Exercise as a Peripheral Circadian Clock Resynchronizer in Vascular and Skeletal Muscle Aging
Abstract
1. Introduction
2. Sarcopenia: Concept and Relationship with Inflammation
2.1. Crosstalk between Skeletal Muscle and Immune Cells
2.2. Inflammation and Circadian Misalignment: A Two-Way Road in Contributing to Sarcopenia
2.3. Vascular Disfunction and Sarcopenia
3. Impact of Exercise on Inflammatory Profile and Association with Clock Genes
4. Impact of Exercise on Circadian Skeletal Muscle Rhythm
5. Impact of Exercise on Vascular Circadian Rhythm
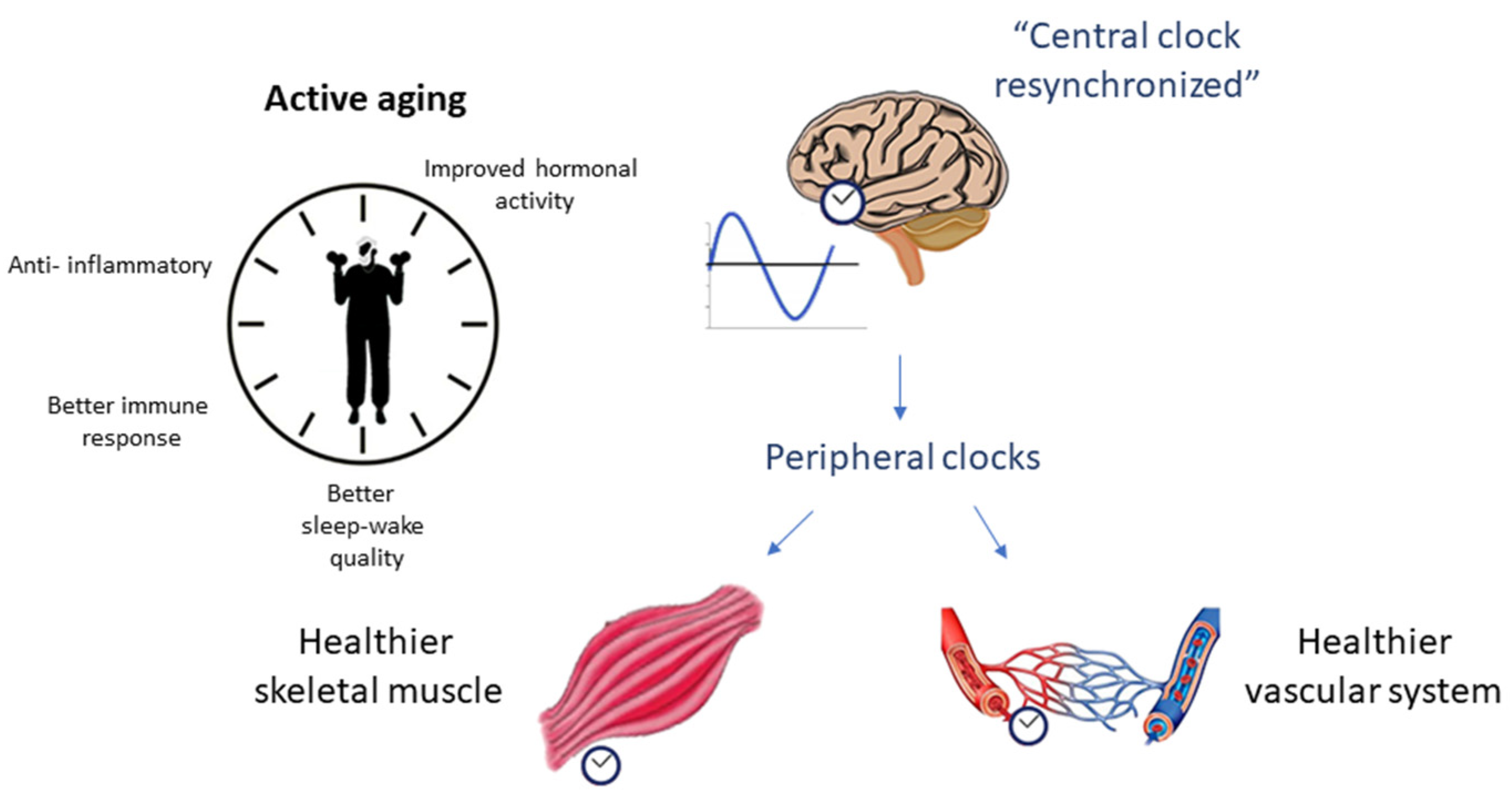
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Bonato, M.; La Torre, A.; Banfi, G. The Role of the Molecular Clock in Promoting Skeletal Muscle Growth and Protecting against Sarcopenia. Int. J. Mol. Sci. 2019, 20, 4318. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Entrainment of the mouse circadian clock: Effects of stress, exercise, and nutrition. Free. Radic. Biol. Med. 2017, 119, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Reddy, A. Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett. 2014, 588, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Kessel, L.; Lundeman, J.H.; Herbst, K.; Andersen, T.V.; Larsen, M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J. Cataract. Refract. Surg. 2010, 36, 308–312. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin and Human Rhythms. Chronobiol. Int. 2006, 23, 21–37. [Google Scholar] [CrossRef]
- Hodge, B.A.; Wen, Y.; Riley, L.A.; Zhang, X.; England, J.H.; Harfmann, B.D.; Schroder, E.A.; Esser, K. A The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 2015, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ma, K. Circadian clock regulation of skeletal muscle growth and repair. F1000Research 2016, 5, 1549. [Google Scholar] [CrossRef]
- Dyar, K.A.; Hubert, M.J.; Mir, A.A.; Ciciliot, S.; Lutter, D.; Greulich, F.; Quagliarini, F.; Kleinert, M.; Fischer, K.; Eichmann, T.O.; et al. Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol. 2018, 16, e2005886. [Google Scholar] [CrossRef]
- Fatima, N.; Rana, S. Metabolic implications of circadian disruption. Pflug. Arch. Eur. J. Physiol. 2020, 472, 513–526. [Google Scholar] [CrossRef]
- Andrews, J.L.; Zhang, X.; McCarthy, J.J.; McDearmon, E.L.; Hornberger, T.A.; Russell, B.; Campbell, K.S.; Arbogast, S.; Reid, M.B.; Walker, J.R.; et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. USA 2010, 107, 19090–19095. [Google Scholar] [CrossRef]
- Li, R.; Xia, J.; Zhang, X.I.; Gathirua-Mwangi, W.G.; Guo, J.; Li, Y.; McKenzie, S.; Song, Y. Associations of Muscle Mass and Strength with All-Cause Mortality among US Older Adults. Med. Sci. Sports Exerc. 2018, 50, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wehling-Henricks, M.; Welc, S.S.; Fisher, A.L.; Zuo, Q.; Tidball, J.G. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Addison, O.; Prior, S.J.; Kundi, R.; Serra, M.C.; Katzel, L.I.; Gardner, A.W.; Ryan, A.S. Sarcopenia in Peripheral Arterial Disease: Prevalence and Effect on Functional Status. Arch. Phys. Med. Rehabil. 2018, 99, 623–628. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, C.S.; Swirski, F.K. Circadian Influence on Metabolism and Inflammation in Atherosclerosis. Circ. Res. 2016, 119, 131–141. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Koster, A.; Schols, J.M.G.A.; Meijers, J.M.M.; Halfens, R.J.G.; Gudnason, V.; Eiriksdottir, G.; Siggeirsdottir, K.; Sigurdsson, S.; Jónsson, P.V.; et al. Physical activity and incidence of sarcopenia: The population-based AGES-Reykjavik Study. Age Ageing 2016, 45, 614–620. [Google Scholar] [CrossRef]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun. Ageing 2018, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2016, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Shardell, M.D.; Seliger, S.L.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Inflammation and Trajectory of Renal Function in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2018, 66, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Cipolletta, D.; Cohen, P.; Spiegelman, B.M.; Benoist, C.; Mathis, D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: Age, diet, and PPARγ effects. Proc. Natl. Acad. Sci. USA 2015, 112, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, C.E.; Wood, E.G.; Loschko, J.; Scagliotti, V.; Cassidy, F.C.; Robinson, M.E.; Feldhahn, N.; Castellano, L.; Voisin, M.-B.; Marelli-Berg, F.; et al. Visceral Adipose Tissue Immune Homeostasis Is Regulated by the Crosstalk between Adipocytes and Dendritic Cell Subsets. Cell Metab. 2018, 27, 588–601.e584. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2017, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor-α With Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. Ser. A 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Cielen, N.; Maes, K.; Gayan-Ramirez, G. Musculoskeletal Disorders in Chronic Obstructive Pulmonary Disease. BioMed Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Edward, F. Adolph Distinguished Lecture. Skeletal muscle atrophy: Multiple pathways leading to a common outcome. J. Appl. Physiol. 2020, 129, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Chen, Y.; John, J.; Moylan, J.; Jin, B.; Mann, D.L.; Reid, M.B. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005, 19, 362–370. [Google Scholar] [CrossRef]
- Hershko, A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005, 12, 1191–1197. [Google Scholar] [CrossRef]
- Madaro, L.; Passafaro, M.; Sala, D.; Etxaniz, U.; Lugarini, F.; Proietti, D.; Alfonsi, M.V.; Nicoletti, C.; Gatto, S.; De Bardi, M.; et al. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nature 2018, 20, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R.A. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 2015, 74, 27–34. [Google Scholar] [CrossRef]
- Chazaud, B.; Brigitte, M.; Yacoub-Youssef, H.; Arnold, L.; Gherardi, R.; Sonnet, C.; Lafuste, P.; Chretien, F. Dual and Beneficial Roles of Macrophages During Skeletal Muscle Regeneration. Exerc. Sport Sci. Rev. 2009, 37, 18–22. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Saclier, M.; Cuvellier, S.; Magnan, M.; Mounier, R.; Chazaud, B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013, 280, 4118–4130. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, S.; McPhee, J.S.; Murgatroyd, C.; Butler-Browne, G.; Stewart, C.E.; Al-Shanti, N. The lymphocyte secretome from young adults enhances skeletal muscle proliferation and migration, but effects are attenuated in the secretome of older adults. Physiol. Rep. 2015, 3, e12518. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef]
- Schiaffino, S.; Pereira, M.G.; Ciciliot, S.; Rovere-Querini, P. Regulatory T cells and skeletal muscle regeneration. FEBS J. 2016, 284, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediat. Inflamm. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; McKenzie, A.I.; Mahmassani, Z.; Petrocelli, J.; Nelson, D.B.; Lindsay, C.C.; Gardner, J.E.; Morrow, V.R.; Keefe, A.C.; Huffaker, T.B.; et al. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am. J. Physiol. Metab. 2019, 317, E85–E98. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018, 17, e12750. [Google Scholar] [CrossRef]
- Cavadini, G.; Petrzilka, S.; Kohler, P.; Jud, C.; Tobler, I.; Birchler, T.; Fontana, A. TNF- suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc. Natl. Acad. Sci. USA 2007, 104, 12843–12848. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Nakai, A.; Kaneshiro, K.; Hashimoto, N.; Suzuki, K.; Uchida, K.; Hashimoto, T.; Kawasaki, Y.; Tateishi, K.; Nakagawa, N.; et al. TNF-α induces expression of the circadian clock gene Bmal1 via dual calcium-dependent pathways in rheumatoid synovial cells. Biochem. Biophys. Res. Commun. 2018, 495, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Mirizio, G.G. Clock Genes, Inflammation and the Immune System-Implications for Diabetes, Obesity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9743. [Google Scholar] [CrossRef] [PubMed]
- Spengler, M.L.; Kuropatwinski, K.K.; Comas, M.; Gasparian, A.V.; Fedtsova, N.; Gleiberman, A.S.; Gitlin, I.I.; Artemicheva, N.M.; Deluca, K.A.; Gudkov, A.V.; et al. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc. Natl. Acad. Sci. USA 2012, 109, 14736–14737. [Google Scholar] [CrossRef] [PubMed]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.A.; Harfmann, B.D.; Zhang, X.; Srikuea, R.; England, J.H.; Hodge, B.A.; Wen, Y.; Riley, L.A.; Yu, Q.; Christie, A.; et al. Intrinsic muscle clock is necessary for musculoskeletal health. J. Physiol. 2015, 593, 5387–5404. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Yan, M.; Huang, R.; Wang, Y.; He, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zhang, F.; et al. CLOCK and BMAL1 Regulate Muscle Insulin Sensitivity via SIRT1 in Male Mice. Endocrinology 2016, 157, 2259–2269. [Google Scholar] [CrossRef]
- Choi, Y.I.; Park, D.K.; Chung, J.-W.; Kim, K.O.; Kwon, K.A.; Kim, Y.J. Circadian rhythm disruption is associated with an increased risk of sarcopenia: A nationwide population-based study in Korea. Sci. Rep. 2019, 9, 12015. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Abbatecola, A.M.; Chiodini, P.; Gallo, C.; Lakatta, E.; Sutton-Tyrrell, K.; Tylavsky, F.A.; Goodpaster, B.; de Rekeneire, N.; Schwartz, A.V.; Paolisso, G.; et al. Pulse wave velocity is associated with muscle mass decline: Health ABC study. AGE 2011, 34, 469–478. [Google Scholar] [CrossRef]
- Kohara, K.; Okada, Y.; Ochi, M.; Ohara, M.; Nagai, T.; Tabara, Y.; Igase, M. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J. Cachex-Sarcopenia Muscle 2017, 8, 557–566. [Google Scholar] [CrossRef]
- Dvoretskiy, S.; Lieblein-Boff, J.C.; Jonnalagadda, S.; Atherton, P.J.; Phillips, B.E.; Pereira, S.L. Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review. Nutrients 2020, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Shin, M.J.; Saini, S.K.; Custodero, C.; Aggarwal, M.; Anton, S.D.; Leeuwenburgh, C.; Mankowski, R.T. Vascular dysfunction as a potential culprit of sarcopenia. Exp. Gerontol. 2021, 145, 111220. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andres, V. Biological Versus Chronological Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, P.X.; Guo, X.H.; Huang, Q.B. Role of moesin, Src, and ROS in advanced glycation end product-induced vascular endothelial dysfunction. Microcirculation 2017, 24, e12358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Xu, M.J.; Cai, Y.; Zhao, G.; Guan, Y.; Kong, W.; Tang, C.; Wang, X. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int. 2011, 79, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Takemura, A.; Iijima, K.; Ota, H.; Son, B.K.; Ito, Y.; Ogawa, S.; Eto, M.; Akishita, M.; Ouchi, Y. Sirtuin 1 Retards Hyperphosphatemia-Induced Calcification of Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2011, 31, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yang, C.; Qu, S.-L.; Shao, Y.-D.; Zhou, C.-Y.; Chao, R.; Huang, L.; Zhang, C. S100 proteins in atherosclerosis. Clin. Chim. Acta 2019, 502, 293–304. [Google Scholar] [CrossRef]
- Al-Aly, Z. Phosphate, oxidative stress, and nuclear factor-κB activation in vascular calcification. Kidney Int. 2011, 79, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Nadra, I.; Mason, J.C.; Philippidis, P.; Florey, O.; Smythe, C.D.; McCarthy, G.M.; Landis, R.C.; Haskard, D.O. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ. Res. 2005, 96, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor Necrosis Factor-α Promotes In Vitro Calcification of Vascular Cells via the cAMP Pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef]
- Duncan, E.R.; Crossey, P.A.; Walker, S.; Anilkumar, N.; Poston, L.; Douglas, G.; Ezzat, V.A.; Wheatcroft, S.B.; Shah, A.M.; Kearney, M.T. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes 2008, 57, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, E.A.; Selby, A.L.; Atherton, P.J.; Patel, R.; Rankin, D.; Smith, K.; Rennie, M.J. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am. J. Clin. Nutr. 2009, 90, 1343–1350. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Cipriano, I.; Costa, T.; Saraiva, M.; Martins, A.; Consortium, A.G.L. Exercise, ageing and cognitive function—Effects of a personalized physical exercise program in the cognitive function of older adults. Physiol. Behav. 2019, 202, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Eng, J.J.; Brasher, P.M.; Madden, K.M.; Mohammadi, A.; Krassioukov, A.V.; Tsang, T.S. Physical Activity Correlates with Arterial Stiffness in Community-dwelling Individuals with Stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 259–266. [Google Scholar] [CrossRef]
- Zanetti, H.R.; Mendes, E.L.; Gonçalves, A.; Lopes, L.T.; Roever, L.; Silva-Vergara, M.L.; Neves, F.F.; Resende, E.S. Effects of exercise training and statin on hemodynamic, biochemical, inflammatory and immune profile of people living with HIV: A randomized, double-blind, placebo-controlled trial. J. Sports Med. Phys. Fit. 2020, 60, 1275–1282. [Google Scholar] [CrossRef]
- Teixeira, M.; Gouveia, M.; Duarte, A.; Ferreira, M.; Simoes, M.I.; Conceicao, M.; Silva, G.; Magalhaes, S.; Ferreira, R.; Nunes, A.; et al. Regular Exercise Participation Contributes to Better Proteostasis, Inflammatory Profile, and Vasoactive Profile in Patients With Hypertension. Am. J. Hypertens. 2019, 33, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Lavin, K.M.; Perkins, R.K.; Jemiolo, B.; Raue, U.; Trappe, S.W.; Trappe, T.A. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J. Appl. Physiol. 2020, 128, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Despeghel, M.; Reichel, T.; Zander, J.; Krüger, K.; Weyh, C. Effects of a 6 Week Low-Dose Combined Resistance and Endurance Training on T Cells and Systemic Inflammation in the Elderly. Cells 2021, 10, 843. [Google Scholar] [CrossRef]
- Mela, V.; Mota, B.C.; Milner, M.; McGinley, A.; Mills, K.H.G.; Kelly, A.M.; Lynch, M.A. Exercise-induced re-programming of age-related metabolic changes in microglia is accompanied by a reduction in senescent cells. Brain Behav. Immun. 2020, 87, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Mathot, E.; Liberman, K.; Cao Dinh, H.; Njemini, R.; Bautmans, I. Systematic review on the effects of physical exercise on cellular immunosenescence-related markers—An update. Exp. Gerontol. 2021, 149, 111318. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, M.J.; Bauerle, T.L. Repetitive seasonal drought causes substantial species-specific shifts in fine-root longevity and spatio-temporal production patterns in mature temperate forest trees. New Phytol. 2021, 231, 974–986. [Google Scholar] [CrossRef]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Farinha, J.B.; Steckling, F.M.; Stefanello, S.T.; Cardoso, M.S.; Nunes, L.S.; Barcelos, R.P.; Duarte, T.; Kretzmann, N.A.; Mota, C.B.; Bresciani, G.; et al. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med. Open 2015, 1, 19. [Google Scholar] [CrossRef]
- Chen, L.; Bai, J.; Li, Y. The Change of Interleukin-6 Level-Related Genes and Pathways Induced by Exercise in Sedentary Individuals. J. Interf. Cytokine Res. 2020, 40, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Svajger, U.; Tesic, N.; Rozman, P. Programmed death ligand 1 (PD-L1) plays a vital part in DC tolerogenicity induced by IFN-gamma. Int. Immunopharmacol. 2021, 99, 107978. [Google Scholar] [CrossRef] [PubMed]
- Rozman, P.; Svajger, U. The tolerogenic role of IFN-gamma. Cytokine Growth Factor Rev. 2018, 41, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Keaney, L.; Dulson, D.K. Acute hyperketonaemia alters T-cell-related cytokine gene expression within stimulated peripheral blood mononuclear cells following prolonged exercise. Eur. J. Appl. Physiol. 2020, 120, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Hasanli, S.; Hojjati, S.; Koushkie Jahromi, M. The Effect of Exercise and Psychological Stress on Anti- and Proinflammatory Cytokines. Neuroimmunomodulation 2020, 27, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghava, A. Behavior of plasma interferon-gamma with graded exercise in individuals with varied body mass index and age: Risk stratification of predisposition to inflammation. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 131–135. [Google Scholar] [CrossRef]
- Papini, C.B.; Nakamura, P.M.; Zorzetto, L.P.; Thompson, J.L.; Phillips, A.C.; Kokubun, E. The Effect of a Community-Based, Primary Health Care Exercise Program on Inflammatory Biomarkers and Hormone Levels. Mediat. Inflamm. 2014, 2014, 185707. [Google Scholar] [CrossRef]
- Liberman, K.; Forti, L.N.; Beyer, I.; Bautmans, I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: A systematic review. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Afsin, A.; Bozyilan, E.; Asoglu, R.; Hosoglu, Y.; Dundar, A.A. Effects of regular exercise on inflammatory biomarkers and lipid parameters in soccer players. J. Immunoass. Immunochem. 2021, 42, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.; Edirappuli, S.D.; Zaman, H.P.; Zaman, R. The Effect of Exercise on Mental Health: A Focus on Inflammatory Mechanisms. Psychiatr. Danub. 2020, 32, 105–113. [Google Scholar]
- González-Gil, E.M.; Santaliestra-Pasías, A.M.; Buck, C.; Gracia-Marco, L.; Lauria, F.; Pala, V.; Molnar, D.; Veidebaum, T.; Iacoviello, L.; Tornaritis, M.; et al. Improving cardiorespiratory fitness protects against inflammation in children: The IDEFICS study. Pediatr. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Bautmans, I.; Salimans, L.; Njemini, R.; Beyer, I.; Lieten, S.; Liberman, K. The effects of exercise interventions on the inflammatory profile of older adults: A systematic review of the recent literature. Exp. Gerontol. 2021, 146, 111236. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Esser, A.K. Exercise timing and circadian rhythms. Curr. Opin. Physiol. 2019, 10, 64–69. [Google Scholar] [CrossRef]
- Dickinson, J.M.; D’Lugos, A.C.; Naymik, M.A.; Siniard, A.L.; Wolfe, A.J.; Curtis, D.R.; Huentelman, M.J.; Carroll, C.C. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J. Appl. Physiol. 2018, 124, 1529–1540. [Google Scholar] [CrossRef]
- Lassiter, D.G.; Sjogren, R.J.O.; Gabriel, B.M.; Krook, A.; Zierath, J.R. AMPK activation negatively regulates GDAP1, which influences metabolic processes and circadian gene expression in skeletal muscle. Mol. Metab. 2018, 16, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Peek, C.B.; Levine, D.C.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian Clock Interaction with HIF1alpha Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2016, 25, 86–92. [Google Scholar] [CrossRef] [PubMed]
- de Souza Teixeira, A.A.; Minuzzi, L.G.; Lira, F.S.; Goncalves, A.; Martinho, A.; Rosa Neto, J.C.; Teixeira, A.M. Improvement in the anti-inflammatory profile with lifelong physical exercise is related to clock genes expression in effector-memory CD4+ T cells in master athletes. Exerc. Immunol. Rev. 2021, 27, 67–83. [Google Scholar] [PubMed]
- Tylutka, A.; Morawin, B.; Gramacki, A.; Zembron-Lacny, A. Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC Geriatr. 2021, 21, 200. [Google Scholar] [CrossRef]
- Popov, D.V.; Makhnovskii, P.A.; Kurochkina, N.S.; Lysenko, E.A.; Vepkhvadze, T.F.; Vinogradova, O.L. Intensity-dependent gene expression after aerobic exercise in endurance-trained skeletal muscle. Biol. Sport 2018, 35, 277–289. [Google Scholar] [CrossRef]
- Zambon, A.C.; McDearmon, E.L.; Salomonis, N.; Vranizan, K.M.; Johansen, K.L.; Adey, D.; Takahashi, J.S.; Schambelan, M.; Conklin, B.R. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 2003, 4, R61. [Google Scholar] [CrossRef]
- Wolff, G.; Esser, K.A. Scheduled Exercise Phase Shifts the Circadian Clock in Skeletal Muscle. Med. Sci. Sports Exerc. 2012, 44, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Zhang, H.; Mey, J.T.; Kirwan, J.P. Exercise Training Impacts Skeletal Muscle Clock Machinery in Prediabetes. Med. Sci. Sports Exerc. 2020, 52, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Hood, D.A. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. J. Appl. Physiol. 2013, 114, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Crnko, S.; Du Pre, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2017, 8, a029819. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hopman, M.T.E.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H.J. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Verheyden, B.; Aubert, E.A.; Fagard, R.H. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J. Hum. Hypertens. 2010, 24, 175–182. [Google Scholar] [CrossRef]
- Ross, M.D.; Malone, E.; Florida-James, G. Vascular Ageing and Exercise: Focus on Cellular Reparative Processes. Oxidative Med. Cell. Longev. 2015, 2016, 3583956. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M. Early vascular aging (EVA): Consequences and prevention. Vasc. Health Risk Manag. 2008, 4, 547–552. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Hashimoto, S.; Masubuchi, S.; Natsubori, A.; Nishide, S.-Y.; Honma, S.; Honma, K.-I. Differential regulation of circadian melatonin rhythm and sleep-wake cycle by bright lights and nonphotic time cues in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R546–R557. [Google Scholar] [CrossRef] [PubMed]
- Leonardo-Mendonça, R.C.; Martinez-Nicolas, A.; Galván, C.D.T.; Ocaña-Wilhelmi, J.; Rusanova, I.; Guerra-Hernández, E.; Escames, G.; Acuña-Castroviejo, D. The benefits of four weeks of melatonin treatment on circadian patterns in resistance-trained athletes. Chronobiol. Int. 2015, 32, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; Davis, H.C.; Lane, A.R. Exercise augments the nocturnal prolactin rise in exercise-trained men. Ther. Adv. Endocrinol. Metab. 2015, 6, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Heaney, J.L.; Carroll, D.; Whittaker, A. Physical Activity, Life Events Stress, Cortisol, and DHEA: Preliminary Findings That Physical Activity May Buffer Against the Negative Effects of Stress. J. Aging Phys. Act. 2014, 22, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Hower, I.M.; Harper, S.A.; Buford, T.W. Circadian Rhythms, Exercise, and Cardiovascular Health. J. Circadian Rhythm. 2018, 16, 7. [Google Scholar] [CrossRef]
- Rossi, A.; Formenti, D.; Vitale, J.A.; Calogiuri, G.; Weydahl, A. The Effect of Chronotype on Psychophysiological Responses during Aerobic Self-Paced Exercises. Percept. Mot. Ski. 2015, 121, 840–855. [Google Scholar] [CrossRef] [PubMed]
- Facer-Childs, E.R.; Brandstaetter, R. The Impact of Circadian Phenotype and Time since Awakening on Diurnal Performance in Athletes. Curr. Biol. 2015, 25, 518–522. [Google Scholar] [CrossRef]
- Fairbrother, K.; Cartner, B.; Alley, J.R.; Curry, C.D.; Dickinson, D.L.; Morris, D.M.; Collier, S.R. Effects of exercise timing on sleep architecture and nocturnal blood pressure in prehypertensives. Vasc. Health Risk Manag. 2014, 10, 691–698. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.S.d.A.; Uzeloto, J.S.; Lira, F.S.; Pereira, T.; Coelho-E-Silva, M.J.; Caseiro, A. Exercise as a Peripheral Circadian Clock Resynchronizer in Vascular and Skeletal Muscle Aging. Int. J. Environ. Res. Public Health 2021, 18, 12949. https://doi.org/10.3390/ijerph182412949
Silva BSdA, Uzeloto JS, Lira FS, Pereira T, Coelho-E-Silva MJ, Caseiro A. Exercise as a Peripheral Circadian Clock Resynchronizer in Vascular and Skeletal Muscle Aging. International Journal of Environmental Research and Public Health. 2021; 18(24):12949. https://doi.org/10.3390/ijerph182412949
Chicago/Turabian StyleSilva, Bruna Spolador de Alencar, Juliana Souza Uzeloto, Fábio Santos Lira, Telmo Pereira, Manuel J. Coelho-E-Silva, and Armando Caseiro. 2021. "Exercise as a Peripheral Circadian Clock Resynchronizer in Vascular and Skeletal Muscle Aging" International Journal of Environmental Research and Public Health 18, no. 24: 12949. https://doi.org/10.3390/ijerph182412949
APA StyleSilva, B. S. d. A., Uzeloto, J. S., Lira, F. S., Pereira, T., Coelho-E-Silva, M. J., & Caseiro, A. (2021). Exercise as a Peripheral Circadian Clock Resynchronizer in Vascular and Skeletal Muscle Aging. International Journal of Environmental Research and Public Health, 18(24), 12949. https://doi.org/10.3390/ijerph182412949








