Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.1.1. Population Cohort
2.1.2. Subsample Cohort
2.2. Mercury Analyses in Hair Specimens
2.3. Intra-Individual Variation Evaluated with Distal Hair Segment Analyses
2.4. Statistical Analyses
2.4.1. Reference Hair %MeHg Range
2.4.2. Predictors of Hair THg, Hair MeHg, and %MeHg
2.4.3. Hair THg-Hair MeHg Correlations
3. Results
3.1. Differences in Hair THg, MeHg, and %MeHg Contents between Communities
3.2. Other Demographic Variables Associated with Exposure
3.3. Factors Associated with Low %MeHg Values in Hair in Mining Communities
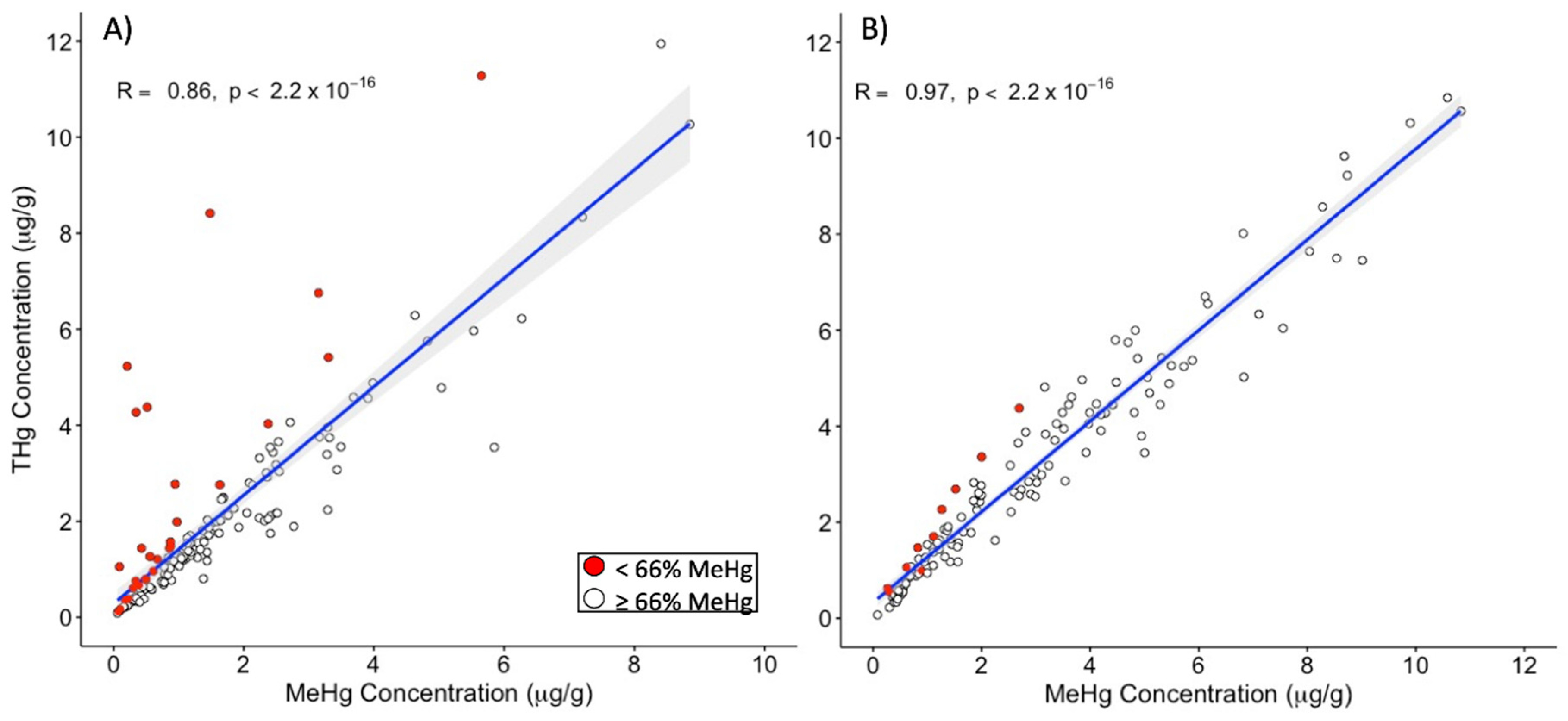
3.4. Hair THg as a Predictor of Hair MeHg Exposure
3.5. Subsample Selection Bias and Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN Environment Programme. Global Mercury Assessment 2018; United Nations: Geneva, Switzerland, 2018. [Google Scholar]
- Veiga, M.M.; Angeloci-Santos, G.; Meech, J.A. Review of barriers to reduce mercury use in artisanal gold mining. Extr. Ind. Soc. 2014, 1, 351–361. [Google Scholar] [CrossRef]
- Evers, D.C.; Keane, S.E.; Basu, N.; Buck, D. Evaluating the effectiveness of the minamata convention on mercury: Principles and recommendations for next steps. Sci. Total Environ. 2016, 569, 888–903. [Google Scholar] [CrossRef]
- UN Environment Programme. UNEP/MC/COP.3/14—Report of the Ad Hoc Technical Expert Group for Effectivess for Evaluation: Proposed Framework for the Effectiveness Evaluation of the Minamata Convention on Mercury; United Nations: Geneva, Switzerland, 2019. [Google Scholar]
- Feingold, B.J.; Berky, A.; Hsu-Kim, H.; Rojas, E.; Pan, W.K. Population-based dietary exposure to mercury through fish consumption in the Southern Peruvian Amazon. Environ. Res. 2019, 183, 108720. [Google Scholar] [CrossRef] [PubMed]
- Hacon, S.D.; Oliveira-Da-costa, M.; Gama, C.D.; Ferreira, R.; Basta, P.C.; Schramm, A.; Yokota, D. Mercury exposure through fish consumption in traditional communities in the Brazilian Northern Amazon. Int. J. Environ. Res. Public Health 2020, 17, 5269. [Google Scholar] [CrossRef]
- Salazar-Camacho, C.; Salas-Moreno, M.; Marrugo-Madrid, S.; Marrugo-Negrete, J.; Díez, S. Dietary human exposure to mercury in two artisanal small-scale gold mining communities of Northwestern Colombia. Environ. Int. 2017, 107, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Straaten, P. Mercury contamination associated with small-scale gold mining in Tanzania and Zimbabwe. Sci. Total Environ. 2000, 259, 105–113. [Google Scholar] [CrossRef]
- Mason, R.P.; Baumann, Z.; Hansen, G.; Yao, K.M.; Coulibaly, M.; Coulibaly, S. An assessment of the impact of artisanal and commercial gold mining on mercury and methylmercury levels in the Environment and Fish in Cote d’Ivoire. Sci. Total Environ. 2019, 665, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J.D.; Williams, T.M.; Breward, N.; Apostol, A.; Miguel, J.; Miranda, C. Mercury contamination associated with artisanal gold mining on the island of Mindanao, the Philippines. Sci. Total Environ. 1999, 228, 95–109. [Google Scholar] [CrossRef]
- Limbong, D.; Kumampung, J.; Rimper, J.; Arai, T.; Miyazaki, N. Emissions and environmental implications of mercury from artisanal gold mining in North Sulawesi, Indonesia. Sci. Total Environ. 2003, 302, 227–236. [Google Scholar] [CrossRef]
- Wyatt, L.; Ortiz, E.J.; Feingold, B.; Berky, A.; Diringer, S.; Morales, A.M.; Jurado, E.R.; Hsu-Kim, H.; Pan, W. Spatial, temporal, and dietary variables associated with elevated mercury exposure in peruvian riverine communities upstream and downstream of artisanal and small-scale gold mining. Int. J. Environ. Res. Public Health 2017, 14, 1582. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Toxicological Effects of Methylmercury; The National Academies Press: Washington, DC, USA, 2000. [CrossRef]
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Drasch, G.; Wanghofer, E.; Roider, G. Are blood, urine, hair, and muscle valid biomonitors for the internal burden of men with the heavy metals mercury, lead and cadmium? An investigation on 150 deceased. Trace Elem. Electrocytes 1997, 14, 116–123. [Google Scholar]
- Cernichiari, E.; Myers, G.J.; Ballatori, N.; Zareba, G.; Vyas, J.; Clarkson, T. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology 2007, 28, 1015–1022. [Google Scholar] [CrossRef]
- Clarkson, T.W. Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 2007, 764, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ASTDR). Toxicological Profile for Mercury; US Department of Health and Human Services: Atlanta, GA, USA, 1999.
- UN Environment Programme. Minamata Convention on Mercury. UNEP/MC/COP.4/INF/12—Guidance on Monitoring of Mercury and Mercury Compounds to Support the Effectiveness Evaluation of the Minamata Convention; United Nations: Geneva, Switzerland, 2021. [Google Scholar]
- Laffont, L.; Maurice, L.; Amouroux, D.; Navarro, P.; Monperrus, M.; Sonke, J.E.; Behra, P. Mercury speciation analysis in human hair by species specific isotope dilution using GC-ICP-MS. Anal. Bioanal. Chem. 2013, 405, 3001–3010. [Google Scholar] [CrossRef]
- Laffont, L.; Sonke, J.E.; Maurice, L.; Monrroy, S.L.; Chincheros, J.; Amouroux, D.; Behra, P. Hg Speciation and stable isotope signatures in human hair as a tracer for dietary and occupational exposure to mercury. Environ. Sci. Technol. 2011, 45, 9910–9916. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, K.L. Review: Interpreting hair mercury levels in individual patients. Ann. Clin. Lab. Sci. 2006, 36, 248–261. [Google Scholar]
- Sherman, L.S.; Blum, J.D.; Basu, N.; Rajaee, M.; Evers, D.C.; Buck, D.G.; Petrlik, J.; DiGangi, J. Assessment of mercury exposure among small-scale gold miners using mercury stable isotopes. Environ. Res. 2015, 137, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nakachi, S.; Cheu, T.; Hamada, H.; Ono, Y.; Tsuda, T.; Yanagida, K.; Kizaki, T.; Ohno, H. Monitoring of mercury pollution in Tanzania: Relation between head hair mercury and health. Sci. Total Environ. 1999, 227, 249–256. [Google Scholar] [CrossRef]
- Akagi, H.; Malm, O.; Branchesb, F.J.P.; Kinjoa, Y.; Kashima, Y.; Guimaraes, J.; Oliveira, R.; Haraguchi, K.; Pfeiffer, W.; Takizawa, Y.; et al. Human exposure to mercury due to goldmining in the Tapajos River basin, Amazon, Brazil: Speciation of mercury in human hair, blood, and urine. Water Air Soil Pollut. 1995, 80, 85–94. [Google Scholar] [CrossRef]
- Ikingura, J.R.; Akagib, H. Monitoring of fish and human exposure to mercury due to gold mining in the Lake Victoria goldfields, Tanzania. Sci. Total Environ. 1996, 9697, 96. [Google Scholar] [CrossRef]
- Malm, O.; Branchesb, F.J.P.; Akagi, H.; Castro, M.; Pfeiffer, W.C.; Haradac, M.; Bastosa, W.R.; Katob, H. Mercury and methylmercury in fish and human hair from the Tapajos River Basin, Brazil. Sci. Total Environ. 1995, 175, 141–150. [Google Scholar] [CrossRef]
- Calao-Ramos, C.; Bravo, A.G.; Paternina-Uribe, R.; Marrugo-Negrete, J.; Díez, S. Occupational human exposure to mercury in artisanal small-scale gold mining communities of Colombia. Environ. Int. 2021, 146, 106216. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; De Galan, S.; De Brauwere, A.; Baeyens, W.; Leermakers, M. Mercury speciation in hair by headspace injection-gas chromatography-atomic fluorescence spectrometry (methylmercury) and combustion-atomic absorption spectrometry (Total Hg). Talanta 2010, 82, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Kehrig, A.; Malm, O.; Akagi, H.; Guimara, J.R.D.; Torres, P.M. Methylmercury in Fish and Hair Samples from the Balbina Reservoir, Brazilian Amazon. Environ. Res. 1998, 77, 84–90. [Google Scholar] [CrossRef]
- Dermelj, M.; Horvat, M.; Byrne, A.R.; Stegnar, P. Mercury, methyl-mercury, and selenium in scalp hair of inhabitants from Mediterranean areas. Chemosphere 1987, 16, 877–886. [Google Scholar] [CrossRef]
- Al-Majed, N.B.; Preston, M.R. Factors influencing the total mercury and methyl mercury in the hair of the fishermen of Kuwait. Environ. Pollut. 2000, 109, 239–250. [Google Scholar] [CrossRef]
- Díez, S.; Delgado, S.; Aguilera, I.; Astray, J.; Pérez-Gómez, B.; Torrent, M.; Sunyer, J.; Bayona, J.M. Prenatal and early childhood exposure to mercury and methylmercury in Spain, a high-fish-consumer country. Arch. Environ. Contam. Toxicol. 2009, 56, 615–622. [Google Scholar] [CrossRef]
- Lebel, J.; Mergler, D.; Branches, F.; Lucotte, M.; Amorim, M.; Larribe, F.; Dolbec, J. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ. Res. 1998, 79, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Soria, M.L.; Sanz, P.; Martínez, D.; López-Artíguez, M.; Garrido, R.; Grilo, A.; Repetto, M. Total mercury and methylmercury in hair, maternal and umbilical blood, and placenta from women in the Seville Area. Bull. Environ. Contam. Toxicol. 1992, 48, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Akagi, H.; Malm, O.; Kinjo, Y.; Harada, M.; Branches, F.J.P.; Pfeiffer, W.C.; Kato, H. Methylmercury pollution in the Amazon, Brazil. Sci. Total Environ. 1995, 175, 85–95. [Google Scholar] [CrossRef]
- Sakamoto, M.; Feng, X.; Li, P.; Qiu, G.; Jiang, H.; Yoshida, M.; Iwata, T.; Liu, X.J.; Murata, K. High exposure of Chinese mercury mine workers to elemental mercury vapor and increased methylmercury levels in their hair. Environ. Health Prev. Med. 2007, 12, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Queipo Abad, S.; Rodríguez-González, P.; García Alonso, J.I. Evidence of the direct adsorption of mercury in human hair during occupational exposure to mercury vapour. J. Trace Elem. Med. Biol. 2016, 36, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, A.; Hachiya, N. Accumulation of inorganic mercury in hair of rats exposed to methylmercury or mercuric chloride. Tohoku J. Exp. Med. 2006, 210, 301–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berglund, M.; Lind, B.; Björnberg, K.A.; Palm, B.; Einarsson, Ö.; Vahter, M. Inter-individual variations of human mercury exposure biomarkers: A cross-sectional assessment. Environ. Health 2005, 11, 20. [Google Scholar] [CrossRef]
- Zareba, G.; Cernichiari, E.; Hojo, R.; Nitt, S.M.; Weiss, B.; Mumtaz, M.M.; Jones, D.E.; Clarkson, T.W. Validity of methyl mercury hair analysis: Mercury monitoring in human scalp/nude mouse model. J. Appl. Toxicol. 2007, 27, 511–518. [Google Scholar] [CrossRef] [PubMed]
- UN Environment Programme. Technical Background Report to the Global Mercury Assessment 2018; Arctic Monitoring and Assessment Programme: Oslo, Norway/UN Environment Programme, Chemicals and Health Branch: Geneva, Switzerland, 2019. [Google Scholar]
- Moody, K.H.; Hasan, K.M.; Aljic, S.; Blakeman, V.M.; Hicks, L.P.; Loving, D.C.; Moore, M.E.; Hammett, B.S.; Silva-González, M.; Seney, C.S.; et al. Mercury emissions from Peruvian gold shops: Potential ramifications for Minamata compliance in artisanal and small-scale gold mining communities. Environ. Res. 2020, 182, 109042. [Google Scholar] [CrossRef]
- Diringer, S.; Feingold, B.J.; Ortiz, E.J.; Gallis, J.A.; Arauo-Flores, J.M.; Berky, A.; Pan, W.K.Y.; Hsu-kim, H. River transport of mercury from artisanal and small-scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru †. Environ. Sci. Process. Impacts 2015, 17, 478–487. [Google Scholar] [CrossRef]
- Diringer, S.E.; Berky, A.J.; Marani, M.; Ortiz, E.J.; Karatum, O.; Plata, D.L.; Pan, W.K.; Hsu-Kim, H. Deforestation due to artisanal and small-scale gold mining exacerbates soil and mercury mobilization in Madre de Dios, Peru. Environ. Sci. Technol. 2020, 54, 286. [Google Scholar] [CrossRef]
- Asner, G.P.; Tupayachi, R. Accelerated losses of protected forests from gold mining in the Peruvian Amazon. Environ. Res. Lett. 2017, 12, 094004. [Google Scholar] [CrossRef]
- Asner, G.P.; Llactayo, W.; Tupayachi, R.; Ráez, E. Elevated rates of gold mining in the amazon revealed through high-resolution monitoring. Proc. Natl. Acad. Sci. USA 2013, 110, 18454–18459. [Google Scholar] [CrossRef] [PubMed]
- Swenson, J.J.; Carter, C.E.; Domec, J.C.; Delgado, C.I. Gold mining in the Peruvian Amazon: Global prices, deforestation, and mercury imports. PLoS ONE 2011, 6, e18875. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; McCord, S.A.; Driscoll, C.T.; Todorova, S.; Wu, S.; Araújo, J.F.; Vega, C.M.; Fernandez, L.E. Mercury contamination in riverine sediments and fish associated with artisanal and small-scale gold mining in Madre de Dios, Peru. Int. J. Environ. Res. Public Health 2018, 15, 1584. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Brush, M.; Rydberg, J.; Gamboa, N.; Storch, I.; Biester, H. Is mercury from small-scale gold mining prevalent in the southeastern Peruvian amazon? Environ. Pollut. 2016, 218, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, C.; Ortiz, E.J.; Berky, A.J.; Bullins, P.; Hare-grogg, J.; Morales, A.; Hsu-kim, H.; Pan, W.K. Hair mercury level is associated with anemia and micronutrient status in children living near artisanal and small-scale gold mining in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2018, 97, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, C.; Gallis, J.A.; Ortiz, E.; Berky, A.J.; Morales, A.M.; Diringer, S.E.; Harrington, J.; Bullins, P.; Rogers, L.; Hare, J.; et al. A population-based mercury exposure assessment near an artisanal and small scale gold mining site in the Peruvian Amazon. JESEE 2019, 31, 126–136. [Google Scholar] [CrossRef]
- Ashe, K. Elevated mercury concentrations in humans of Madre de Dios, Peru. PLoS ONE 2012, 7, e33305. [Google Scholar] [CrossRef] [PubMed]
- Yard, E.E.; Horton, J.; Schier, J.G.; Caldwell, K.; Sanchez, C.; Lewis, L.; Gastaňaga, C. Mercury exposure among artisanal gold miners in Madre de Dios, Peru: A cross-sectional study. J. Med. Toxicol. 2012, 8, 441–448. [Google Scholar] [CrossRef]
- Gonzalez, D.J.X.; Arain, A.; Fernandez, L.E. Mercury exposure, risk factors, and perceptions among women of childbearing age in an artisanal gold mining region of the Peruvian Amazon. Environ. Res. 2019, 179, 108786. [Google Scholar] [CrossRef]
- Reuben, A.; Frischtak, H.; Berky, A.; Ortiz, E.J.; Morales, A.M.; Hsu-Kim, H.; Pendergast, L.; Pan, W.K. Elevated Hair Mercury Levels Are Associated with Neurodevelopmental Deficits in Children Living near Artisanal and Small-Scale Gold Mining in Peru. GeoHealth 2020, 4, e2019GH000222. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.M.; Baker, R.F. Protocols for Environmental and Health Assessment of Mercury Released by Artisanal and Small-Scale Gold Miners; Global Mercury Project: Vienna, Austria, 2004. [Google Scholar]
- World Health Organization. Assessment of Prenatal Exposure to Mercury: Standard Operating Procedures; WHO Regional Office for Europe: Copenhagen, Denmark, 2018. [Google Scholar]
- Drasch, G.; Reilly, S.B.; Beinhoff, C.; Roider, G.; Maydl, S. The Mt. Diwata study on the philippines 1999—Assessing mercury intoxication of the population by small scale gold mining. Sci. Total Environ. 2001, 267, 151–168. [Google Scholar] [CrossRef]
- Morton, J.; Carolan, V.A.; Gardiner, P.H.E. Removal of exogenously bound elements from human hair by various washing procedures and determination by inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2002, 455, 23–34. [Google Scholar] [CrossRef]
- Li, Y.F.; Chen, C.; Li, B.; Wang, J.; Gao, Y.; Zhao, Y.; Chai, Z. Scalp hair as a biomarker in environmental and occupational mercury exposed populations: Suitable or not? Environ. Res. 2008, 107, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Schindler, B.K.; Jiménez, J.A.; Koch, H.M.; Angerer, J.; Rosado, M.; Gómez, S.; Casteleyn, L.; Kolossa-Gehring, M.; Becker, K.; et al. Mercury analysis in hair: Comparability and quality assessment within the transnational COPHES/DEMOCOPHES project. Environ. Res. 2015, 141, 24–30. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Jardim, W.; Dórea, J.G.; Fosberg, B.; Souza, J. Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro River Basin, Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2001, 40, 439–444. [Google Scholar] [CrossRef]
- Basu, N.; Clarke, E.; Green, A.; Calys-Tagoe, B.; Chan, L.; Dzodzomenyo, M.; Fobil, J.; Long, R.N.; Neitzel, R.L.; Obiri, S.; et al. Integrated assessment of artisanal and small-scale gold mining in Ghana-part 1: Human health review. Int. J. Environ. Res. Public Health 2015, 12, 5143–5176. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.J.; Veiga, M.M.; Veiga, A. Clean artisanal gold mining: A utopian approach? J. Clean. Prod. 2003, 11, 99–115. [Google Scholar] [CrossRef]
- Fraser, B. Peruvian gold rush threatens health and the environment. Environ. Sci. Technol. 2009, 43, 7162–7164. [Google Scholar] [CrossRef][Green Version]
- Marinho, J.S.; Lima, M.O.; De Oliveira Santos, E.C.; De Jesus, I.M.; Pinheiro, M.D.C.N.; Alves, C.N.; Muller, R.C.S. Mercury speciation in hair of children in three communities of the Amazon, Brazil. BioMed Res. Int. 2014, 2014, 945963. [Google Scholar] [CrossRef]
- Basu, N.; Horvat, M.; Evers, D.C.; Zastenskaya, I.; Weihe, P.; Tempowski, J. A state-of-the-science review of mercury biomarkers in human populations worldwide between 2000 and 2018. Environ. Health Perspect. 2018, 126, 106001. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Hopps, H. The biologic bases for using hair and nail for analyses of trace elements. Sci. Total Environ. 1977, 7, 71–89. [Google Scholar] [CrossRef]
- UN Environment Programme. Minamata Convention on Mercury; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Congreso de la República del Perú. Ley Para Fortalecer La Prevención, Mitigación y Atención de La Salud Afectada Por La Contaminación Con Metales Pesados y Otras. El Peurano 2021, 16001, 3. [Google Scholar]
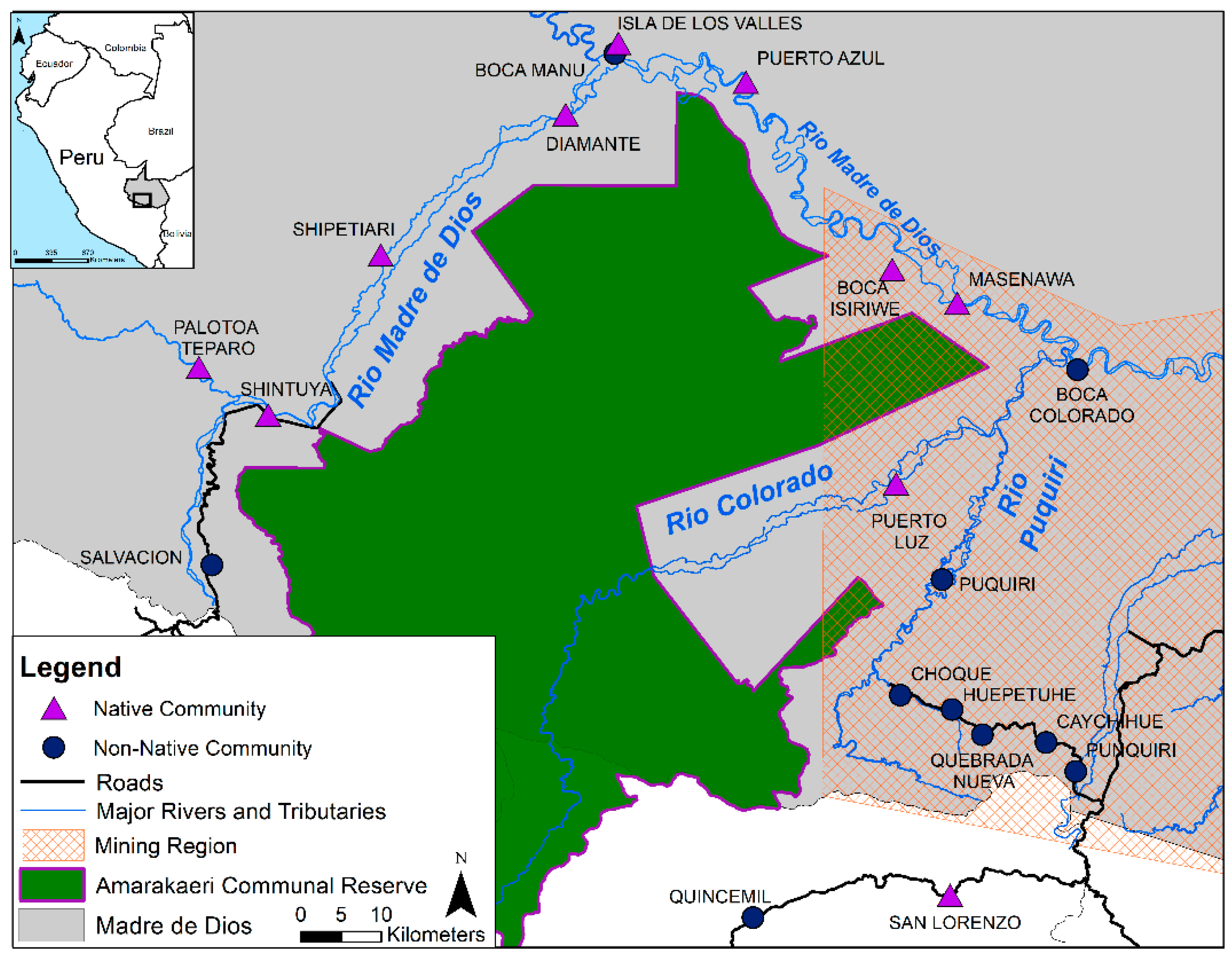

| Location | n | Males | Females | Age (Years) | MeHg (μg/g) | THg (μg/g) | %MeHg | Number of Individuals with %MeHg > 66% (Percentage of n) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Avg. ± s.d. | Min. | Max. | Avg. ± s.d. | Min. | Max. | Avg. ± s.d. | ||||||
| Outside Mining | 137 | 38 | 99 | 35.8 ± 10.4 | 0.080 | 10.8 | 2.87 ± 2.45 * | 0.070 | 10.8 | 3.04 ± 2.37 * | 16.0 | 145 | 91.3 ± 20.0 * | 127 (92.7%) |
| Within Mining | 150 | 46 | 104 | 36.2 ± 10.9 | 0.059 | 8.85 | 1.64 ± 1.58 | 0.090 | 11.9 | 2.14 ± 2.08 | 4.00 | 171 | 81.0 ± 25.9 | 121 (80.7%) |
| Native | 88 | 28 | 60 | 33.6 ± 8.96 | 0.45 | 10.8 | 3.92 ± 2.41 * | 0.403 | 11.3 | 4.15 ± 2.38 * | 34.0 | 145 | 93.4 ± 20.2 * | 81 (92.0%) |
| Non-Native | 199 | 56 | 143 | 37.0 ± 11.1 | 0.059 | 8.85 | 1.47 ± 1.47 | 0.070 | 11.9 | 1.87 ± 1.83 | 4.00 | 171 | 82.6 ± 24.7 | 167 (83.9) |
| Overall | 287 | 84 | 203 | 36.0 ± 10.6 | 0.059 | 10.8 | 2.22 ± 2.13 | 0.07 | 11.9 | 2.56 ± 2.27 | 4.00 | 171 | 86.0 ± 23.9 | 248 (86.4%) |
| Variable | %MeHg < 66% (N = 29) | %MeHg ≥ 66% (N = 121) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 12 | 41 | 34 | 28 |
| Female | 17 | 59 | 87 | 72 |
| Age | ||||
| <31 | 10 | 34 | 43 | 36 |
| 31–50 | 14 | 48 | 51 | 42 |
| >50 | 5 | 18 | 27 | 22 |
| Native Ethnicity | ||||
| Yes | 2 | 7 | 15 | 12 |
| No | 27 | 93 | 106 | 88 |
| Occupation | ||||
| Mining | 1 | 3 | 12 | 10 |
| Agriculture/Fishing | 2 | 7 | 4 | 3 |
| Other Outdoor | 1 | 3 | 4 | 3 |
| Professional/Urban | 6 | 21 | 41 | 34 |
| Self-employed/Other | 5 | 18 | 22 | 18 |
| No Job/Not Reported | 14 | 48 | 38 | 32 |
| Length of Residence | ||||
| Born Here | 4 | 14 | 19 | 16 |
| ≤5 years | 4 | 14 | 20 | 17 |
| >5 years | 16 | 54 | 73 | 60 |
| NA | 5 | 18 | 9 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koenigsmark, F.; Weinhouse, C.; Berky, A.J.; Morales, A.M.; Ortiz, E.J.; Pierce, E.M.; Pan, W.K.; Hsu-Kim, H. Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. Int. J. Environ. Res. Public Health 2021, 18, 13350. https://doi.org/10.3390/ijerph182413350
Koenigsmark F, Weinhouse C, Berky AJ, Morales AM, Ortiz EJ, Pierce EM, Pan WK, Hsu-Kim H. Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. International Journal of Environmental Research and Public Health. 2021; 18(24):13350. https://doi.org/10.3390/ijerph182413350
Chicago/Turabian StyleKoenigsmark, Faye, Caren Weinhouse, Axel J. Berky, Ana Maria Morales, Ernesto J. Ortiz, Eric M. Pierce, William K. Pan, and Heileen Hsu-Kim. 2021. "Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru" International Journal of Environmental Research and Public Health 18, no. 24: 13350. https://doi.org/10.3390/ijerph182413350
APA StyleKoenigsmark, F., Weinhouse, C., Berky, A. J., Morales, A. M., Ortiz, E. J., Pierce, E. M., Pan, W. K., & Hsu-Kim, H. (2021). Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. International Journal of Environmental Research and Public Health, 18(24), 13350. https://doi.org/10.3390/ijerph182413350






