The Obesity Paradox Predicts the Second Wave of COVID-19 to Be Severe in Western Countries
Abstract
1. Introduction
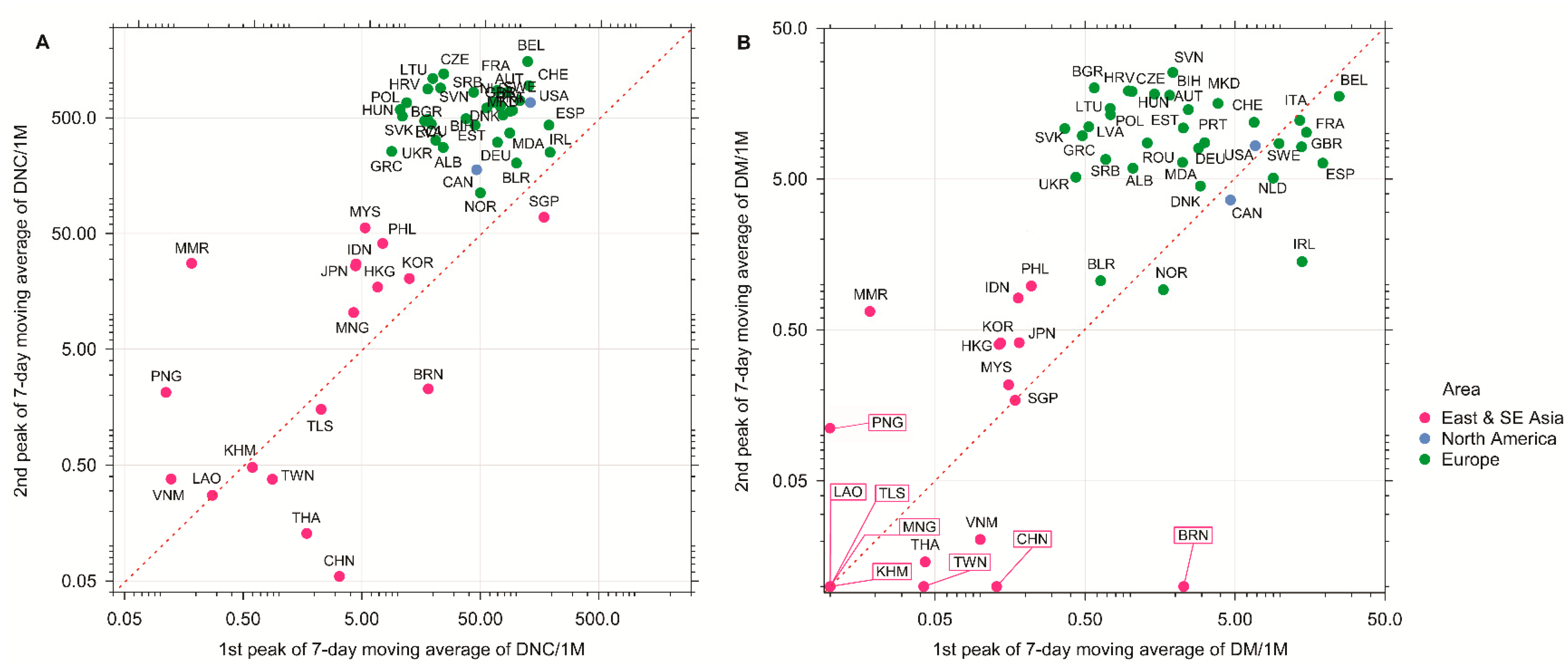
2. The Politics of Lockdown and the COVID-19 Infection
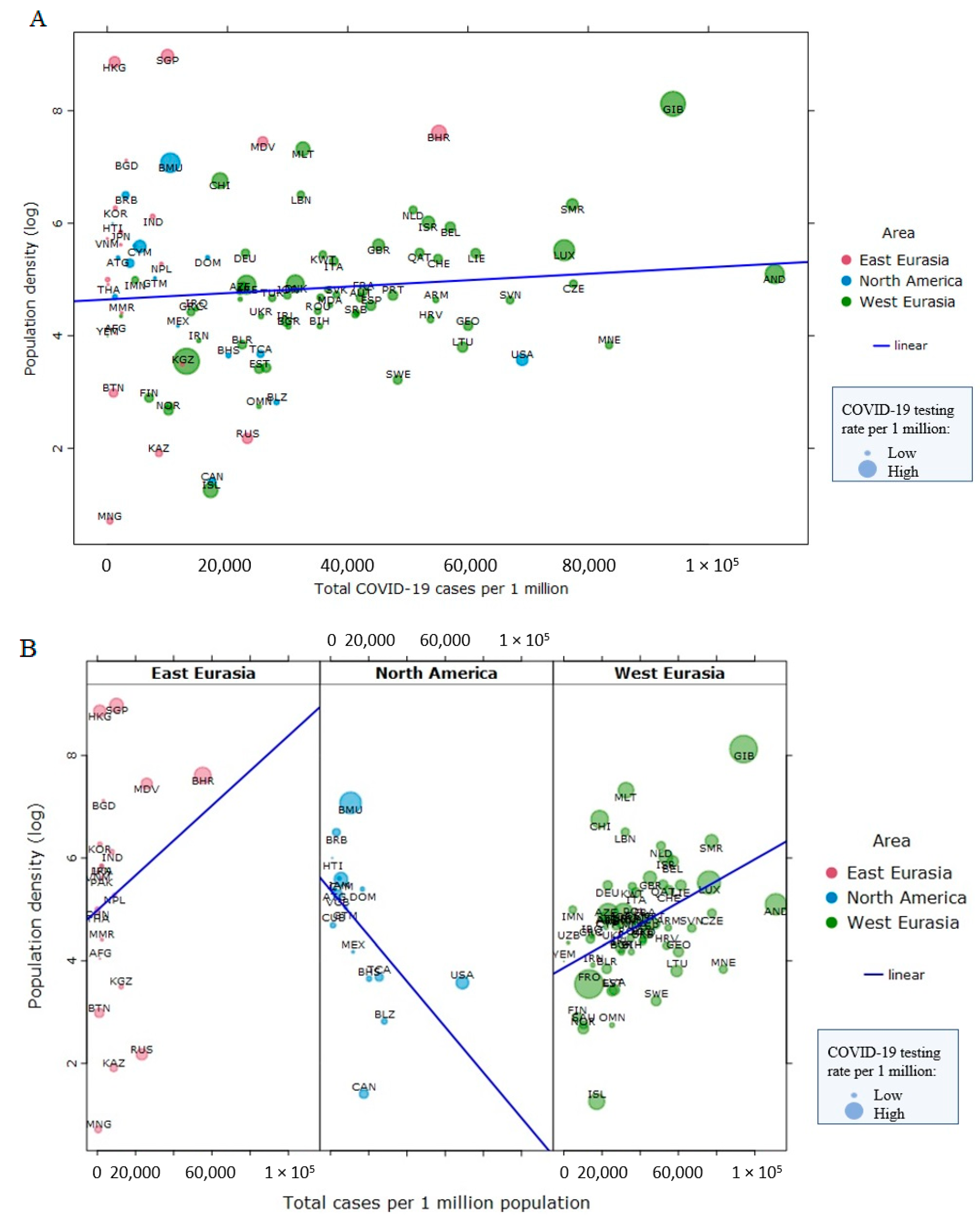
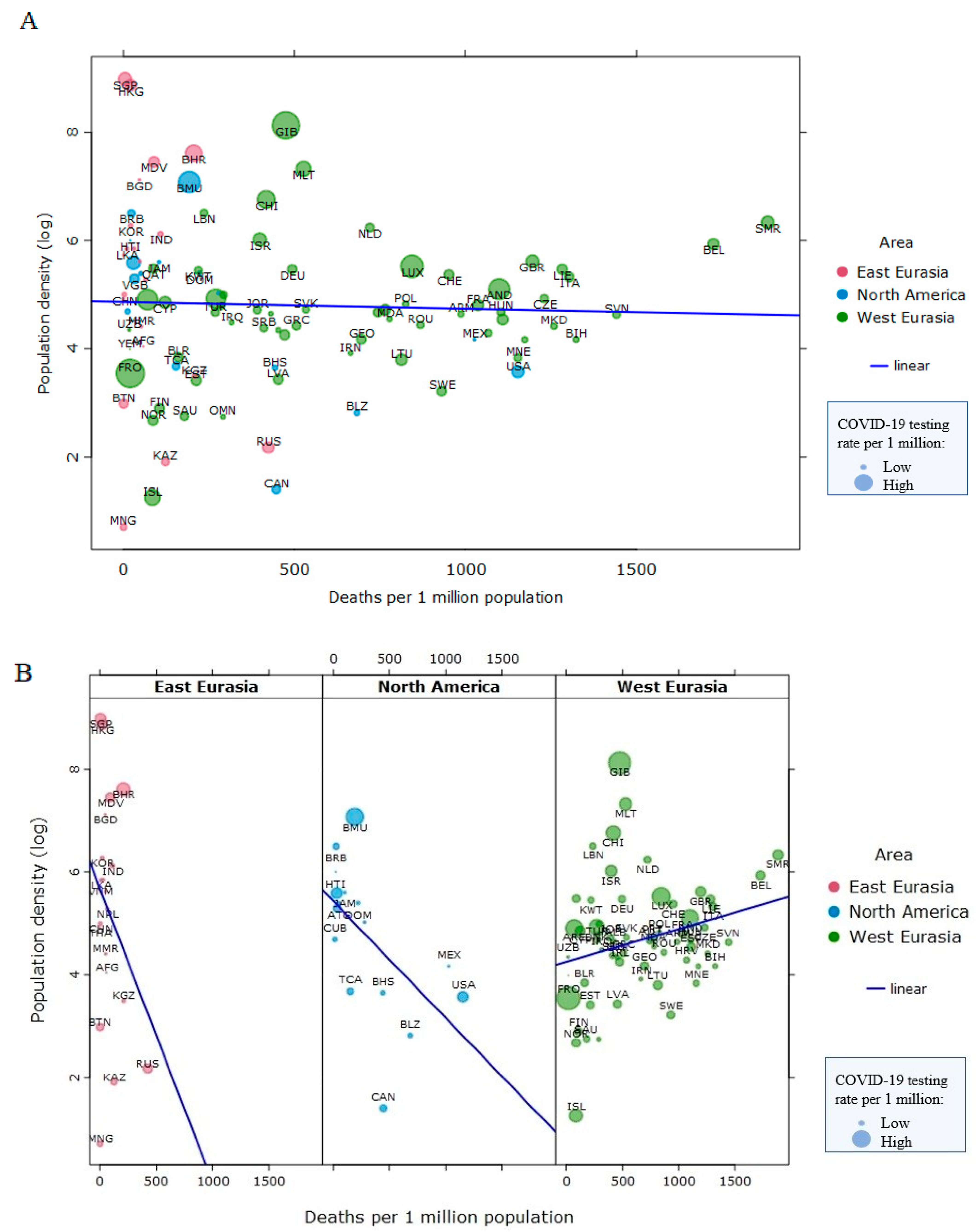
3. The Obesity Paradox, Visceral Fat, and COVID-19 Outcomes
4. Pandemic-Induced Weight Gain and COVID-19
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burki, T. China’s Successful Control of COVID-19. Lancet Infect. Dis. 2020, 20, 1240–1241. [Google Scholar] [CrossRef]
- Coelho, M.T.P.; Rodrigues, J.F.M.; Medina, A.M.; Scalco, P.; Terribile, L.C.; Vilela, B.; Diniz-Filho, J.A.F.; Dobrovolski, R. Global Expansion of COVID-19 Pandemic Is Driven by Population Size and Airport Connections. Peer J. 2020, 8, e9708. [Google Scholar] [CrossRef]
- Speakman, J.R. The Evolution of Body Fatness: Trading off Disease and Predation Risk. J. Exp. Biol. 2018, 221, jeb167254. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.D.; Racine, R.; Wittmer, S.T.; Harston, L.; Papillion, A.M.; Dishaw, L.M.; Randall, T.D.; Woodland, D.L.; Winslow, G.M. The Omentum Is a Site of Protective IgM Production during Intracellular Bacterial Infection. Infect. Immun. 2015, 83, 2139–2147. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, X.; Shi, W. Impacts of Social and Economic Factors on the Transmission of Coronavirus Disease 2019 (COVID-19) in China. J. Popul. Econ. 2020, 33, 1127–1172. [Google Scholar] [CrossRef]
- Wilson, S. Pandemic Leadership: Lessons from New Zealand’s Approach to COVID-19. Leadership 2020, 16, 279–293. [Google Scholar] [CrossRef]
- Krams, I.A.; Luoto, S.; Rantala, M.J.; Jõers, P.; Krama, T. Covid-19: Fat, Obesity, Inflammation, Ethnicity, and Sex Differences. Pathogens 2020, 9, 887. [Google Scholar] [CrossRef]
- Singanayagam, A.; Singanayagam, A.; Chalmers, J.D. Obesity Is Associated with Improved Survival in Community-Acquired Pneumonia. Eur. Respir. J. 2013, 42, 180–187. [Google Scholar] [CrossRef]
- Lavie, C.J.; Osman, A.F.; Milani, R.V.; Mehra, M.R. Body Composition and Prognosis in Chronic Systolic Heart Failure: The Obesity Paradox. Am. J. Cardiol. 2003, 91, 891–894. [Google Scholar] [CrossRef]
- Curtis, J.P.; Selter, J.G.; Wang, Y.; Rathore, S.S.; Jovin, I.S.; Jadbabaie, F.; Kosiborod, M.; Portnay, E.L.; Sokol, S.I.; Bader, F.; et al. The Obesity Paradox: Body Mass Index and Outcomes in Patients with Heart Failure. Arch. Intern. Med. 2005, 165, 55. [Google Scholar] [CrossRef]
- Bagheri, M.; Speakman, J.R.; Shabbidar, S.; Kazemi, F.; Djafarian, K. A Dose-Response Meta-Analysis of the Impact of Body Mass Index on Stroke and All-Cause Mortality in Stroke Patients: A Paradox within a Paradox: BMI Effects on Stroke and Overall Death. Obes. Rev. 2015, 16, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Speakman, J.R.; Shemirani, F.; Djafarian, K. Renal Cell Carcinoma Survival and Body Mass Index: A Dose–Response Meta-Analysis Reveals Another Potential Paradox within a Paradox. Int. J. Obes. 2016, 40, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Kristan, D.M. Chronic Calorie Restriction Increases Susceptibility of Laboratory Mice (Mus Musculus) to a Primary Intestinal Parasite Infection: Calorie Restriction and Intestinal Parasites. Aging Cell 2007, 6, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Kristan, D.M. Calorie Restriction and Susceptibility to Intact Pathogens. AGE 2008, 30, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Krams, I.; Rantala, M.J.; Luoto, S.; Krama, T. Fat Is Not Just an Energy Store. J. Exp. Biol. 2018, 221, jeb183756. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Bhopal, R.; Prabhakaran, D.; Sarrafzadegan, N.; Tchernof, A. Why Might South Asians Be so Susceptible to Central Obesity and Its Atherogenic Consequences? The Adipose Tissue Overflow Hypothesis. Int. J. Epidemiol. 2007, 36, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the Art Paper Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef]
- Han, S.-J.; Glatman Zaretsky, A.; Andrade-Oliveira, V.; Collins, N.; Dzutsev, A.; Shaik, J.; Morais da Fonseca, D.; Harrison, O.J.; Tamoutounour, S.; Byrd, A.L.; et al. White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity 2017, 47, 1154–1168.e6. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Tittelbach, T.J.; Berman, D.M.; Nicklas, B.J.; Ryan, A.S.; Goldberg, A.P. Racial Differences in Adipocyte Size and Relationship to the Metabolic Syndrome in Obese Women. Obes. Res. 2004, 12, 990–998. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral Fat Adipokine Secretion Is Associated with Systemic Inflammation in Obese Humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Qiang, G.; Kong, H.W.; Fang, D.; McCann, M.; Yang, X.; Du, G.; Blüher, M.; Zhu, J.; Liew, C.W. The Obesity-Induced Transcriptional Regulator TRIP-Br2 Mediates Visceral Fat Endoplasmic Reticulum Stress-Induced Inflammation. Nat. Commun. 2016, 7, 11378. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine Release Syndrome in Severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Freitas, I.; Plank, L.D. Body Size, Body Composition and Fat Distribution: Comparative Analysis of European, Maori, Pacific Island and Asian Indian Adults. BJN 2009, 102, 632. [Google Scholar] [CrossRef]
- Wells, J.C.K. Ethnic Variability in Adiposity and Cardiovascular Risk: The Variable Disease Selection Hypothesis. Int. J. Epidemiol. 2009, 38, 63–71. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-Converting Enzyme 2 Is a Functional Receptor for the SARS Coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Heialy, S.A.; Hachim, M.; Senok, A.; Tayoun, A.A.; Hamoudi, R.; Alsheikh-Ali, A.; Hamid, Q. Regulation of Angiotensin Converting Enzyme 2 (ACE2) in Obesity: Implications for COVID-19. Front. Physiol. 2020. [Google Scholar] [CrossRef]
- Blume, C.; Jackson, C.L.; Spalluto, C.M.; Legebeke, J.; Nazlamova, L.; Conforti, F.; Perotin, J.-M.; Frank, M.; Butler, J.; Crispin, M.; et al. A Novel ACE2 Isoform Is Expressed in Human Respiratory Epithelia and Is Upregulated in Response to Interferons and RNA Respiratory Virus Infection. Nat. Genet. 2021. [Google Scholar] [CrossRef]
- Al-Benna, S. Association of High Level Gene Expression of ACE2 in Adipose Tissue with Mortality of COVID-19 Infection in Obese Patients. Obes. Med. 2020, 19, 100283. [Google Scholar] [CrossRef] [PubMed]
- AlGhatrif, M.; Cingolani, O.; Lakatta, E.G. The Dilemma of Coronavirus Disease 2019, Aging, and Cardiovascular Disease: Insights from Cardiovascular Aging Science. JAMA Cardiol. 2020, 5, 747. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative Genetic Analysis of the Novel Coronavirus (2019-NCoV/SARS-CoV-2) Receptor ACE2 in Different Populations. Cell Discov. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Favre, G.; Frey, S.; Esnault, V.; Gugenheim, J.; Bouam, S.; Schiavo, L.; Tran, A.; Alifano, M. Obesity and COVID-19: ACE 2, the Missing Tile. Obes. Surg. 2020, 30, 4615–4617. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef]
- Gouilh, M.A.; Puechmaille, S.J.; Gonzalez, J.-P.; Teeling, E.; Kittayapong, P.; Manuguerra, J.-C. SARS-Coronavirus Ancestor’s Foot-Prints in South-East Asian Bat Colonies and the Refuge Theory. Infect. Genet. Evolut. 2011, 11, 1690–1702. [Google Scholar] [CrossRef]
- Hekman, R.M.; Hume, A.J.; Goel, R.K.; Abo, K.M.; Huang, J.; Blum, B.C.; Werder, R.B.; Suder, E.L.; Paul, I.; Phanse, S.; et al. Actionable Cytopathogenic Host Responses of Human Alveolar Type 2 Cells to SARS-CoV-2. Mol. Cell 2020, 80, 1104–1122.e9. [Google Scholar] [CrossRef]
- Keel, P.K.; Gomez, M.M.; Harris, L.; Kennedy, G.A.; Ribeiro, J.; Joiner, T.E. Gaining “The Quarantine 15:” Perceived versus Observed Weight Changes in College Students in the Wake of COVID -19. Int J. Eat. Disord. 2020, 53, 1801–1808. [Google Scholar] [CrossRef]
- Pearl, R.L. Weight Stigma and the “Quarantine-15”. Obesity 2020, 28, 1180–1181. [Google Scholar] [CrossRef]
- Hernandez-Rodas, M.; Valenzuela, R.; Videla, L. Relevant Aspects of Nutritional and Dietary Interventions in Non-Alcoholic Fatty Liver Disease. IJMS 2015, 16, 25168–25198. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Uranga, J.A.; López-Miranda, V.; Lombó, F.; Abalo, R. Food, Nutrients and Nutraceuticals Affecting the Course of Inflammatory Bowel Disease. Pharmacol. Rep. 2016, 68, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Krama, T.; Krams, I. Eating Disorders: An Evolutionary Psychoneuroimmunological Approach. Front. Psychol. 2019, 10, 2200. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Krams, I.; Karlsson, H. Depression Subtyping Based on Evolutionary Psychiatry: Proximate Mechanisms and Ultimate Functions. Brain Behav. Immun. 2018, 69, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Borráz-León, J.I.; Krams, I. Bipolar Disorder: An Evolutionary Psychoneuroimmunological Approach. Neurosci. Biobehav. Rev. 2021, S0149763420307065. [Google Scholar] [CrossRef]
- Clemmensen, C.; Petersen, M.B.; Sørensen, T.I.A. Will the COVID-19 Pandemic Worsen the Obesity Epidemic? Nat. Rev. Endocrinol. 2020, 16, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Pinti, M.; Farinetti, A.; Nasi, M. Obesity Risk during Collective Quarantine for the COVID-19 Epidemic. Obes. Med. 2020, 20, 100263. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, R.; Guío-Carrión, A.; Zapatero-Gaviria, A.; Martínez, P.; Blasco, M.A. Shorter Telomere Lengths in Patients with Severe COVID-19 Disease. Aging 2021. [Google Scholar] [CrossRef]
- Green, W.D.; Beck, M.A. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann. ATS 2017, 14, S406–S409. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity Is Associated with Impaired Immune Response to Influenza Vaccination in Humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Paich, H.A.; Sheridan, P.A.; Handy, J.; Karlsson, E.A.; Schultz-Cherry, S.; Hudgens, M.G.; Noah, T.L.; Weir, S.S.; Beck, M.A. Overweight and Obese Adult Humans Have a Defective Cellular Immune Response to Pandemic H1N1 Influenza a Virus: Obese/Overweight Response to Influenza. Obesity 2013, 21, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Coetzee, V.; Moore, F.R.; Skrinda, I.; Kecko, S.; Krama, T.; Kivleniece, I.; Krams, I. Adiposity, Compared with Masculinity, Serves as a More Valid Cue to Immunocompetence in Human Mate Choice. Proc. R. Soc. B. 2013, 280, 20122495. [Google Scholar] [CrossRef]
- Eastwood, S.V.; Tillin, T.; Dehbi, H.-M.; Wright, A.; Forouhi, N.G.; Godsland, I.; Whincup, P.; Sattar, N.; Hughes, A.D.; Chaturvedi, N. Ethnic Differences in Associations between Fat Deposition and Incident Diabetes and Underlying Mechanisms: The SABRE Study: Adiposity Measures and Incident Diabetes. Obesity 2015, 23, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral Fat Shows the Strongest Association with the Need of Intensive Care in Patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef] [PubMed]
- Cacciapaglia, G.; Cot, C.; Sannino, F. Second Wave COVID-19 Pandemics in Europe: A Temporal Playbook. Sci. Rep. 2020, 10, 15514. [Google Scholar] [CrossRef]
- Ortenzi, F.; Albanese, E.; Fadda, M. A Transdisciplinary Analysis of COVID-19 in Italy: The Most Affected Country in Europe. Int. J. Environ. Res. Public Health 2020, 17, 9488. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krams, I.A.; Jõers, P.; Luoto, S.; Trakimas, G.; Lietuvietis, V.; Krams, R.; Kaminska, I.; Rantala, M.J.; Krama, T. The Obesity Paradox Predicts the Second Wave of COVID-19 to Be Severe in Western Countries. Int. J. Environ. Res. Public Health 2021, 18, 1029. https://doi.org/10.3390/ijerph18031029
Krams IA, Jõers P, Luoto S, Trakimas G, Lietuvietis V, Krams R, Kaminska I, Rantala MJ, Krama T. The Obesity Paradox Predicts the Second Wave of COVID-19 to Be Severe in Western Countries. International Journal of Environmental Research and Public Health. 2021; 18(3):1029. https://doi.org/10.3390/ijerph18031029
Chicago/Turabian StyleKrams, Indrikis A., Priit Jõers, Severi Luoto, Giedrius Trakimas, Vilnis Lietuvietis, Ronalds Krams, Irena Kaminska, Markus J. Rantala, and Tatjana Krama. 2021. "The Obesity Paradox Predicts the Second Wave of COVID-19 to Be Severe in Western Countries" International Journal of Environmental Research and Public Health 18, no. 3: 1029. https://doi.org/10.3390/ijerph18031029
APA StyleKrams, I. A., Jõers, P., Luoto, S., Trakimas, G., Lietuvietis, V., Krams, R., Kaminska, I., Rantala, M. J., & Krama, T. (2021). The Obesity Paradox Predicts the Second Wave of COVID-19 to Be Severe in Western Countries. International Journal of Environmental Research and Public Health, 18(3), 1029. https://doi.org/10.3390/ijerph18031029






