Challenges and Adaptation of a European Influenza Vaccine Effectiveness Study Platform in Response to the COVID-19 Emergence: Experience from the DRIVE Project
Abstract
1. Introduction
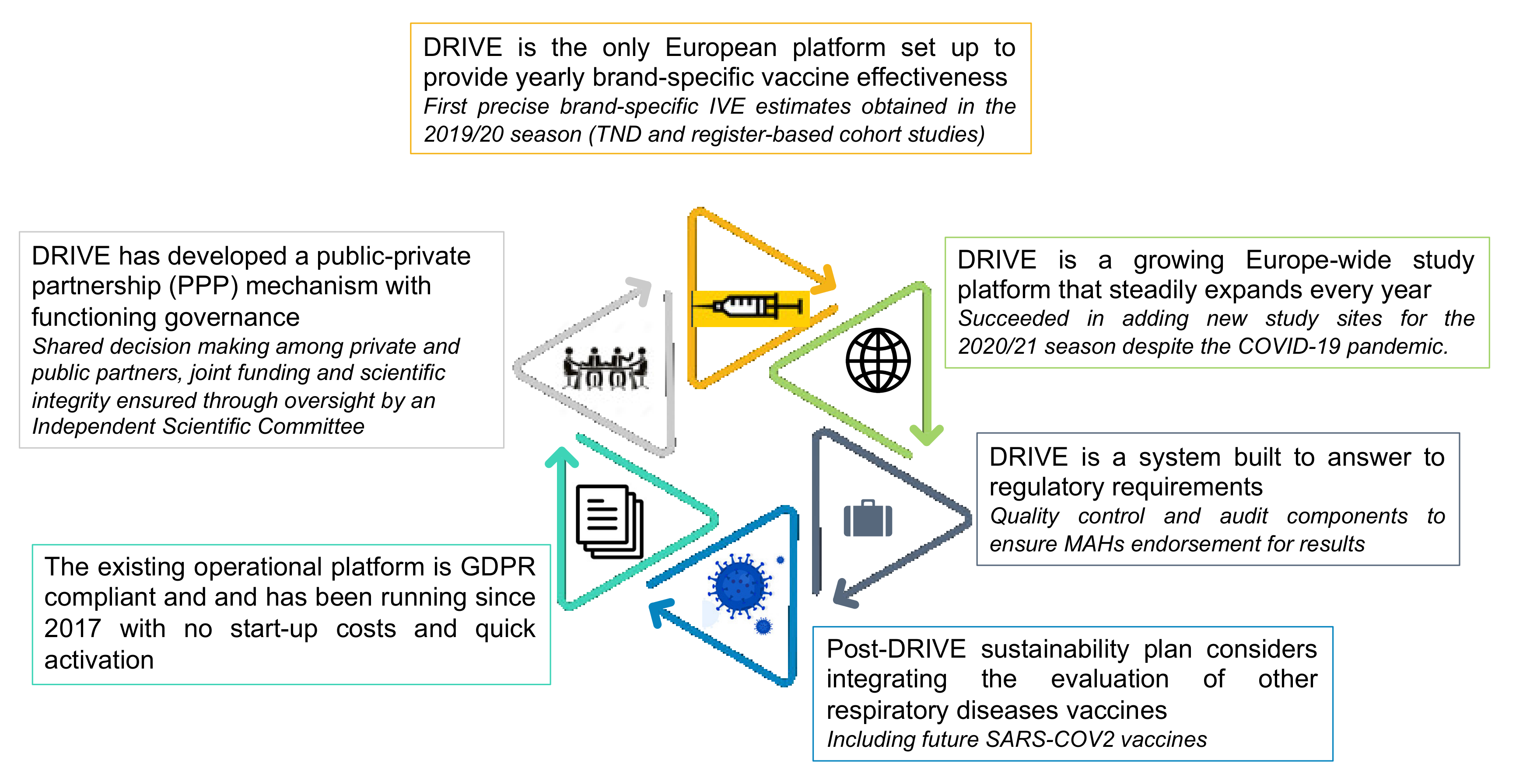
1.1. DRIVE Project, a Public–Private Partnership to Monitor Influenza Vaccine Effectiveness
1.2. DRIVE Influenza Vaccine Effectiveness Studies through the Years
2. New Challenges Encountered at DRIVE Study Platform Level Due to COVID-19 Pandemic
2.1. DRIVE IVE Study Platform and COVID-19: Actions Taken in the 2019/2020 Season
2.2. DRIVE Study Sites Experience: Management of the DRIVE Study during the COVID-19 Pandemic
2.2.1. Interuniversity Research Center on Influenza and Other Transmissible Infections (CIRI-IT, Italy)
2.2.2. Helsinki University Hospital, Jorvi Hospital (HUS, Finland)
3. Anticipating the Impact of COVID-19 on the IVE Studies for the Upcoming Influenza Seasons: Next Steps
3.1. DRIVE Study Platform Adaptation for COVID-19 for 2020/2021 Season
- SARS-CoV-2 virus infection could potentially be a confounder for the association between influenza vaccination and influenza disease [11].
- In potential co-circulation of influenza and SARS-CoV-2, efforts to increase rates of influenza vaccination are important to reduce the additional influenza-related burden, particularly among groups at high risk of influenza complications and severe COVID-19 disease. Thus, COVID-19 might influence the influenza vaccine recommendations or its acceptance, increasing the differences between the vaccinated and unvaccinated [12].
- There may also be differences in healthcare-seeking behavior for influenza vs. COVID-19 (e.g., at-risk individuals and severity of the new disease) leading to under- or over-estimation of IVE.
- Co-infection may affect clinical outcomes and individual response to vaccine-derived protection for influenza.
- The standard of care and access to influenza vaccination might be impacted in the case of SARS-CoV-2 virus circulation.
3.2. Adaptation of DRIVE TND Protocol to Include COVID-19 Components
- COVID-19 positivity in the current season (RT-PCR test)
- Date of COVID-19 test
- COVID-19 positivity in the previous season
- Use of COVID-19 treatments and type (e.g., antivirals)
- Clinical symptoms to distinguish between influenza and COVID-19, such as anosmia, ageusia, etc.
- Co-morbidities to identify risk-specific groups for COVID-19
- Morbidity related to COVID-19
3.3. DRIVE Study Site Operations and Data Collection for the 2020/2021 Season
4. DRIVE beyond Influenza: COVID-19 Vaccine Effectiveness Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIRI-IT | Interuniversity Research Center on Influenza and other Transmissible Infections |
| COVID-19 | Coronavirus disease 2019 |
| DRIVE | Development of Robust and Innovative Vaccine Effectiveness |
| ECDC | European Centre for Disease Prevention and Control |
| EMA | European Medicines Agency |
| EU | European Union |
| FISABIO | Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana |
| GP | General practice |
| GTPUH | Germans Trias I Pujol University Hospital |
| HUS | Helsinki University Hospital, Jorvi Hospital |
| HUVH | Hospital Universitari Vall D’Hebron |
| ICU | Intensive care unit |
| ILI | Influenza-like illness |
| IMI | Innovative Medicines Initiative |
| IREIVAC | Innovative Clinical Research Network in Vaccinology |
| IVE | Influenza vaccine effectiveness |
| LCI | Lab-confirmed influenza |
| LPUH | La Paz University Hospital |
| MUV | Medical University Vienna |
| NIID | National Institute for Infectious Disease “Prof. Dr. Matei Bals” |
| PPE | Personal protective equipment |
| RSV | Respiratory syncytial virus |
| RT-PCR | Reverse-transcriptase polymerase chain reaction |
| SAP | Statistical analysis plan |
| SARI | Severe acute respiratory illness |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TND | Test-negative design |
| VAHNSI | Valencia Hospital Surveillance Network for the Study of Influenza and Respiratory Viruses Disease |
References
- Committee for Medicinal Products for Human Use. Guideline on Influenza Vaccines—Non-clinical and Clinical Module. Eur. Med. Agency EMA/CHMP/VWP/457259/2014 2016, 44, 1–31. [Google Scholar]
- DRIVE Consortium. DRIVE IVE Results Section. DRIVE Website. 2020. Available online: https://www.drive-eu.org/index.php/results/ (accessed on 28 December 2020).
- Stuurman, A.L.; De Smedt, T.; Alexandridou, M.; Biccler, J.; Domingo, J.D.; Nohynek, H.; Rizzo, C.; Turunen, T.; Riera-Montes, M. Vaccine effectiveness against laboratory-confirmed influenza in Europe—Results from the DRIVE network during season 2018/19. Vaccine 2020, 38, 6455–6463. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Cole, S.R.; Platt, R.W. Overadjustment Bias and Unnecessary Adjustment in Epidemiologic Studies. Epidemiology 2009, 20, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.; Carville, K.; Pierse, N.; Kelly, H. Seasonal influenza vaccine effectiveness estimates: Development of a parsimonious case test negative model using a causal approach. Vaccine 2016, 34, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Flu News Europe. 2019/20 Season Overview. Available online: https://flunewseurope.org/ (accessed on 28 December 2020).
- DRIVE Consortium. DRIVE Webannex of IVE Results Report 2019/2020. P-95 Webannex. 2020. Available online: https://apps.p-95.com/drivewebapp/ (accessed on 28 December 2020).
- Auvinen, R.; Nohynek, H.; Syrjänen, R.; Ollgren, J.; Kerttula, T.; Mäntylä, J.; Ikonen, N.; Loginov, R.; Haveri, A.; Kurkela, S.; et al. Comparison of the clinical characteristics and outcomes of hospitalized adult COVID-19 and influenza patients—A prospective observational study. Infect. Dis. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kulkarni, D.; Dozier, M.; Hartnup, K.; Paget, J.; Campbell, H.; Nair, H. Influenza vaccination strategies for 2020-21 in the context of COVID-19. J. Glob. Health Dec. 2020, 10, 021102. [Google Scholar] [CrossRef]
- ECDC. COVID-19 Situation Update for the EU/EEA and the UK, as of Week 50 2020. Available online: https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea2020 (accessed on 28 December 2020).
- Stowe, J.; Tessier, E.; Zhao, H.; Guy, R.; Muller-Pebody, B.; Zambon, M.; Andrews, N.; Ramsay, M.; Bernal, J.L. Interactions between SARS-CoV-2 and Influenza and the impact of coinfection on disease severity: A test negative design. medRxiv 2020. [Google Scholar] [CrossRef]
- WHO. WHO SAGE Seasonal Influenza Vaccination Recommendations during the COVID-19 Pandemic—Interim Guidance. Available online: https://www.who.int/immunization/policy/position_papers/Interim_SAGE_influenza_vaccination_recommendations.pdf?ua=12020 (accessed on 28 December 2020).
- ECDC. Key Aspects Regarding the Introduction and Prioritisation of COVID-19 Vaccination in the EU/EEA and the UK. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/key-aspects-regarding-introduction-and-prioritisation-covid-19-vaccination2020 (accessed on 28 December 2020).
- European Comission. Communication: “Coronavirus: Commission Unveils EU Vaccines Strategy”. EC Website. 2020. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_20_1103 (accessed on 28 December 2020).
- ECDC. Operational Considerations for Influenza Surveillance in the WHO European Region during COVID-19: Interim Guidance. ECDC Website. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/operational-considerations-influenza-surveillance-european-region-during-covid-19 (accessed on 28 December 2020).
- WHO. Maintaining Surveillance of Influenza and Monitoring SARS-CoV-2—Adapting Global Influenza Surveillance and Response System (GISRS) and Sentinel Systems during the COVID-19 Pandemic. World Health Organization (WHO) Website. 2020. Available online: https://www.who.int/publications/i/item/maintaining-surveillance-of-influenza-and-monitoring-sars-cov-2-adapting-global-influenza-surveillance-and-response-system-(gisrs)-and-sentinel-systems-during-the-covid-19-pandemic (accessed on 28 December 2020).
| Season 2017/2018 | Season 2018/2019 | Season 2019/2020 | |
|---|---|---|---|
| Comments | Pilot season, severe influenza season | Mild influenza season | COVID-19 impact (shorter study period) and mild influenza season |
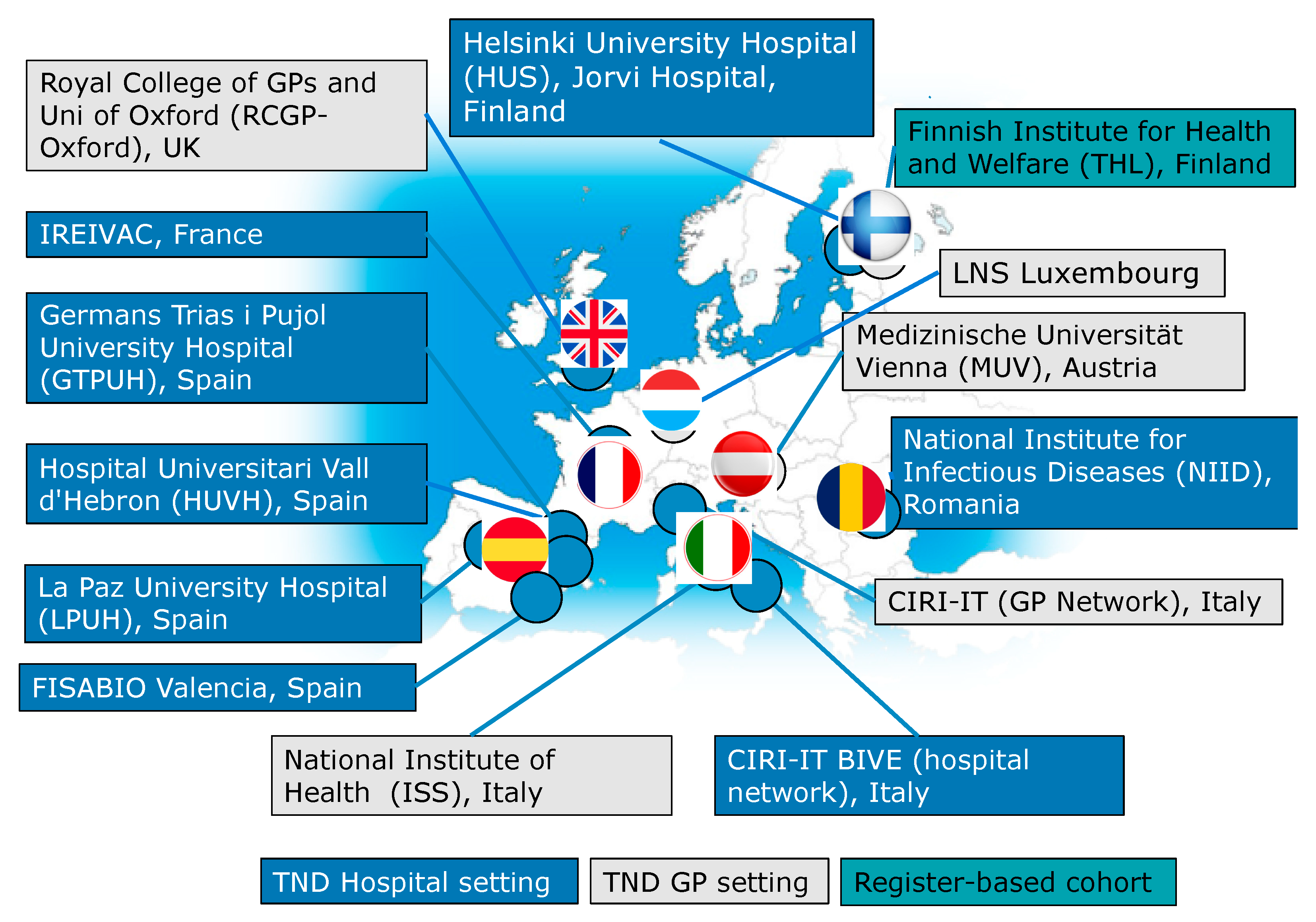
| Study platform | 5 sites 4 countries | 13 sites 7 countries | 14 sites 8 countries |
| Countries | Austria, Finland, Italy and Spain | Added: Luxembourg, Romania and United Kingdom | Added: France |
| TND Hospital | 4 hospitals 2.882 SARI 436 LCI | 12 hospitals 4.884 SARI 1.442 LCI | 19 hospitals 4.121 SARI 1.296 LCI |
| TND GP | 361 GP 4.343 ILI 2.419 LCI | 378 GP 5.008 ILI 2.159 LCI | 388 GP 4.958 ILI 2.235 LCI |
| Register-based cohort | 1.326.097 subjects 5.708 LCI | 1.516.946 subjects 6379 LCI | 1.550.000 subjects 2427 LCI |
| Influenza vaccine brands | 11 on market 10 in DRIVE dataset | 10 on market 7 in DRIVE dataset | 11 licensed 8 in DRIVE dataset |
| DRIVE Study Sites 2019/2020 | Characteristics | Was Testing for Influenza Impacted? | Did Triage Strategy Change? | Date of Last Swab Positive for Influenza | DRIVE Data Collection |
|---|---|---|---|---|---|
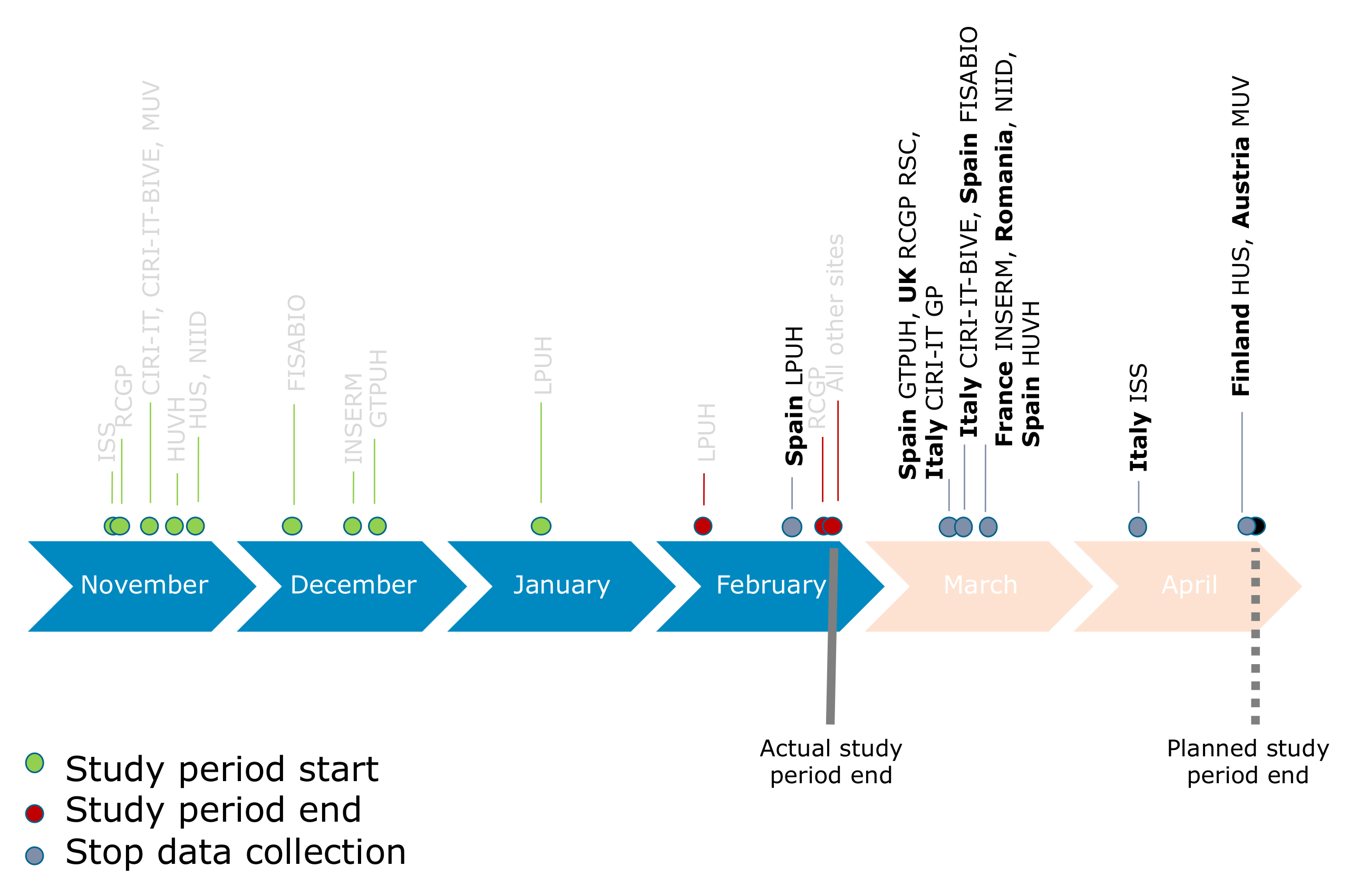
| Fisabio (Spain) | Hospital setting (4 hospitals) 19 children * 157 adults ** 486 older adults *** | No, only samples that tested negative for influenza (and the other respiratory viruses included in VAHNSI multiplex PCR) were frozen and re-analyzed for SARS-CoV-2. | No | 13 March 2020 | Stopped (13 March 2020) The collection of samples for DRIVE stopped in early March to prevent risk of infection of nurses, as well as because of the added complexity to access isolated patients for informed consent/questionnaires. |
| Hospital Germans Trias i Pujol (Spain) | Hospital setting (1 hospital) 25 children * 68 adults ** 89 older adults *** | No, all samples from SARI patients were tested for both SARS-CoV-2 and influenza virus with multiplex PCR. | No | 12 March 2020 | Continued data collection until 30 April 2020 (DRIVE initial data cut-off) Since there was one staff member dedicated exclusively to reviewing and entering the DRIVE data, the study site could collect all the information needed for the project. |
| Hospital La Paz (Spain) | Hospital setting (1 hospital) 15 adults ** 21 older adults *** | Yes, samples from all SARI cases admitted to the hospital were only tested for SARS-CoV-2 and no subsequent PCR testing for influenza was performed. | Yes, all SARI cases arriving to the hospital were managed as COVID-19 cases, so they were isolated and immediately tested for SARS-CoV-2 (and not for influenza). | 24 February 2020 | Stopped (first week of March) |
| Hospital Universitari Vall d’Hebron (Spain) | Hospital setting (1 hospital) 78 children * 110 adults ** 100 older adults *** | No, every patient suspected of COVID-19 was tested for both SARS-CoV-2 and influenza virus. | No, screening of SARI cases was not affected during the COVID-19 pandemic. | 16 March 2020 | Continued data collection until 30 April 2020 (DRIVE initial data cut-off) |
| I-REIVAC (France) | Hospital setting (5 hospitals) 134 adults ** 246 older adults *** | No, however, the FLUVAC study protocol did not implement routinely SARS-Cov-2 testing, so patients hospitalized with SARI were not tested for SARS-CoV-2 at admission and the information on SARS-CoV-2 positivity was initially missing. However, all samples were stored to be re-analyzed for SARS-CoV-2 later on. | Yes, the algorithm for hospital screening of admitted patients was different between eligible SARI and suspected COVID-19 individuals, who were isolated. Thus, clinical research assistants of the IREIVAC centers did not get access to the samples of suspected COVID-19 patients for influenza testing. | 16 March 2020 | Stopped (20 March 2020) The French national public health agency stopped influenza surveillance at the country level by mid-March and switched to specific COVID-19 monitoring. |
| ISS (Italy) | Primary Care setting (245 GP) 938 children * 863 adults ** 119 older adults *** | Yes, simultaneous test for influenza and SARS-CoV-2 was not implemented, so ILIs were tested only for SARS-CoV-2 and not for influenza. Samples were stored and re-analyzed later on for influenza. | Yes, ILI cases arriving to the GP during the COVID-19 pandemic were considered COVID-19 suspects and tested only for SARS-CoV-2. | 7 April 2020 | Continued data collection until 30 April 2020 (DRIVE initial data cut-off) |
| CIRI-IT BIVE (Italy) | Hospital setting (5 hospitals) 770 children * 296 adults ** 584 older adults *** | Yes. At the beginning of the COVID-19 pandemic (March 2020), the impossibility of performing the simultaneous test for influenza and SARS-CoV-2 was real. So, all SARI cases were initially tested for SARS-CoV-2, samples stored, and then re-analyzed for influenza. | Yes, ILI cases arriving to the emergency department during the COVID-19 pandemic were considered COVID-19 suspects and tested initially only for SARS-CoV2. | 13 March 2020 | Stopped (15 March 2020) |
| CIRI-IT GP (Italy) | Primary Care setting (35 GP) 698 children * 524 adults ** 146 older adults *** | Yes. At the beginning of the COVID-19 pandemic (March 2020), the impossibility of performing the simultaneous test for influenza and SARS-CoV-2 was real. So, all ILI cases were initially tested for SARS-CoV-2, samples stored, and then re-analyzed for influenza. | Yes, ILI cases consulting the physicians during the COVID-19 pandemic were considered COVID-19 suspects, and to minimize the possibility of contagion, physicians had limited access to their clinics to patients with ILI. ILI cases were initially tested for SARS-CoV-2, samples stored, and then re-analyzed for influenza. | 4 March 2020 | Stopped (13 March 2020) |
| MUV (Austria) | Primary Care setting (96 GP) 639 children * 673 adults ** 47 older adults *** | No, Austria efficiently switched from influenza to SARS-CoV-2 surveillance and MUV implemented the SARS-CoV-2 testing into the sentinel system on 24 February 2020, meaning that each sample was tested both for influenza and SARS-CoV-2 by PCR. | Yes, with the beginning of the COVID-19 pandemic in Austria, the ICU units were separated in COVID ICUs and non- COVID ICUs, preventing nosocomial infections. | 13 March 2020 | Continued data collection until 30 April 2020 (DRIVE initial data cut-off) Routine influenza screening at the primary care level almost stopped, as each patient requiring ICU admission got tested for SARS-CoV-2 only. This testing was performed in the respective hospitals and not at the GPs’; therefore, MUV was not able to get these samples for influenza testing. |
| NIID (Romania) | Hospital setting (1 hospital) 500 children * 221 adults ** 78 older adults *** | In the beginning of the COVID-19 pandemic all samples from SARI patients were tested for both SARS-CoV-2 and influenza simultaneously. However, from 22 March, SARI cases were only tested for influenza after a positive test for SARS-CoV-2. From 22 March to 30 April 2020, NIID only identified 1 coinfection of SARS-CoV-2 + influenza B. | In the initial stages of the COVID-19 pandemic, NIID was the designated facility for SARS-CoV-2 diagnosis and surveillance in Romania. All SARI cases admitted to the hospital were tested for both SARS-CoV-2 and influenza, so the recruitment of cases for DRIVE studies did not stop. | 16 March 2020 | Continued data collection until 30 April 2020 (DRIVE initial data cut-off) As of 22 March 2020, NIID switched to a COVID-19 hospital, so only COVID-19 cases were admitted. The screening for influenza was only performed for patients positive for SARS-CoV-2. Thus, the vast majority of the influenza cases were admitted to other hospitals. |
| DRIVE Study Sites 2020/2021 | Characteristics | Planned Triage Influenza/COVID-19 | Laboratory Testing | Possibility to Collect COVID-19 Variables |
|---|---|---|---|---|
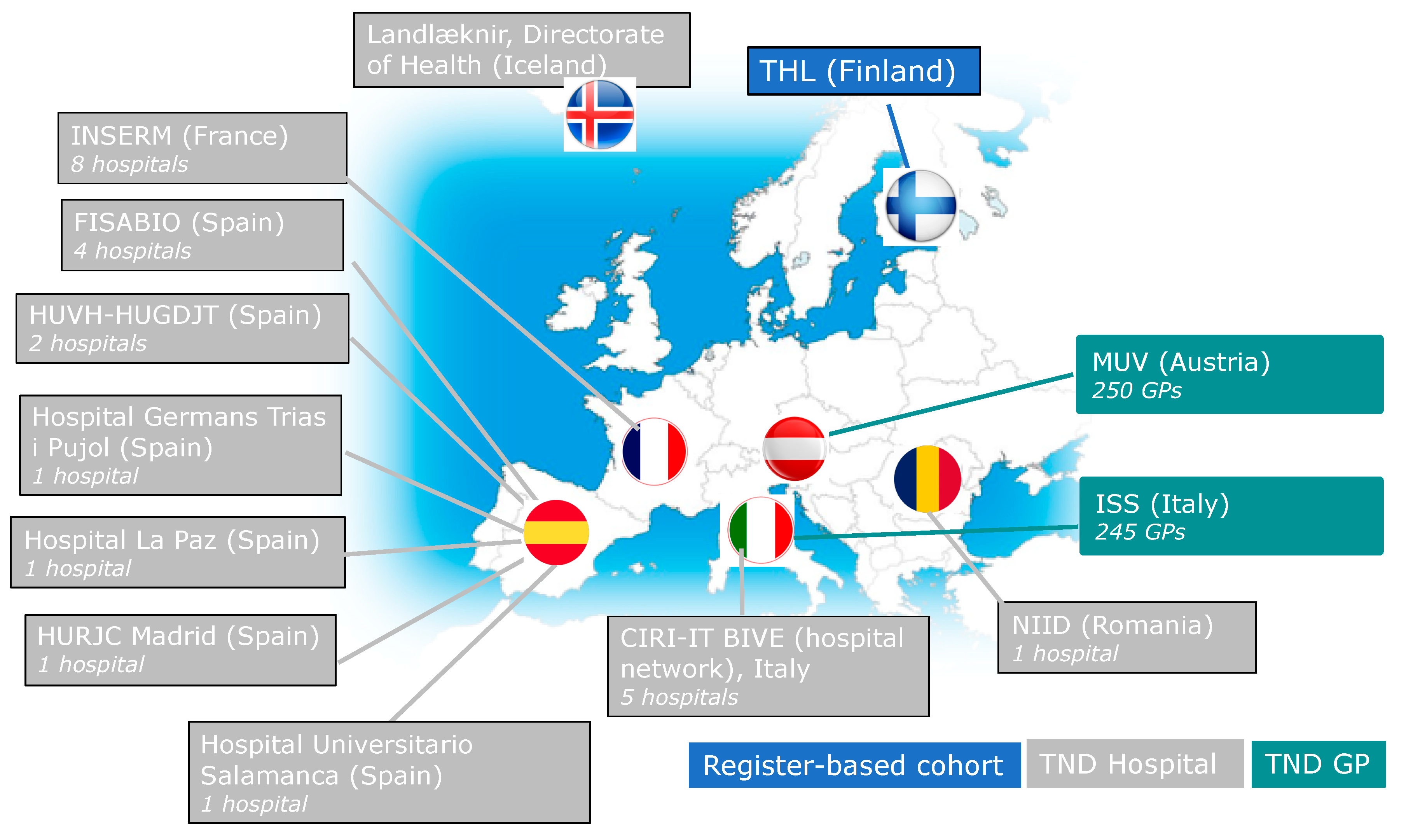
| Fisabio (Spain) | Hospital setting (4 hospitals) | Common triage (all ILI/SARI cases admitted). | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza, from the same swab. | Yes
|
| Hospital Germans Trias i Pujol (Spain) | Hospital setting (1 hospital) | Common triage (all SARI cases admitted). | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza, not yet established if it will be from the same swab. | Yes
|
| Hospital Universitari Vall d’Hebron (Spain) | Hospital setting (2 hospitals) | Common triage (all SARI cases admitted). This study site avoids many of the problems generated by prospective studies and risk of COVID-19 infections by selecting the cases and controls from the influenza surveillance electronic system of Catalonia, so there will be no problem accessing patient data if isolated. | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza, from the same swab. | Yes
|
| CIRI-IT (Italy) | Hospital setting (5 hospitals) | Common triage of all SARI cases admitted. | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza, from the same swab. | Yes
|
| MUV (Austria) | Primary Care setting (250 GP) | Different triage for ILI cases suspected case of COVID-19: if a suspected COVID-19 case, patient stays at home and physicians come to take the swab. If ILI case is suspected of influenza, normal GP appointment. In all ILI cases, swabs are tested for both influenza and SARS-CoV-2. | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza, from the same swab. | Yes, but limited to
|
| IREIVAC (France) | Hospital setting (8 hospitals) | Common triage of all SARI cases admitted. | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza, from the same swab. | Yes
|
| Directorate of Health (Iceland) | Hospital and primary care settings | Common triage at the primary care and hospital level. | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza, from the same swab. | Yes
|
| NIID (Romania) | Hospital setting (1 COVID-19– only hospital) | Not common triage of SARIs arriving to the hospital: only SARS-CoV-2 positives are admitted to the hospital (as it is a COVID-19–only hospital). SARS-CoV-2 negatives are diverted to other hospitals. | Simultaneous RT-PCR testing for SARS-CoV-2 and influenza. Swabbing case-by-case: if the patient is already confirmed as having COVID-19, another swab will be taken for influenza. | Yes
|
| Hospital U. Salamanca (Spain) | Hospital setting (1 hospital) | Common triage of all SARI cases admitted. | All subjects are tested for both SARS-CoV-2 and influenza, but testing for influenza will be done at the end of the season, from the same swab. | Yes
|
| Hospital U. La Paz (Spain) | Hospital setting (1 hospital) | Common triage of all SARI cases admitted. | Swab will initially be tested for SARS-CoV2, and subsequently tested for influenza and other respiratory viruses (same swab). | Yes
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, A.; Muñoz-Quiles, C.; Stuurman, A.; Descamps, A.; Mira-Iglesias, A.; Torcel-Pagnon, L.; Díez-Domingo, J. Challenges and Adaptation of a European Influenza Vaccine Effectiveness Study Platform in Response to the COVID-19 Emergence: Experience from the DRIVE Project. Int. J. Environ. Res. Public Health 2021, 18, 1058. https://doi.org/10.3390/ijerph18031058
Carmona A, Muñoz-Quiles C, Stuurman A, Descamps A, Mira-Iglesias A, Torcel-Pagnon L, Díez-Domingo J. Challenges and Adaptation of a European Influenza Vaccine Effectiveness Study Platform in Response to the COVID-19 Emergence: Experience from the DRIVE Project. International Journal of Environmental Research and Public Health. 2021; 18(3):1058. https://doi.org/10.3390/ijerph18031058
Chicago/Turabian StyleCarmona, Antonio, Cintia Muñoz-Quiles, Anke Stuurman, Alexandre Descamps, Ainara Mira-Iglesias, Laurence Torcel-Pagnon, and Javier Díez-Domingo. 2021. "Challenges and Adaptation of a European Influenza Vaccine Effectiveness Study Platform in Response to the COVID-19 Emergence: Experience from the DRIVE Project" International Journal of Environmental Research and Public Health 18, no. 3: 1058. https://doi.org/10.3390/ijerph18031058
APA StyleCarmona, A., Muñoz-Quiles, C., Stuurman, A., Descamps, A., Mira-Iglesias, A., Torcel-Pagnon, L., & Díez-Domingo, J. (2021). Challenges and Adaptation of a European Influenza Vaccine Effectiveness Study Platform in Response to the COVID-19 Emergence: Experience from the DRIVE Project. International Journal of Environmental Research and Public Health, 18(3), 1058. https://doi.org/10.3390/ijerph18031058






