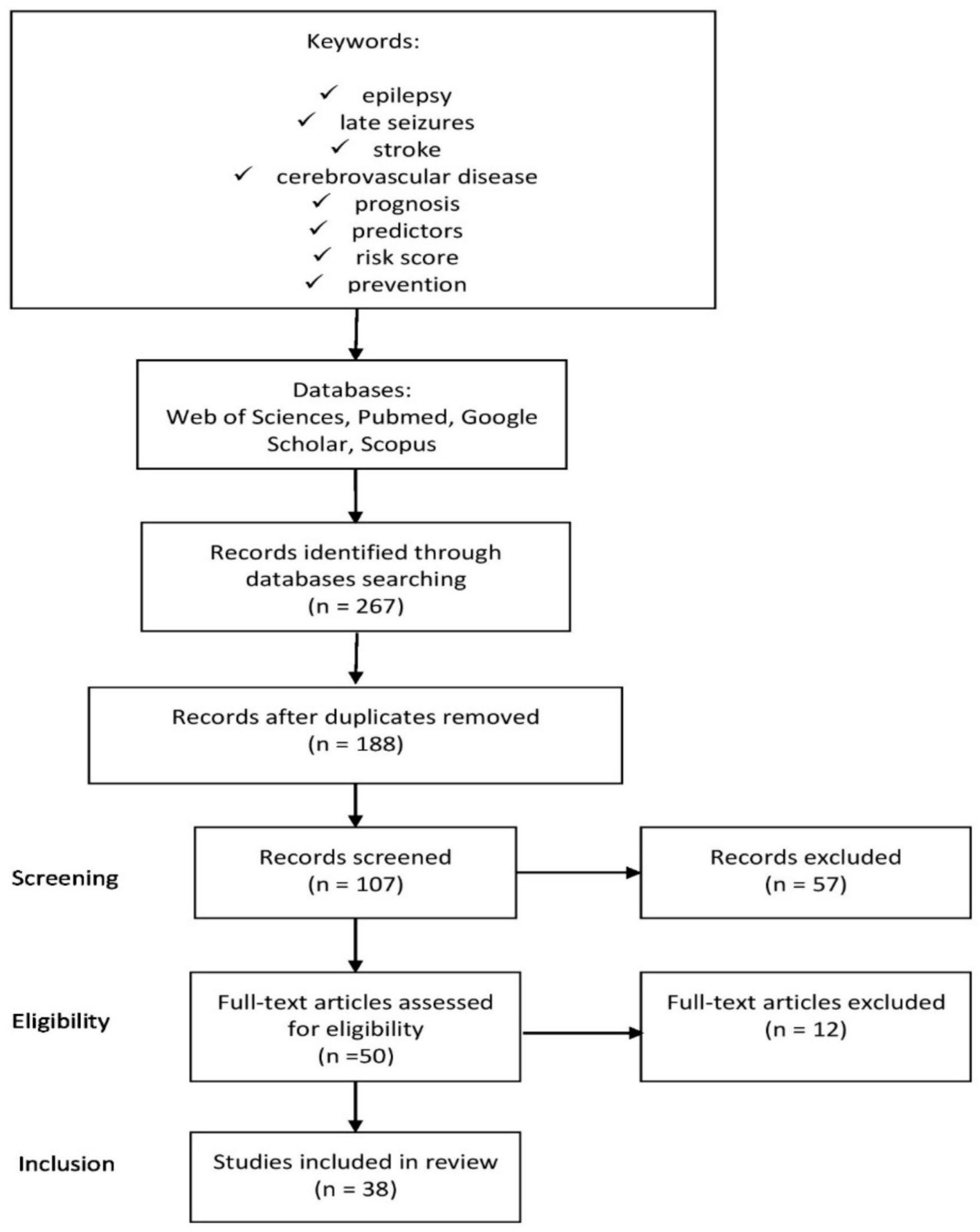
2. Materials and Methods
3. Predictors
3.1. Clinical Predictors
3.2. Radiological Predictors
3.3. Prognostic Models Based on Combinations of the Above Predictors
3.4. Meta-Analysis Related to Clinical and Radiological Predictors
3.5. Electroencephalographic Predictors
3.6. Genetic Predictors
3.7. Laboratory Predictors
3.8. Predictors Related to Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, T.F.; Rabinstein, A.A.; Middlebrooks, E.H.; Haranhalli, N.; Silliman, S.L.; Meschia, J.F.; Tawk, R.G. Diagnosis and management of acute ischemic stroke. Mayo Clin. Proc. 2018, 93, 523–538. [Google Scholar] [CrossRef]
- Krueger, H.; Koot, J.; Hall, R.E.; O’Callaghan, C.; Bayley, M.; Corbett, D. Prevalence of individuals experiencing the effects of stroke in Canada: Trends and projections. Stroke 2015, 46, 2226–2231. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Silverman, I.E.; Restrepo, L.; Mathews, G.C. Poststroke seizures. Arch. Neurol. 2002, 59, 195–201. [Google Scholar] [CrossRef]
- Marchi, N.; Granata, T.; Ghosh, C.; Janigro, D. Blood-brain barrier dysfunction and epilepsy: Pathophysiologic role and therapeutic approaches. Epilepsia 2012, 53, 1877–1886. [Google Scholar] [CrossRef]
- Pitkänen, A.; Lukasiuk, K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011, 10, 173–186. [Google Scholar] [CrossRef]
- Feyissa, A.M.; Hasan, T.F.; Meschia, J.F. Stroke-related epilepsy. Eur. J. Neurol. 2019, 26, 18-e3. [Google Scholar] [CrossRef]
- Huang, C.-W.; Saposnik, G.; Fang, J.; Steven, D.A.; Burneo, J.G. Influence of seizures on stroke outcomes: A large multicenter study. Neurology 2014, 82, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Hesdorffer, D.C.; Benn, E.K.; Cascino, G.D.; Hauser, W.A. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009, 50, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Burneo, J.G.; Fang, J.; Saposnik, G. Investigators of the Registry of the Canadian Stroke Network: Impact of seizures on morbidity and mortality after stroke: A Canadian multicentre cohort study. Eur. J. Neurol. 2010, 17, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bladin, C.F.; Alexandrov, A.V.; Bellavance, A.; Bornstein, N.; Chambers, B.; Cote, R.; Lebrun, L.; Pirisi, A.; Norris, J.W. Seizures after stroke: A prospective multicenter study. Arch. Neurol. 2000, 57, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.S.; Crichton, S.; Koutroumanidis, M.; Wolfe, C.D.; Rudd, A.G. Incidence and associations of poststroke epilepsy: The prospective South London Stroke Register. Stroke 2013, 44, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.; Pawlowski, M.; Dogan, M.; Kovac, S.; Ritter, M.A.; Evers, S. Seizures after cerebrovascular events: Risk factors and clinical features. Seizure 2013, 22, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kammersgaard, L.P.; Olsen, T.S. Poststroke epilepsy in the Copenhagen stroke study: Incidence and predictors. J. Stroke Cereb. Dis. 2005, 14, 210–214. [Google Scholar] [CrossRef] [PubMed]
- De Reuck, J.; Goethals, M.; Vonck, K.; van Maele, G. Clinical predictors of late-onset seizures and epilepsy in patients with cerebrovascular disease. Eur. Neurol. 2005, 54, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Tsoi, T.H.; Au-Yeung, M.; Tang, A.S. Epileptic seizure after stroke in Chinese patients. J. Neurol. 2003, 250, 839–843. [Google Scholar] [CrossRef]
- Roivainen, R.; Haapaniemi, E.; Putaala, J.; Kaste, M.; Tatlisumak, T. Young adult ischaemic stroke related acute symptomatic and late seizures: Risk factors. Eur. J. Neurol. 2013, 20, 1247–1255. [Google Scholar] [CrossRef]
- Wang, G.; Jia, H.; Chen, C.; Lang, S.; Liu, X.; Xia, C.; Sun, Y.; Zhang, J. Analysis of risk factors for first seizure after stroke in Chinese patients. BioMed Res. Int. 2013, 2013, 702871. [Google Scholar] [CrossRef]
- Serafini, A.; Gigli, G.L.; Gregoraci, G.; Janes, F.; Cancelli, I.; Novello, S.; Valente, M. Are early seizures predictive of epilepsy after a stroke? Results of a population-based study. Neuroepidemiology 2015, 45, 50–58. [Google Scholar] [CrossRef]
- Jungehulsing, G.J.; Heuschmann, P.U.; Holtkamp, M.; Schwab, S.; Kolominsky-Rabas, P.L. Incidence and predictors of post-stroke epilepsy. Acta Neurol. Scand. 2013, 127, 427–430. [Google Scholar] [CrossRef]
- Lossius, M.I.; Rønning, O.M.; Slapoe, G.D.; Mowinckel, P.; Gjerstad, L. Poststroke epilepsy: Occurrence and predictors. A long-term, prospective controlled study (Akershus stroke study). Epilepsia 2005, 46, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Churilov, L.; Koome, M.; Chen, Z.; Naylor, L.; Kwan, P.; Yan, B. Post-stroke seizures is associated with low alberta stroke program early CT score. Cereb. Dis. 2017, 43, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Takano, S.; Ueno, M.; Hamaguchi, H.; Kanda, F. Clinical features of late-onset poststroke seizures. J. Stroke Cereb. Dis. 2012, 21, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Strzelczyk, A.; Haag, A.; Raupach, H.; Herrendorf, G.; Hamer, H.M.; Rosenow, F. Prospective evaluation of a post-stroke epilepsy risk scale. J. Neurol. 2010, 257, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, E.; Strbian, D.; Rossi, C.; Putaala, J.; Sipi, T.; Mustanoja, S.; Sairanen, T.; Curtze, S.; Satopaa, J.; Roivainen, R.; et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014, 45, 1971–1976. [Google Scholar] [CrossRef]
- Kwon, S.J.; Obeidat, A.Z.; Sekar, P.; Moomaw, C.J.; Osborne, J.; Testai, F.D.; Koch, S.; Lowe, M.R.; Demel, S.; Coleman, E.R.; et al. Risk factors for seizures after intracerebral hemorrhage: Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) Study. Clin. Neurol. Neurosurg. 2020, 192, 105731. [Google Scholar] [CrossRef]
- Galovic, M.; Dohler, N.; Erdelyi-Canavese, B.; Felbecker, A.; Siebel, P.; Conrad, J.; Evers, S.; Winklerhner, M.; von Oertzen, T.J.; Haring, H.P.; et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): A multivariable prediction model development and validation study. Lancet Neurol. 2018, 17, 143–152. [Google Scholar] [CrossRef]
- Benbir, G.; Ince, B.; Bozluolcay, M. The epidemiology of post-stroke epilepsy according to stroke subtypes. Acta Neurologica Scandinavica 2006, 114, 8–12. [Google Scholar] [CrossRef]
- Ferlazzo, E.; Gasparini, S.; Beghi, E.; Sueri, C.; Russo, E.; Leo, A.; Labate, A.; Gambardella, A.; Belcastro, V.; Striano, P.; et al. Epilepsy in cerebrovascular diseases: Review of experimental and clinical data with meta-analysis of risk factors. Epilepsia 2016, 57, 1205–1214. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, Y.; Zhang, J.; Hu, W.; Ge, M.; Zhang, K.; Shao, X. Risk factors for post-stroke seizures: A systematic review and meta-analysis. Epilepsy Res. 2014, 108, 1806–1816. [Google Scholar] [CrossRef]
- Bentes, C.; Martins, H.; Peralta, A.R.; Morgado, C.; Casimiro, C.; Franco, A.C.; Fonseca, A.C.; Geraldes, R.; Canhao, P.; E Melo, T.P.; et al. Early EEG predicts poststroke epilepsy. Epilepsia Open 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- De Reuck, J.; Goethals, M.; Claeys, I.; van Maele, G.; De Clerk, M. EEG findings after a cerebral territorial infarct in patients who develop early-and late-onset seizures. Eur. Neurol. 2006, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Onder, H.; Arsava, E.M.; Topcuoglu, M.A.; Dericioglu, N. Do video-EEG monitoring findings in ICU patients with acute stroke predict development of seizures and survival during follow-up? Clin. EEG Neurosci. 2017, 48, 417–421. [Google Scholar] [CrossRef]
- Leone, M.A.; Tonini, M.C.; Bogliun, G.; Gionco, M.; Tassinari, T.; Bottacchi, E.; Beghi, E.; ARES Study Group. Risk factors for a first epileptic seizure after stroke: A case control study. J. Neurol. Sci. 2009, 277, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, Z.; Yang, G.P.; Zhang, B.K.; Chen, M.; Wu, T.; Guo, R. The ALDH2 rs671 polymorphism affects post-stroke epilepsy susceptibility and plasma 4-HNE levels. PLoS ONE 2014, 9, e109634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, M.; Yang, H.; Wu, T.; Song, C.; Guo, R. Evidence for involvement of the CD40/CD40L system in post-stroke epilepsy. Neurosci. Lett. 2014, 567, 6–10. [Google Scholar] [CrossRef]
- Abraira, L.; Santamarina, E.; Cazorla, S.; Bustamante, A.; Quintana, M.; Toledo, M.; Fonseca, E.; Grau-Lopez, E.; Jimenez, M.; Ciurans, J.; et al. Blood biomarkers predictive of epilepsy after an acute stroke event. Epilepsia 2020, 61, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Hobohm, C.; Zeynalova, S.; Classen, J.; Baum, P. Does treatment with t-PA increase the risk of developing epilepsy after stroke? J. Neurol. 2015, 262, 2364–2372. [Google Scholar] [CrossRef]
- Naylor, J.; Thevathasan, A.; Churilov, L.; Guo, R.; Xiong, Y.; Koome, M.; Chen, Z.; Chen, Z.; Liu, X.; Kwan, P.; et al. Association between different acute stroke therapies and development of post stroke seizures. BMC Neurol. 2018, 18, 61. [Google Scholar] [CrossRef]
- Bentes, C.; Martins, H.; Peralta, A.R.; Morgado, C.; Casimiro, C.; Franco, A.C.; Fonseca, A.C.; Geraldes, R.; Canhao, P.; E Melo, T.P.; et al. Epileptic manifestations in stroke patients treated with intravenous alteplase. Eur. J. Neurol. 2017, 24, 755–761. [Google Scholar] [CrossRef]
- Tan, M.L.; Ng, A.; Pandher, P.S.; Sashindranath, M.; Hamilton, J.A.; Davis, S.M.; O’Brien, T.J.; Medcalf, R.L.; Yan, B.; Jones, N.C. Tissue plasminogen activator does not alter development of acquired epilepsy. Epilepsia 2012, 53, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Nesselroth, D.; Gilad, R.; Namneh, M.; Avishay, S.; Eilam, A. Estimation of seizures prevalence in ischemic strokes after thrombolytic therapy. Seizure 2018, 62, 91–94. [Google Scholar] [CrossRef] [PubMed]
| Article (Year) [Reference] | Type | Sample Size | Follow-Up | Clinical Predictors | Radiological Findings (Predictors) |
|---|---|---|---|---|---|
| Graham et al. (2013) [12] | prospective | 3310 | 12 years | young age of <65 years TACI, subarachnoidal hemorrhage, intracranial hemorrhage, lower scores on Glasgow Coma Scale and Barthel Index | n/a |
| Conrad et al. (2013) [13] | cohort | 593 | 3.5 years | young age, severe initial clinical condition estimated by NIHSS, thrombotic (macroangiopathic) etiology of stroke, intracranial hemorrhage | no significant impact of multiple changes in neuroimaging, cortical involvement, anterior or posterior localization of stroke |
| Kammersgaard et al. (2005) [14] | prospective, observational | 1195 | 7 years | younger age, initial severe clinical status (measured by Scandinavian Stroke Scale), cerebral hemorrhage and incidence of early seizures after stroke | large lesion (increase by >1 cm in diameter) |
| Bladin et al. (2000) [11] | prospective, cohort | 1897 | 9 months | hemorrhagic stroke, initial stroke disability measured by Canadian Neurological Scale, long-term unfavorable functional outcome measured by modified Rankin Scale (mRS) | cortical location and larger size of ischemic focus in computed tomography |
| De Reuck et al. (2005) [15] | retrospective, observational | 476 | 4 years | PACI stroke | n/a |
| Cheung et al. (2003) [16] | retrospective | 1000 | 12 months | male sex, older age, partial anterior and total anterior circulation infarctions | cortical location and large lesion size |
| Roivainen et al. (2013) [17] | observational, cohort | 978 | 10 years | partial anterior and total anterior circulation infarctions, hemorrhagic infarct, male sex, hyperglycemia and history of early seizures after the onset of stroke, | n/a |
| Wang et al. (2013) [18] | retrospective, multicenter | 2474 | 2 years | older age among stroke subjects | cortical involvement and large lesion size (>3.5 cm in diameter) |
| Serafini et al. (2015) [19] | prospective | 782 | 2 years | younger age, early epileptic seizures (only among hemorrhagic stroke) | cortical involvement |
| Jungenhulsing et al. (2013) [20] | prospective | 1815 | 2 years | stroke severity (assessed on 5–7 days after the stroke onset on Barthel index) | n/a |
| Lossius et al. (2005) [21] | prospective | 484 | 8 years | stroke severity on admission (measured by Scandinavian Stroke Scale) | no significant impact of cortical involvement |
| Chen et al. (2017) [22] | prospective, cohort | 348 | 3 years | n/a | the extent of cerebral ischemia, measured by ASPECTS, cortical involvement at 24 h after the onset of stroke |
| Okuda et al. (2012) [23] | retrospective | 448 | 18 months | n/a | cortical localization of ischemic stroke and large infarcts involving middle cerebral artery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewski, A.; Jatužis, D. Multifactorial Predictors of Late Epileptic Seizures Related to Stroke: Evaluation of the Current Possibilities of Stratification Based on Existing Prognostic Models—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 1079. https://doi.org/10.3390/ijerph18031079
Wiśniewski A, Jatužis D. Multifactorial Predictors of Late Epileptic Seizures Related to Stroke: Evaluation of the Current Possibilities of Stratification Based on Existing Prognostic Models—A Comprehensive Review. International Journal of Environmental Research and Public Health. 2021; 18(3):1079. https://doi.org/10.3390/ijerph18031079
Chicago/Turabian StyleWiśniewski, Adam, and Dalius Jatužis. 2021. "Multifactorial Predictors of Late Epileptic Seizures Related to Stroke: Evaluation of the Current Possibilities of Stratification Based on Existing Prognostic Models—A Comprehensive Review" International Journal of Environmental Research and Public Health 18, no. 3: 1079. https://doi.org/10.3390/ijerph18031079
APA StyleWiśniewski, A., & Jatužis, D. (2021). Multifactorial Predictors of Late Epileptic Seizures Related to Stroke: Evaluation of the Current Possibilities of Stratification Based on Existing Prognostic Models—A Comprehensive Review. International Journal of Environmental Research and Public Health, 18(3), 1079. https://doi.org/10.3390/ijerph18031079







