Uterine Fibroids and Diet
Abstract
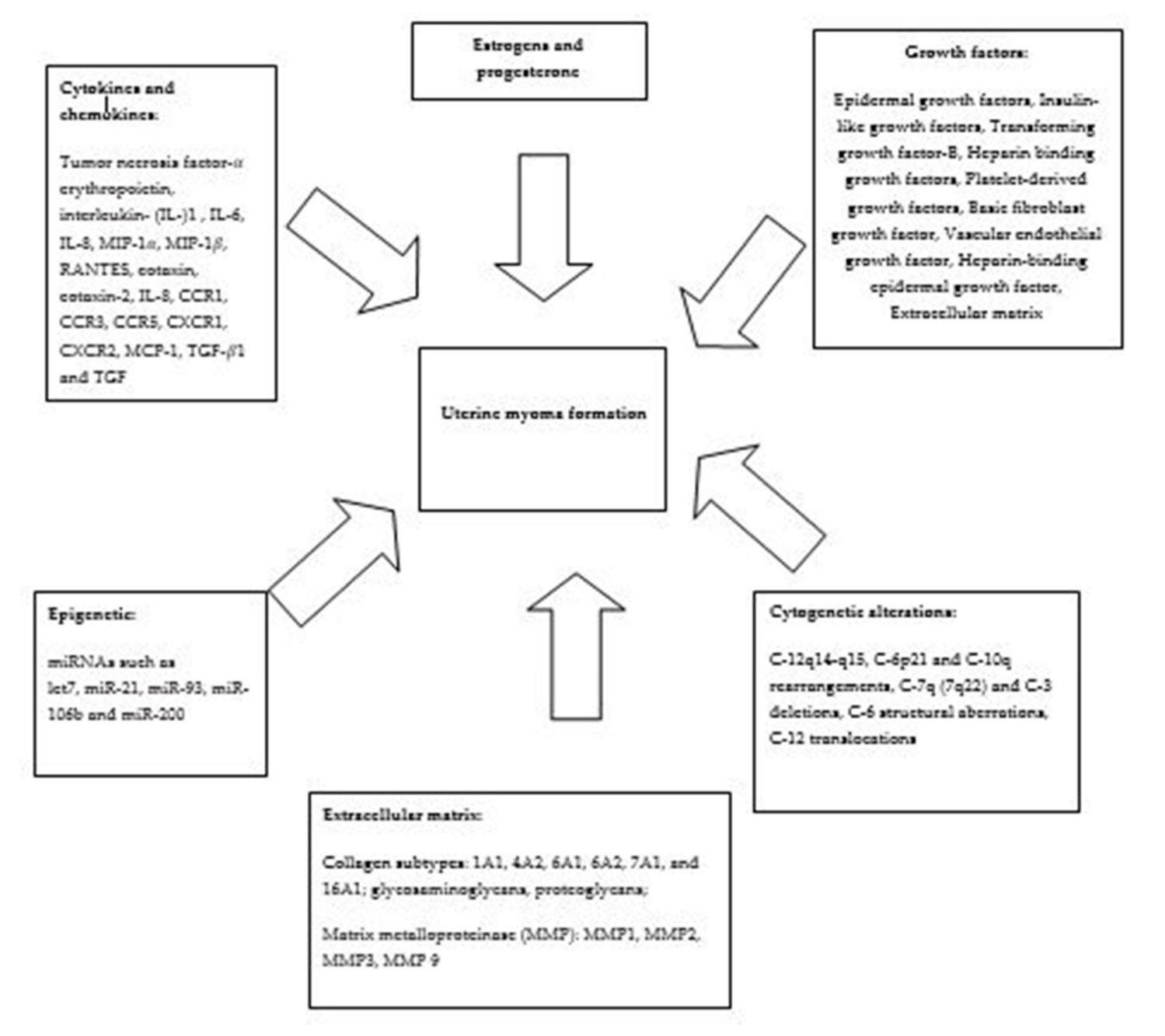
1. Introduction
2. Materials and Methods
3. Results
3.1. Glycemic Index, Dietary Fibers and Cereals Intake and Uterine Myoma
3.2. Soya Intake and Uterine Myoma
3.3. Dietary Fat, Meat and Fish Intake
3.4. Fruit and Vegetable Intake and Uterine Myoma Risk
3.5. Alcohol, Coffee, and Tea Consumption and Uterine Myoma
3.6. Dairy Products and Uterine Myoma
3.7. Vitamins and Uterine Myoma Risk
3.8. The Food Pollutants and Uterine Myoma Risk
3.9. Metaloestrogens and Uterine Myoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Drayer, S.M.; Catherino, W.H. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int. J. Gynecol. Obstet. 2015, 131, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.-M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Prim. 2016, 2, 16043. [Google Scholar] [CrossRef]
- Guo, X.C.; Segars, J.H. The Impact and Management of Fibroids for Fertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 521–533. [Google Scholar] [CrossRef]
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012, 206, 211.e1–211.e9. [Google Scholar] [CrossRef]
- Ciavattini, A.; Di Giuseppe, J.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, S.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine Fibroids: Pathogenesis and Interactions with Endometrium and Endomyometrial Junction. Obstet. Gynecol. Int. 2000, 108, 791–793. [Google Scholar] [CrossRef]
- Rein, M.S. Advances in Uterine Leiomyoma Research: The Progesterone Hypothesis. Environ. Health Perspect. 2000, 108, 791–793. [Google Scholar] [CrossRef]
- Richards, P.A.; Tiltman, A.J. Anatomical variation of the oestrogen receptor in the non-neoplastic myometrium of fibromyomatous uteri. Virchows Archiv. 1996, 428, 347–351. [Google Scholar] [CrossRef]
- Stewart, E.A.; Nowak, R.A. Leiomyoma-related bleeding: A classic hypothesis updated for the molecular era. Hum. Reprod. Updat. 1996, 2, 295–306. [Google Scholar] [CrossRef]
- Dixon, D.; Flake, G.P.; Moore, A.B.; He, H.; Haseman, J.K.; Risinger, J.I.; Lancaster, J.M.; Berchuck, A.; Barrett, C.J.; Robboy, S.J. Cell proliferation and apoptosis in human uterine leiomyomas and myometria. Virchows Archiv. 2002, 441, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lindner, V.; Reidy, M.A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA 1991, 88, 3739–3743. [Google Scholar] [CrossRef]
- Mangrulkar, R.S.; Ono, M.; Ishikawa, M.; Takashima, S.; Klagsbrun, M.; Nowak, R.A. Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol. Reprod. 1995, 53, 636–646. [Google Scholar] [CrossRef] [PubMed]
- 1Sparic, R.; Mirkovic, L.; Malvasi, A.; Tinelli, A. Epidemiology of Uterine Myomas: A Review. Int. J. Fertil. Steril. 2016, 9, 424–435. [Google Scholar] [CrossRef]
- Masala, G.; Assedi, M.; Bendinelli, B.; Ermini, I.; Sieri, S.; Grioni, S.; Sacerdote, C.; Ricceri, F.; Panico, S.; Mattiello, A.; et al. Fruit and vegetables consumption and breast cancer risk: The EPIC Italy study. Breast Cancer Res. Treat. 2012, 132, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Schulze, M.B.; Hu, F.B.; Hankinson, S.E.; Holmes, M.D. A dietary pattern derived to correlate with estrogens and risk of postmenopausal breast cancer. Breast Cancer Res. Treat. 2012, 132, 1157–1162. [Google Scholar] [CrossRef]
- Grassi, P.; Fattore, E.; Generoso, C.; Fanelli, R.; Arvati, M.; Zuccato, E. Polychlorobiphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in fruit and vegetables from an industrial area in northern Italy. Chemosphere 2010, 79, 292–298. [Google Scholar] [CrossRef]
- La Rocca, C.; Mantovani, A. From environment to food: The case of PCB. Ann. Ist. Super. Sanita 2006, 42, 410–416. [Google Scholar]
- Wallach, E.E.; Vlahos, N.F. Uterine Myomas: An Overview of Development, Clinical Features, and Management. Obstet. Gynecol. 2004, 104, 393–406. [Google Scholar] [CrossRef]
- He, Y.; Zeng, Q.; Dong, S.; Qin, L.; Li, G.; Wang, P. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: A case-control study in China. Asia Pac. J. Clin. Nutr. 2013, 22, 109–117. [Google Scholar] [CrossRef]
- Nagata, C.; Nakamura, K.; Oba, S.; Hayashi, M.; Takeda, N.; Yasuda, K. Association of intakes of fat, dietary fibre, soya isoflavones and alcohol with uterine fibroids in Japanese women. Br. J. Nutr. 2009, 101, 1427. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Kumanyika, S.K.; Boggs, D.A.; Rosenberg, L. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am. J. Clin. Nutr. 2011, 94, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Parazzyini, F.; Vecchial La, C.; Cheatenoud, L.; Di Cintio, E.; Marsico, S. Diet and Uterine Myomas. Obstet. Gynecol. 1999, 94, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Lampe, J.W.; Scholes, D.; Chen, C.; Wähälä, K.; Schwartz, S.M. Lignan and isoflavone excretion in relation to uterine fibroids: A case-control study of young to middle-aged women in the United States. Am. J. Clin. Nutr. 2006, 84, 587–593. [Google Scholar] [CrossRef]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Kumanyika, S.K.; Rosenberg, L. A Prospective Study of Dairy Intake and Risk of Uterine Leiomyomata. Am. J. Epidemiol. 2010, 171, 221–232. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Q.; Xu, J.; Ren, M.L.; Cai, Y.L. Environmental exposure and risk of uterine leiomyoma: An epidemiologic survey. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3249–3256. [Google Scholar]
- Gao, M.; Wang, H. Frequent milk and soybean consumption are high risks for uterine leiomyoma. Medicine 2018, 97, e12009. [Google Scholar] [CrossRef]
- Upson, K.; Harmon, Q.E.; Baird, D.D. Soy-Based Infant Formula Feeding and Ultrasound-Detected Uterine Fibroids among Young African-American Women with No Prior Clinical Diagnosis of Fibroids. Environ. Health Perspect. 2016, 124, 769–775. [Google Scholar] [CrossRef]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Rosenberg, L. Association of intrauterine and early life factors with uterine leiomyomata in black women. Ann. Epidemiol. 2012, 22, 847–854. [Google Scholar] [CrossRef] [PubMed]
- D’Aloisio, A.A.; Baird, D.D.; DeRoo, L.A.; Sandler, D.P. Association of Intrauterine and Early-Life Exposures with Diagnosis of Uterine Leiomyomata by 35 Years of Age in the Sister Study. Environ. Health Perspect. 2010, 118, 375–381. [Google Scholar] [CrossRef]
- D’Aloisio, A.A.; Baird, D.D.; DeRoo, L.A.; Sandler, D.P. Early-Life Exposures and Early-Onset Uterine Leiomyomata in Black Women in the Sister Study. Environ. Health Perspect. 2012, 120, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Abraham, K.; Appel, K.E.; Lampen, A. Risks and benefits of dietary isoflavones for cancer. Crit. Rev. Toxicol. 2011, 41, 463–506. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; Bethea, T.N.; Wesselink, A.K.; Wegienka, G.R.; Baird, D.D.; Wise, L.A. Dietary Fat Intake and Risk of Uterine Leiomyomata: A Prospective Ultrasound Study. Am. J. Epidemiol. 2020, 189, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Eliassen, A.H.; Doody, D.R.; Terry, K.L.; Missmer, S.A. Dietary fat intake, erythrocyte fatty acids, and risk of uterine fibroids. Fertil. Steril. 2020, 114, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Pike, M.C.; Stram, D.O. Meta-analysis: Dietary Fat Intake, Serum Estrogen Levels, and the Risk of Breast Cancer. JNCI J. Natl. Cancer Inst. 1999, 91, 529–534. [Google Scholar] [CrossRef]
- Wegienka, G. Are uterine leiomyoma a consequence of a chronically inflammatory immune system? Med. Hypotheses 2012, 79, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Pischon, T.; Hankinson, S.E.; Rifai, N.; Joshipura, K.; Willett, W.C.; Rimm, E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004, 79, 606–612. [Google Scholar] [CrossRef]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual Dietary Intake of n-3 and n-6 Fatty Acids in Relation to Inflammatory Markers Among US Men and Women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef]
- Islam, S.; Castellucci, C.; Fiorini, R.; Greco, S.; Gagliardi, R.; Zannotti, A.; Giannubilo, S.R.; Ciavattini, A.; Frega, N.G.; Pacetti, D.; et al. Omega-3 fatty acids modulate the lipid profile, membrane architecture, and gene expression of leiomyoma cells. J. Cell. Physiol. 2018, 233, 7143–7156. [Google Scholar] [CrossRef]
- Lambertino, A.; Turyk, M.; Anderson, H.; Freels, S.; Persky, V. Uterine leiomyomata in a cohort of Great Lakes sport fish consumers. Environ. Res. 2011, 111, 565–572. [Google Scholar] [CrossRef]
- Bredhult, C.; Bäcklin, B.-M.; Bignert, A.; Olovsson, M. Study of the relation between the incidence of uterine leiomyomas and the concentrations of PCB and DDT in Baltic gray seals. Reprod. Toxicol. 2008, 25, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, Y.; Lu, Q.; Ren, M. Vegetarian diet and reduced uterine fibroids risk: A case-control study in Nanjing, China. J. Obstet. Gynaecol. Res. 2016, 42, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Akhtar, M.M.; Ciavattini, A.; Giannubilo, S.R.; Protic, O.; Janjusevic, M.; Procopio, A.D.; Segars, J.H.; Castellucci, M.; Ciarmela, P. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: Promising options for prevention and treatment of uterine fibroids? Mol. Nutr. Food Res. 2014, 58, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Giampieri, F.; Janjusevic, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Greco, S.; Giannubilo, S.R.; Ciavattini, A.; Mezzetti, B.; et al. An anthocyanin rich strawberry extract induces apoptosis and ROS while decreases glycolysis and fibrosis in human uterine leiomyoma cells. Oncotarget 2017, 8, 23575–23587. [Google Scholar] [CrossRef]
- Giampieri, F.; Islam, S.; Greco, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Carpini, G.D.; Giannubilo, S.R.; Ciavattini, A.; Mezzetti, B.; Mazzoni, L.; et al. Romina: A powerful strawberry with in vitro efficacy against uterine leiomyoma cells. J. Cell. Physiol. 2019, 234, 7622–7633. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Mendoza, M.; Payson, M.; Catherino, W.H. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril. 2009, 91, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Tsuiji, K.; Takeda, T.; Li, B.; Wakabayashi, A.; Kondo, A.; Kimura, T.; Yaegashi, N. Inhibitory effect of curcumin on uterine leiomyoma cell proliferation. Gynecol. Endocrinol. 2010, 27, 512–517. [Google Scholar] [CrossRef]
- Greco, S.; Islam, S.; Zannotti, A.; Carpini, G.D.; Giannubilo, S.R.; Ciavattini, A.; Petraglia, F.; Ciarmela, P. Quercetin and indole-3-carbinol inhibit extracellular matrix expression in human primary uterine leiomyoma cells. Reprod. Biomed. Online 2020, 40, 593–602. [Google Scholar] [CrossRef]
- Sahin, K.; Ozercan, R.; Onderci, M.; Sahin, N.; Khachik, F.; Seren, S.; Kucuk, O. Dietary Tomato Powder Supplementation in the Prevention of Leiomyoma of the Oviduct in the Japanese Quail. Nutr. Cancer 2007, 59, 70–75. [Google Scholar] [CrossRef]
- Sahin, K.; Ozercan, R.; Onderci, M.; Sahin, K.; Gursu, M.F.; Khachik, F.; Sarkar, F.H.; Munkarah, A.; Ali-Fehmi, R.; Kmak, D.; et al. Lycopene Supplementation Prevents the Development of Spontaneous Smooth Muscle Tumors of the Oviduct in Japanese Quail. Nutr. Cancer 2004, 50, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Yang, Y.-C.; Chin, Y.-T.; Chou, S.-Y.; Chen, Y.-R.; Shih, Y.-J.; Whang-Peng, J.; Changou, C.A.; Liu, H.-L.; Lin, S.-J.; et al. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin αvβ3 and IGF-1R. Food Chem. Toxicol. 2018, 120, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, P.H.; Shih, Y.H.; Wang, K.L.; Hong, Y.H.; Shieh, T.M. Natural Antioxidant Resveratrol Suppresses Uterine Fibroid Cell Growth and Extracellular Matrix Formation In Vitro and In Vivo. Antioxidants 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum. Reprod. 2004, 19, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Templeman, C.; Marshall, S.F.; Clarke, C.A.; Henderson, K.D.; Largent, J.; Neuhausen, S.; Reynolds, P.; Ursin, G.; Bernstein, L. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil. Steril. 2009, 92, 1436–1446. [Google Scholar] [CrossRef]
- Hankinson, S.E.; Willett, W.C.; Manson, J.E.; Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Longcope, C.; Speizer, F.E. Alcohol, Height, and Adiposity in Relation to Estrogen and Prolactin Levels in Postmenopausal Women. J. Natl. Cancer Inst. 1995, 87, 1297–1302. [Google Scholar] [CrossRef]
- Wise, L.A. Reproductive Factors, Hormonal Contraception, and Risk of Uterine Leiomyomata in African-American Women: A Prospective Study. Am. J. Epidemiol. 2004, 159, 113–123. [Google Scholar] [CrossRef]
- Lucero, J.; Harlow, B.L.; Barbieri, R.L.; Sluss, P.; Cramer, D.W. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil. Steril. 2001, 76, 723–729. [Google Scholar] [CrossRef]
- Leonard, T.K.; Watson, R.R.; Mohs, M.E. The effects of caffeine on various body systems: A review. J. Am. Diet. Assoc. 1987, 87, 1048–1053. [Google Scholar]
- Spiller, G.A. Basic Metabolism and Physiological Effects of the Methylxanthines. In Caffeine; CRC Press: Boca Raton, FL, USA, 1998; pp. 225–231. [Google Scholar]
- Nowak, R.; Mora, S.; Diehl, T.; Rhoades, A.; Stewart, E. Prolactin Is an Autocrine or Paracrine Growth Factor for Human Myometrial and Leiomyoma Cells. Gynecol. Obstet. Investig. 1999, 48, 127–132. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 2010, 202, 289.e1–289.e9. [Google Scholar] [CrossRef]
- Ahmed, R.S.I.; Liu, G.; Renzetti, A.; Farshi, P.; Yang, H.; Soave, C.; Saed, G.; El-Ghoneimy, A.A.; El-Banna, H.A.; Foldes, R.; et al. Biological and Mechanistic Characterization of Novel Prodrugs of Green Tea Polyphenol Epigallocatechin Gallate Analogs in Human Leiomyoma Cell Lines. J. Cell. Biochem. 2016, 117, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Rajaratnam, V.; Al-Hendy, O.; Halder, S.; Al-Hendy, A. Green Tea Extract Inhibition of Human Leiomyoma Cell Proliferation Is Mediated via Catechol-O-Methyltransferase. Gynecol. Obstet. Investig. 2014, 78, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Women’s Health 2013, 5, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Parodi, P.W. Dairy Product Consumption and the Risk of Breast Cancer. J. Am. Coll. Nutr. 2005, 24, 556–568. [Google Scholar] [CrossRef]
- Jacobson, E.A.; James, K.A.; Newmark, H.L.; Carroll, K.K. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989, 49, 6300–6303. [Google Scholar]
- Terry, K.; Missmer, S.A.; Hankinson, S.E.; Willett, W.C.; De Vivo, I. Lycopene and other carotenoid intake in relation to risk of uterine leiomyomata. Am. J. Obstet. Gynecol. 2008, 198, 37.e1–37.e8. [Google Scholar] [CrossRef]
- Martin, C.L.; Huber, L.R.B.; Thompson, M.E.; Racine, E.F. Serum Micronutrient Concentrations and Risk of Uterine Fibroids. J. Women’s Health 2011, 20, 915–922. [Google Scholar] [CrossRef]
- Catherino, W.H.; Malik, M. Uterine leiomyomas express a molecular pattern that lowers retinoic acid exposure. Fertil. Steril. 2007, 87, 1388–1398. [Google Scholar] [CrossRef]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin D and the Risk of Uterine Fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Halder, S.K.; Allah, A.S.A.; Roshdy, E.; Rajaratnam, V.; Sabry, M. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Women’s Health 2013, 5, 93–100. [Google Scholar] [CrossRef]
- Mitro, S.D.; Zota, A.R. Vitamin D and uterine leiomyoma among a sample of US women: Findings from NHANES, 2001–2006. Reprod. Toxicol. 2015, 57, 81–86. [Google Scholar] [CrossRef]
- Paffoni, A.; Somigliana, E.; Vigano’, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Fedele, L. Vitamin D Status in Women With Uterine Leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef]
- Singh, V.; Barik, A.; Imam, N. Vitamin D3 Level in Women with Uterine Fibroid: An Observational Study in Eastern Indian Population. J. Obstet. Gynecol. India 2019, 69, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Oskovi Kaplan, Z.A.; Taşçi, Y.; Topçu, H.O.; Erkaya, S. 25-Hydroxy vitamin D levels in premenopausal Turkish women with uterine leiomyoma. Gynecol. Endocrinol. 2018, 34, 261–264. [Google Scholar] [CrossRef]
- Li, S.; Chen, B.; Sheng, B.; Wang, J.; Zhu, X. The associations between serum vitamin D, calcium and uterine fibroids in Chinese women: A case-controlled study. J. Int. Med. Res. 2020, 48, 030006052092349. [Google Scholar] [CrossRef]
- Srivastava, P.; Gupta, H.P.; Singhi, S.; Khanduri, S.; Rathore, B. Evaluation of 25-hydroxy vitamin D3 levels in patients with a fibroid uterus. J. Obstet. Gynaecol. 2020, 40, 710–714. [Google Scholar] [CrossRef]
- Ciavattini, A.; Carpini, G.D.; Serri, M.; Vignini, A.; Sabbatinelli, J.; Tozzi, A.; Aggiusti, A.; Clemente, N. Hypovitaminosis D and “small burden” uterine fibroids. Medicine 2016, 95, e5698. [Google Scholar] [CrossRef]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 Treatment Shrinks Uterine Leiomyoma Tumors in the Eker Rat Model1. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Diamond, M.P.; Boyer, T.G.; Halder, S.K. Vitamin D3 Inhibits Wnt/β-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells. J. Clin. Endocrinol. Metab. 2016, 101, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Diamond, M.P.; El-Sohemy, A.; Halder, S.K. 1,25-Dihydroxyvitamin D3 Regulates Expression of Sex Steroid Receptors in Human Uterine Fibroid Cells. J. Clin. Endocrinol. Metab. 2015, 100, e572–e582. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum. Reprod. 2013, 28, 2407–2416. [Google Scholar] [CrossRef]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. Hypovitaminosis D exacerbates the DNA damage load in human uterine fibroids, which is ameliorated by vitamin D3 treatment. Acta Pharmacol. Sin. 2019, 40, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.J.; Urbanetz, A.A. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assist. Reprod. 2019, 23, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, Q.; Ren, M.; Feng, X.; Cai, Y.; Gao, Y. Measurement of Phenolic Environmental Estrogens in Women with Uterine Leiomyoma. PLoS ONE 2013, 8, e79838. [Google Scholar] [CrossRef]
- Jeong, E.H.; Hong, G.Y.; Kim, B.R.; Park, S.N.; Lee, H.-H.; Na, Y.-J.; Namkung, J. The Relationship between Uterine Myoma Growth and the Endocrine Disruptor in Postmenopausal Women. J. Menopausal Med. 2013, 19, 130–134. [Google Scholar] [CrossRef]
- Han, M.S.; Byun, J.C.; Park, J.E.; Kim, J.Y.; Chung, J.Y.; Kim, J.M. Bisphenol-A Concentrations from Leiomyoma Patients by LC/MS. Toxicol. Res. 2011, 27, 49–52. [Google Scholar] [CrossRef]
- Weuve, J.; Hauser, R.; Calafat, A.M.; Missmer, S.A.; Wise, L.A. Association of Exposure to Phthalates with Endometriosis and Uterine Leiomyomata: Findings from NHANES, 1999–2004. Environ. Health Perspect. 2010, 118, 825–832. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kho, Y.; Chun, K.-C.; Koh, J.W.; Park, J.W.; Bunderson-Schelvan, M.; Cho, Y.H. Increased Urinary Phthalate Levels in Women with Uterine Leiomyoma: A Case-Control Study. Int. J. Environ. Res. Public Health 2016, 13, 1247. [Google Scholar] [CrossRef]
- Zota, A.R.; Geller, R.J.; Calafat, A.M.; Marfori, C.Q.; Baccarelli, A.A.; Moawad, G.N. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: A preliminary study. Fertil. Steril. 2019, 111, 112–121. [Google Scholar] [CrossRef]
- Zota, A.R.; Geller, R.J.; Vannoy, B.N.; Marfori, C.Q.; Tabbara, S.; Hu, L.Y.; Baccarelli, A.A.; Moawad, G.N. Phthalate Exposures and MicroRNA Expression in Uterine Fibroids: The FORGE Study. Epigenetics Insights 2019, 13, 251686572090405. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Diamond, M.P.; Al-Hendy, A. Early Life Adverse Environmental Exposures Increase the Risk of Uterine Fibroid Development: Role of Epigenetic Regulation. Front. Pharmacol. 2016, 7, 40. [Google Scholar] [CrossRef]
- Baird, D.D.; Newbold, R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod. Toxicol. 2005, 20, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Mahalingaiah, S.; Hart, J.E.; Wise, L.A.; Terry, K.L.; Boynton-Jarrett, R.; Missmer, S.A. Prenatal Diethylstilbestrol Exposure and Risk of Uterine Leiomyomata in the Nurses’ Health Study II. Am. J. Epidemiol. 2014, 179, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Rowlings, K.; Kaufman, R.H.; Herbst, A.L.; Noller, K.L.; Titus-Ernstoff, L.; Troisi, R.; Hatch, E.E.; Robboy, S.J. Risk of Benign Gynecologic Tumors in Relation to Prenatal Diethylstilbestrol Exposure. Obstet. Gynecol. 2005, 105, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, E.; Louis, G.M.B.; Parsons, P.J.; Steuerwald, A.J.; Palmer, C.D.; Chen, Z.; Sun, L.; Hammoud, A.O.; Dorais, J.; Peterson, C.M. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod. Toxicol. 2014, 49, 27–32. [Google Scholar] [CrossRef]
- Ye, S.; Chung, H.W.; Jeong, K.; Sung, Y.-A.; Lee, H.; Lee, H.; Kim, H.; Ha, E.H. Blood cadmium and volume of uterine fibroids in premenopausal women. Ann. Occup. Environ. Med. 2017, 29, 22. [Google Scholar] [CrossRef]
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Metals and Breast Cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 63–73. [Google Scholar] [CrossRef]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M.B. Activation of Estrogen Receptor-α by the Heavy Metal Cadmium. Mol. Endocrinol. 2000, 14, 545–553. [Google Scholar] [CrossRef]
- Jackson, L.W.; Zullo, M.D.; Goldberg, J.M. The association between heavy metals, endometriosis and uterine myomas among premenopausal women: National Health and Nutrition Examination Survey 1999-2002. Hum. Reprod. 2008, 23, 679–687. [Google Scholar] [CrossRef]
- He, Q.; Ma, R.; Tang, Y. Determination of trace element Cu, Zn, Mg, Cr in serum of women with barrenness and hysteromyoma disease. Guang Pu Xue Yu Guang Pu Fen Xi 2002, 22, 685–686. [Google Scholar] [PubMed]
- Tuzcu, M.; Sahin, N.; Ozercan, I.; Seren, S.; Sahin, K.; Kucuk, O. The Effects of Selenium Supplementation on the Spontaneously Occurring Fibroid Tumors of Oviduct, 8-Hydroxy-2′-Deoxyguanosine Levels, and Heat Shock Protein 70 Response in Japanese Quail. Nutr. Cancer 2010, 62, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.; Tuzcu, M.; Ozercan, İ.; Sahin, K.; Prasad, A.S.; Kucuk, O. Zinc Picolinate in the Prevention of Leiomyoma in Japanese Quail. J. Med. Food 2009, 12, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
| Food | Related Investigations | Influence on Uterine Myoma |
|---|---|---|
| Whole food | He, Y. et al. [20], Nagata, C. et al. [21], Wise, L.A. et al. [22], Chiaffarino, F. et al. [23] | No significant association |
| Soya | He, Y. et al. [20], Nagata, C. et al. [21], Atkinson, C. et al. [24], Wise, L.A. et al. [22] | No significant association |
| Shen, Y. et al. [26], Gao, M. et al. [27] | Increased the risk | |
| Dietary fat | He, Y. et al. [20], Nagata, C. et al. [21], Chiaffarino, F. et al. [23] | Increased the risk |
| Meat | Chiaffarino, F. et al. [23] | Increased the risk |
| He, Y. et al. [20] | No significant association | |
| Fish | Lambertino, A. et al. [40] | Increased the risk |
| Fruit | He, Y. et al. [20], Wise, L.A. et al. [22], Shen, Y. et al. [42] | Decreased the risk |
| Vegetables | He, Y. et al. [20], Shen, Y. et al. [42] | Decreased the risk |
| Alcohol | Wise, L.A. et al. [53], Templeman, C. et al. [54] | Increased the risk |
| He, Y. et al. [20], Chiaffarino F et al. [23] | No significant influence | |
| Coffee | Wise, L.A. et al. [53] | Increased the risk |
| Chiaffarino, F. et al. [23] | No significant influence | |
| Dairy products | Wise, L.A. et al. [25] | Decreased the risk |
| Gao, M. et al. [27] | Increased the risk |
| Natural Compounds | Influence on Uterine Myoma | Mechanism of Action |
|---|---|---|
| Strawberry extract Islam, M. et al. [44], Giampieri, F. et al. [45] | Increase apoptosis of uterine myoma cells | Increases oxygen species. Decreases extracellular acidification rate, activin collagen 1A1, fibronectin, versican mRNA expression |
| Curcumin Malik, M. et al. [46], Tsuiji, K. et al. [47] | Increase apoptosis of uterine myoma risk | Decreases fibronectin production, stimulating caspase-3 and caspase-9 expression; inhibits extracellular signal-regulated kinase 1 (ERK1) and ERK2 and nuclear factor kappa B (NF-κB); activates of peroxisome proliferator-activated receptor-γ |
| Quercetin and indole-3-carbinol Greco, S. et al. [48] | Antiproliferative effect | Reduces expression of fibronectin collagen 1A1 |
| Lycopene Sahin, K. et al. [49], Sahin, K. et al. [50] | Decreases size and incidence of uterine myoma | Modulates the expression of cell cycle regulatory proteins, modulates the IGF-1/IGFBP-3 system, up-regulates tumor suppressor protein Cx43, increases gap junctional intercellular communication, modulates re-dox signaling, prevents oxidative DNA damage, modulates carcinogen-metabolizing enzymes. |
| Resveratrol Ho, Y. et al. [51], Chen, H.Y. et al. [52] | Decreases myoma cell proliferation | Modulates cross-talk between integrin αvβ3 and IGF-1R, increases expression of cyclooxygenase (COX)-2, p21 and CDKN2, decreases expression of fibronectin, proliferates cell nuclear antigen α-smooth muscle actin, upregulates the ratio of Bcl-2-associated X and B-cell lymphoma/leukemia 2 in vivo collagen type 1 and α-SMA and protein levels of β-catenin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinelli, A.; Vinciguerra, M.; Malvasi, A.; Andjić, M.; Babović, I.; Sparić, R. Uterine Fibroids and Diet. Int. J. Environ. Res. Public Health 2021, 18, 1066. https://doi.org/10.3390/ijerph18031066
Tinelli A, Vinciguerra M, Malvasi A, Andjić M, Babović I, Sparić R. Uterine Fibroids and Diet. International Journal of Environmental Research and Public Health. 2021; 18(3):1066. https://doi.org/10.3390/ijerph18031066
Chicago/Turabian StyleTinelli, Andrea, Marina Vinciguerra, Antonio Malvasi, Mladen Andjić, Ivana Babović, and Radmila Sparić. 2021. "Uterine Fibroids and Diet" International Journal of Environmental Research and Public Health 18, no. 3: 1066. https://doi.org/10.3390/ijerph18031066
APA StyleTinelli, A., Vinciguerra, M., Malvasi, A., Andjić, M., Babović, I., & Sparić, R. (2021). Uterine Fibroids and Diet. International Journal of Environmental Research and Public Health, 18(3), 1066. https://doi.org/10.3390/ijerph18031066







