The Disproportionate Impact of COVID-19 among Undocumented Immigrants and Racial Minorities in the US
Abstract
1. Introduction
2. Methods
2.1. Time Frames
2.2. Data Preprocessing
2.3. Spearman Rank Order Correlation
- The Spearman correlation coefficient between each meteorological feature, and daily COVID-19 prevalence/mortality of the counties was calculated for each time segment. In particular, we present the Spearman Correlation results of mean, maximum, and minimum temperature, total precipitation, and relative humidity against prevalence (Figure S3) and mortality (Figure S4);
- The Spearman correlation coefficient between the cumulative number of people vaccinated (per hundred) daily (predictor) and daily COVID-19 prevalence/mortality was calculated for the vaccination period;
- The Spearman correlation coefficient between socio-demographic, comorbidities features, unauthorized population and COVID-19 prevalence/mortality was calculated for each time segment;
- The Spearman correlation coefficient between each socio-demographic, comorbidities feature, and the number of people vaccinated per hundred (aggregated for the whole study period) was calculated for the vaccination period.
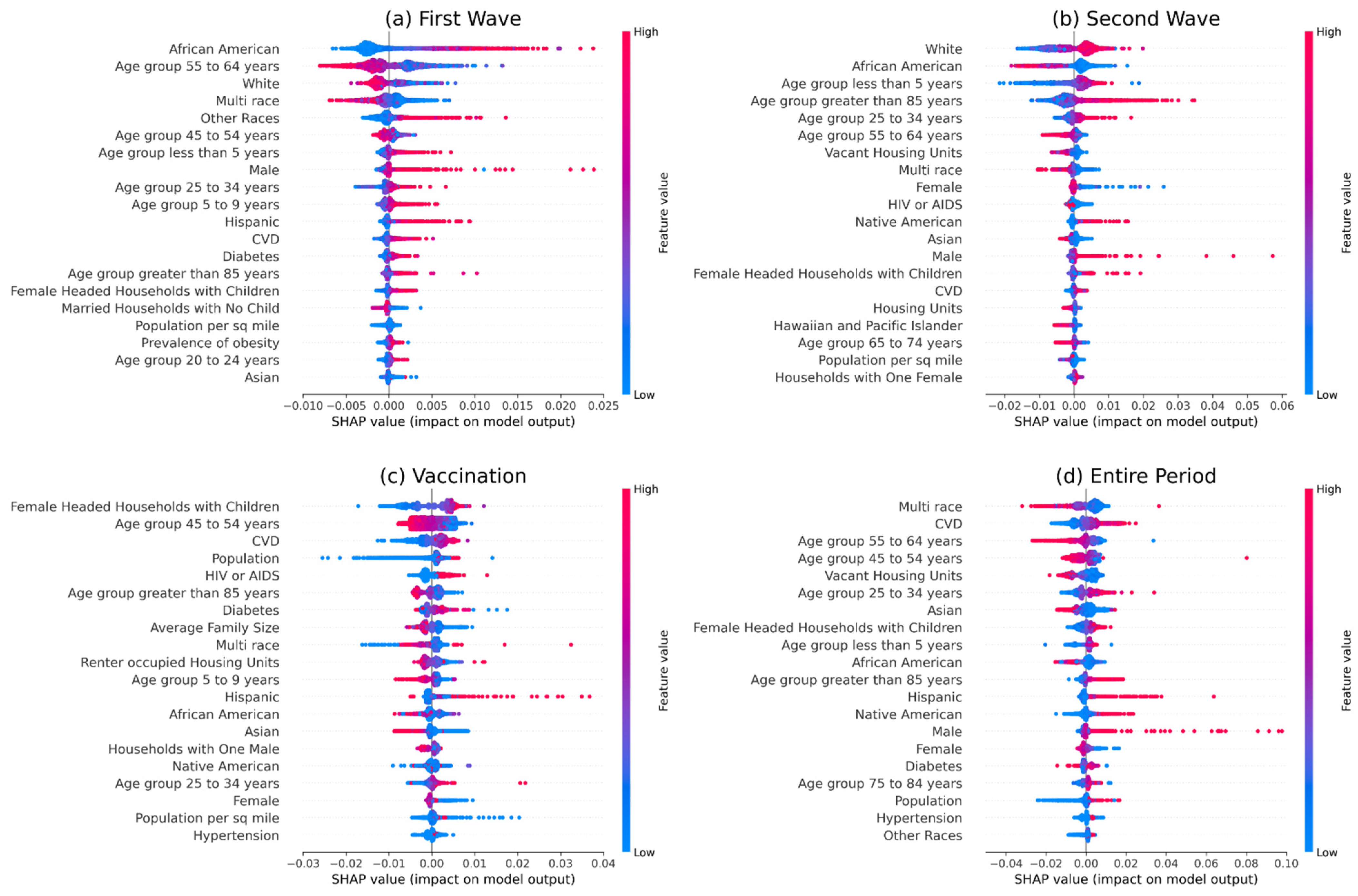
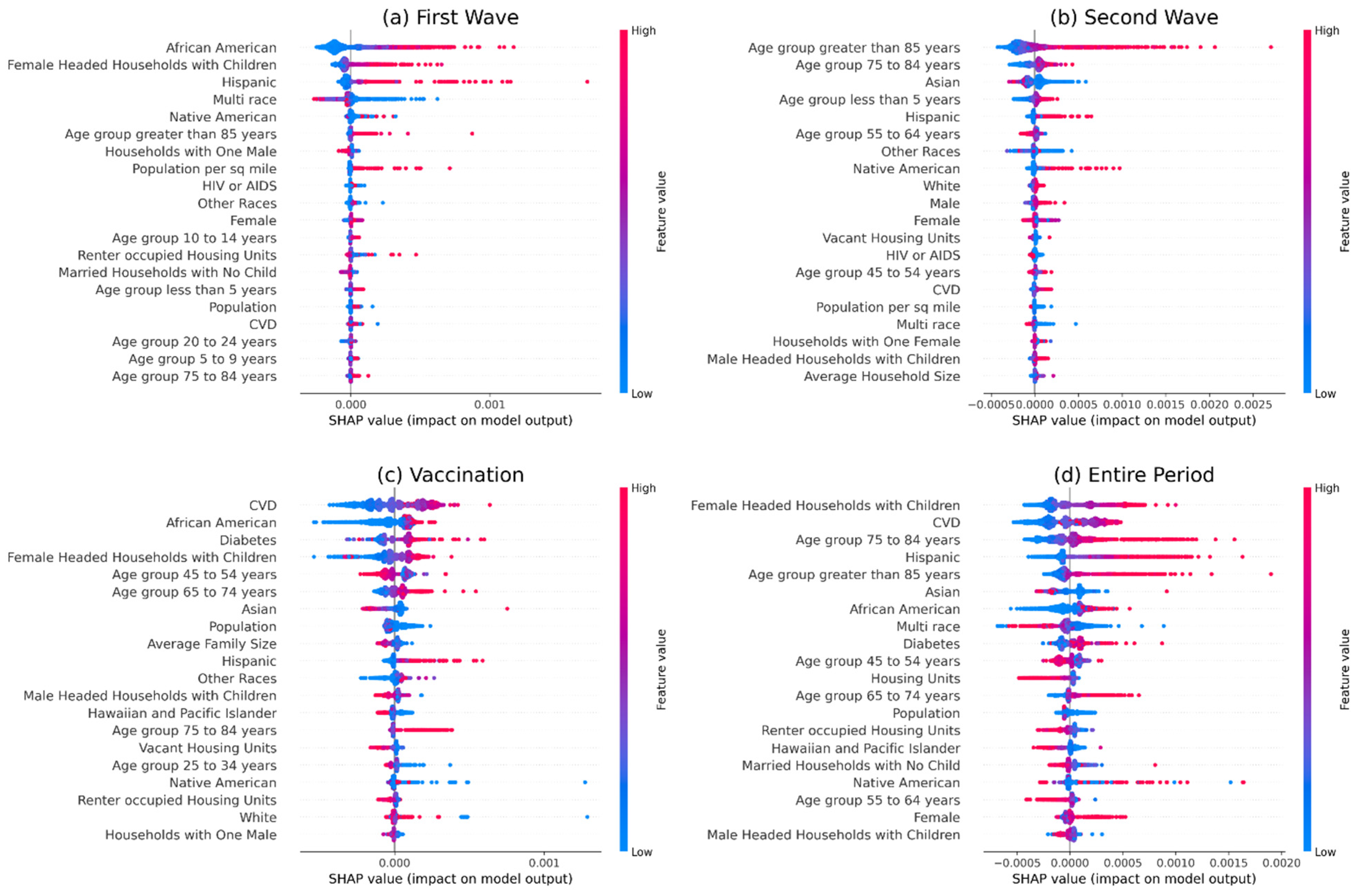
2.4. Feature Importance
2.5. Codes, Environment, and Availability
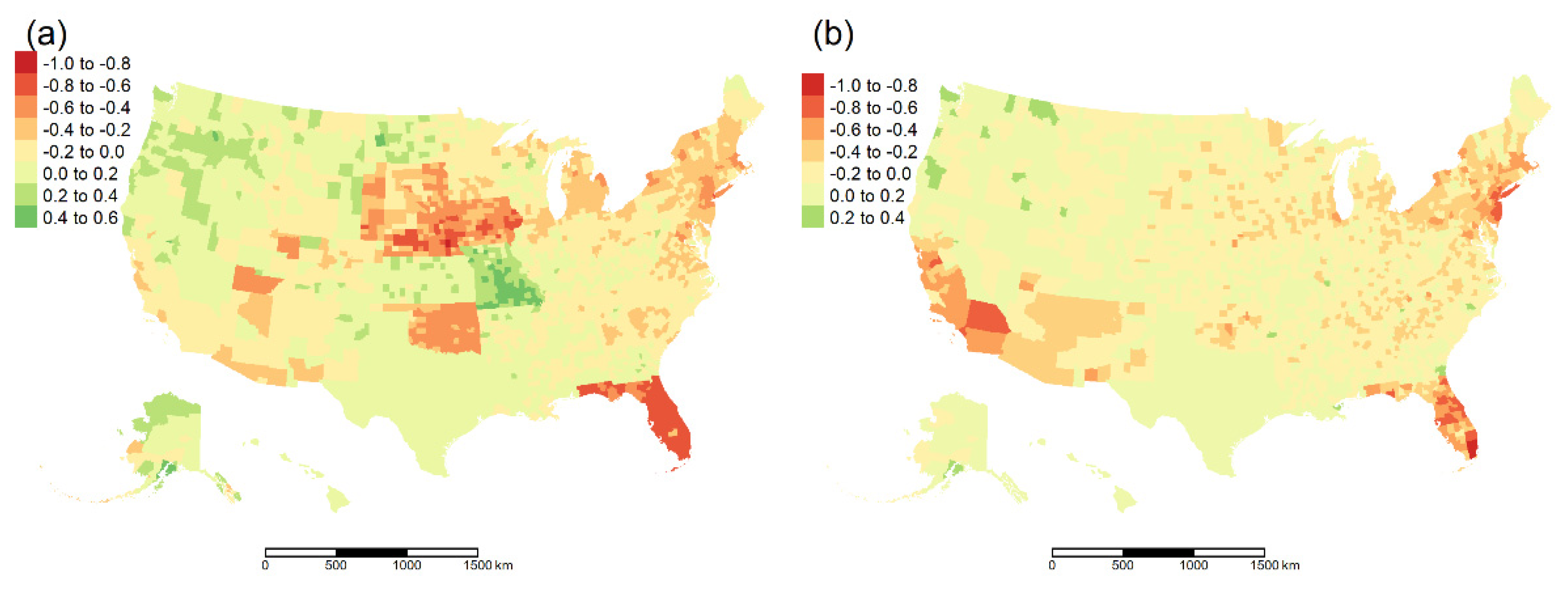
3. Results
3.1. Spearman Rank Order Correlation
3.2. Analysis of Vaccination Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mude, W.; Oguoma, V.M.; Nyanhanda, T.; Mwanri, L.; Njue, C. Racial disparities in COVID-19 pandemic cases, hospitalisations, and deaths: A systematic review and meta-analysis. J Glob Health 2021, 11, 05015. [Google Scholar] [CrossRef]
- Shadmi, E.; Chen, Y.; Dourado, I.; Faran-Perach, I.; Furler, J.; Hangoma, P.; Hanvoravongchai, P.; Obando, C.; Petrosyan, V.; Rao, K.D.; et al. Health equity and COVID-19: Global perspectives. Int. J. Equity Health 2020, 19, 104. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Shah, G.H.; Shankar, P.; Schwind, J.S.; Sittaramane, V. The Detrimental Impact of the COVID-19 Crisis on Health Equity and Social Determinants of Health. J. Public Health Manag. Pract. 2020, 26, 317–319. [Google Scholar] [CrossRef]
- Shrestha, N.; Shad, M.Y.; Ulvi, O.; Khan, M.H.; Karamehic-Muratovic, A.; Nguyen, U.D.T.; Baghbanzadeh, M.; Wardrup, R.; Aghamohammadi, N.; Cervantes, D.; et al. The impact of COVID-19 on globalization. One Health 2020, 11, 100180. [Google Scholar] [CrossRef] [PubMed]
- Peeri, N.C.; Shrestha, N.; Rahman, M.S.; Zaki, R.; Tan, Z.; Bibi, S.; Baghbanzadeh, M.; Aghamohammadi, N.; Zhang, W.; Haque, U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020, 49, 717–726. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, T.; Mui, D.; Ferrari, V.; Jagasia, D.; Scherrer-Crosbie, M.; Chen, Y.; Han, Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart 2020, 106, 1132–1141. [Google Scholar] [CrossRef]
- Zaki, N.; Alashwal, H.; Ibrahim, S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: A systematic review. Diabetes Metab. Syndr. 2020, 14, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, A.; Rahimi, L.; Ismail-Beigi, F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J. Diabetes 2020, 12, 895–908. [Google Scholar] [CrossRef]
- Aggarwal, G.; Lippi, G.; Lavie, C.J.; Henry, B.M.; Sanchis-Gomar, F. Diabetes mellitus association with coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. J. Diabetes 2020, 12, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.B.G.; Shah, A.; Doubeni, C.A.; Sia, I.G.; Wieland, M.L. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin. Infect. Dis. 2021, 72, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; Felix, S.E.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, January 22–May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Killerby, M.E.; Link-Gelles, R.; Haight, S.C.; Schrodt, C.A.; England, L.; Gomes, D.J.; Shamout, M.; Pettrone, K.; O’Laughlin, K.; Kimball, A.; et al. Characteristics Associated with Hospitalization Among Patients with COVID-19—Metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 790–794. [Google Scholar] [CrossRef]
- Rossen, L.M.; Ahmad, F.B.; Anderson, R.N.; Branum, A.M.; Du, C.; Krumholz, H.M.; Li, S.-X.; Lin, Z.; Marshall, A.; Sutton, P.D.; et al. Disparities in Excess Mortality Associated with COVID-19—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1114–1119. [Google Scholar] [CrossRef]
- Van Dyke, M.E.; Mendoza, M.C.; Li, W.; Parker, E.M.; Belay, B.; Davis, E.M.; Quint, J.J.; Penman-Aguilar, A.; Clarke, K.E.N. Racial and Ethnic Disparities in COVID-19 Incidence by Age, Sex, and Period Among Persons Aged <25 Years—16 U.S. Jurisdictions, January 1–December 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 382–388. [Google Scholar] [CrossRef]
- Page, K.R.; Venkataramani, M.; Beyrer, C.; Polk, S. Undocumented U.S. Immigrants and Covid-19. N. Engl. J. Med. 2020, 382, e62. [Google Scholar] [CrossRef] [PubMed]
- Galvan, T.; Lill, S.; Garcini, L.M. Another Brick in the Wall: Healthcare Access Difficulties and Their Implications for Undocumented Latino/a Immigrants. J. Immigr. Minor. Health 2021, 23, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Bozio, C.H.; Grannis, S.J.; Naleway, A.L.; Ong, T.C.; Butterfield, K.A.; DeSilva, M.B.; Natarajan, K.; Yang, D.; Rao, S.; Klein, N.P.; et al. Laboratory-Confirmed COVID-19 among Adults Hospitalized with COVID-19–Like Illness with Infection-Induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity—Nine States, January–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Fredricks, K.; Woc-Colburn, L.; Bottazzi, M.E.; Weatherhead, J. Disproportionate impact of the COVID-19 pandemic on immigrant communities in the United States. PLoS Negl. Trop. Dis. 2020, 14, e0008484. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Fu, S.; Wang, B.; Zhou, J.; Xu, X.; Liu, J.; Ma, Y.; Li, L.; He, X.; Li, S.; Niu, J.; et al. Meteorological factors, governmental responses and COVID-19: Evidence from four European countries. Environ. Res. 2021, 194, 110596. [Google Scholar] [CrossRef]
- Kerr, G.H.; Badr, H.S.; Gardner, L.M.; Perez-Saez, J.; Zaitchik, B.F. Associations between meteorology and COVID-19 in early studies: Inconsistencies, uncertainties, and recommendations. One Health 2021, 12, 100225. [Google Scholar] [CrossRef]
- Khan, I.M.; Haque, U.; Zhang, W.; Zafar, S.; Wang, Y.; He, J.; Sun, H.; Lubinda, J.; Rahman, M.S. COVID-19 in China: Risk Factors and R0 Revisited. Acta Trop. 2021, 213, 105731. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, J.; Yao, J.; Zhang, X.; Li, L.; Xu, X.; He, X.; Wang, B.; Fu, S.; Niu, T.; et al. Impact of meteorological factors on the COVID-19 transmission: A multi-city study in China. Sci. Total Environ. 2020, 726, 138513. [Google Scholar] [CrossRef]
- Vollert, N. Gaussian Process Surrogates with Increased Flexibility For Computer Simulation Output. Ph.D. Thesis, AAU Klagenfurt, Klagenfurt, Austria, 2020. [Google Scholar]
- Mesinger, F.; DiMego, G.; Kalnay, E.; Mitchell, K.; Shafran, P.C.; Ebisuzaki, W.; Jović, D.; Woollen, J.; Rogers, E.; Berbery, E.H.; et al. North American regional reanalysis. Bull. Am. Meteorol. Soc. 2006, 87, 343–360. [Google Scholar] [CrossRef]
- Gramacy, R.B. Surrogates. Gaussian Process Modeling, Design, and Optimization for the Applied Sciences; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Shabbir, W.; Pilz, J. Bayesian Spatio-temporal Analysis for Dengue Fever in Major Cities of Pakistan (2006–2017). In Proceedings of the 12th RSEP International Social Sciences Conference, Barcelona, Spain, 23–25 April 2019; Veysel, M., Chodnicka-Jaworska, P., Eds.; pp. 1–9, ISBN 978-605-80676-0-8. ISSN 2547-9385. [Google Scholar]
- Wikle, C.K.; Zammit-Mangion, A.; Cressie, N. Spatio-Temporal Statistics with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Tsang, T.K.; Wu, P.; Lin, Y.; Lau, E.H.Y.; Leung, G.M.; Cowling, B.J. Effect of changing case definitions for COVID-19 on the epidemic curve and transmission parameters in mainland China: A modelling study. Lancet Public Health 2020, 5, e289–e296. [Google Scholar] [CrossRef]
- Griffin, S. Covid-19: Second wave death rate is doubling fortnightly but is lower and slower than in March. BMJ 2020, 371, m4092. [Google Scholar] [CrossRef] [PubMed]
- Iftimie, S.; Lopez-Azcona, A.F.; Vallverdu, I.; Hernandez-Flix, S.; de Febrer, G.; Parra, S.; Hernandez-Aguilera, A.; Riu, F.; Joven, J.; Andreychuk, N.; et al. First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. PLoS ONE 2021, 16, e0248029. [Google Scholar] [CrossRef]
- Rothman, K.J. No adjustments are needed for multiple-comparisons. Epidemiology 1990, 1, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Batalova, J.; Hooker, S.; Capps, R. DACA at the Two-Year Mark: A National and State Profile of Youth Eligible and Applying for Deferred Action; MPI: Washington, DC, USA, 2014; Available online: https://www.migrationpolicy.org/research/daca-two-year-mark-national-and-state-profile-youth-eligible-and-applying-deferred-action (accessed on 4 November 2021).
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. Available online: https://arxiv.org/pdf/1705.07874.pdf (accessed on 4 November 2021).
- Shapley, L.S. 17. A Value for n-Person Games. In Contributions to the Theory of Games (AM-28); Princeton University Press: Princeton, NJ, USA, 2016; Volume II, pp. 307–318. [Google Scholar]
- Appice, A.; Gel, Y.R.; Iliev, I.; Lyubchich, V.; Malerba, D. A Multi-Stage Machine Learning Approach to Predict Dengue Incidence: A Case Study in Mexico. IEEE Access 2020, 8, 52713–52725. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Esri: How Hot Spot Analysis (Getis-Ord Gi*) Works. Available online: http://help.arcgis.com/en/arcgisdesktop/10.0/help/index.html#/How_Hot_Spot_Analysis_Getis_Ord_Gi_works/005p00000011000000/] (accessed on 1 November 2021).
- Hacker, K.; Anies, M.; Folb, B.L.; Zallman, L. Barriers to health care for undocumented immigrants: A literature review. Risk Manag. Healthc. Policy 2015, 8, 175–183. [Google Scholar] [CrossRef]
- Wallace, S.P.; Rodriguez, M.; Padilla-Frausto, I.; Arredondo, A.; Orozco, E. Improving access to health care for undocumented immigrants in the United States. Salud Publica Mex. 2013, 55, S508–S514. [Google Scholar] [CrossRef]
- Maldonado-Cabrera, A.; Angulo-Molina, A.; Haque, U.; Velazquez, C.; Alvarez-Villasenor, A.S.; Santacruz-Gomez, K.J.; Gallego-Hernandez, A.L. Acute Inflammatory Mediators in Young Adult Patients with COVID-19 in Mexico. Pathogens 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Mangla, S.; Pathak, A.K.; Arshad, M.; Ghosh, D.; Sahoo, P.K.; Garg, V.K.; Haque, U. Impact of Environmental Indicators on the COVID-19 Pandemic in Delhi, India. Pathogens 2021, 10, 1003. [Google Scholar] [CrossRef]
- Hill, J.; Rodriguez, D.X.; McDaniel, P.N. Immigration status as a health care barrier in the USA during COVID-19. J. Migr. Health 2021, 4, 100036. [Google Scholar] [CrossRef]
- Page, K.R.; Flores-Miller, A. Lessons We’ve Learned—Covid-19 and the Undocumented Latinx Community. N. Engl. J. Med. 2021, 384, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mangla, S.; Zohra Makkia, F.T.; Pathak, A.K.; Robinson, R.; Sultana, N.; Koonisetty, K.S.; Karamehic-Muratovic, A.; Nguyen, U.S.D.; Rodriguez-Morales, A.J.; Sanchez-Duque, J.A.; et al. COVID-19 Vaccine Hesitancy and Emerging Variants: Evidence from Six Countries. Behav. Sci. 2021, 11, 148. [Google Scholar] [CrossRef]
- Jony, S.S.R.; Haque, U.; Webb, N.J.; Spence, E.; Rahman, M.S.; Aghamohammadi, N.; Lie, Y.; Angulo-Molina, A.; Ananth, S.; Ren, X.; et al. Analyzing Predictors of Control Measures and Psychosocial Problems Associated with COVID-19 Pandemic: Evidence from Eight Countries. Behav. Sci. 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; KaramehicMuratovic, A.; Amrin, M.; Chowdhury, A.H.; Mondol, M.S.; Haque, U.; Ali, P. COVID-19 Epidemicin Bangladesh among Rural and Urban Residents: An Online Cross-Sectional Survey of Knowledge, Attitudes, and Practices. Epidemiologia 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Polverino, F.; Stern, D.A.; Ruocco, G.; Balestro, E.; Bassetti, M.; Candelli, M.; Cirillo, B.; Contoli, M.; Corsico, A.; D’Amico, F.; et al. Comorbidities, Cardiovascular Therapies, and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO). Front. Cardiovasc. Med. 2020, 7, 585866. [Google Scholar] [CrossRef] [PubMed]
- Michelozzi, P.; de’Donato, F.; Scortichini, M.; Pezzotti, P.; Stafoggia, M.; De Sario, M.; Costa, G.; Noccioli, F.; Riccardo, F.; Bella, A.; et al. Temporal dynamics in total excess mortality and COVID-19 deaths in Italian cities. BMC Public Health 2020, 20, 1238. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Axfors, C.; Contopoulos-Ioannidis, D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020, 188, 109890. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, S.; Holt, C.; Schultz, J.; Rabinowitz, P. Section 22: Using small area analysis to uncover disparities. In Chapter 3: Assessing Community Needs and Resources; Published 2016; Available online: http://ctb.ku.edu/en/table-of-contents/assessment/assessing-community-needs-and-resources/small-area-analysis/main. (accessed on 8 May 2017).
- Talbot, T.O.; Haley, V.B.; Dimmick, W.F.; Paulu, C.; Talbott, E.O.; Rager, J. Developing consistent data and methods to measure the public health impacts of ambient air quality for Environmental Public Health Tracking: Progress to date and future directions. Air Qual. Atmos. Health 2009, 2, 199–206. [Google Scholar] [CrossRef][Green Version]

| Feature | Description |
|---|---|
| Mean Temperature | The average temperature of the day in degrees Celsius |
| Min Temperature | Minimum temperature of the day in degree Celsius |
| Max Temperature | Maximum temperature of the day in degree Celsius |
| Total Precipitation | Total precipitation of the day in millimeter |
| Relative Humidity | Relative humidity of the day in percentage |
| Outcome Variable | Time Segment | Best Model |
|---|---|---|
| COVID-19 prevalence | First wave | Random Forest with 500 estimators |
| Second wave | Random Forest with 500 estimators | |
| Vaccination | XGBoost with 300 estimators | |
| The entire study period | XGBoost with 300 estimators | |
| COVID-19 mortality | First wave | Random Forest with 500 estimators |
| Second wave | Random Forest with 300 estimators | |
| Vaccination | XGBoost with 150 estimators | |
| The entire study period | XGBoost with 180 estimators | |
| People vaccinated with one dose (per hundred) | Vaccination | XGBoost with 700 estimators |
| People vaccinated with two doses (per hundred) | XGBoost with 550 estimators |
| Feature (County Level) | Time Segment | Spearman Correlation Coefficient |
|---|---|---|
| COVID-19 prevalence | First wave | 0.83 |
| Second wave | 0.75 | |
| Vaccination | 0.72 | |
| The entire study period | 0.77 | |
| COVID-19 mortality | First wave | 0.74 |
| Second wave | 0.68 | |
| Vaccination | 0.71 | |
| The entire study period | 0.75 |
| Predictor | Spearman Coefficient |
|---|---|
| Asian | 0.42 |
| Population | 0.40 |
| CVD | −0.35 |
| Population per sq. mile | 0.31 |
| Diabetes | −0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan Bhuiyan, M.T.; Mahmud Khan, I.; Rahman Jony, S.S.; Robinson, R.; Nguyen, U.-S.D.T.; Keellings, D.; Rahman, M.S.; Haque, U. The Disproportionate Impact of COVID-19 among Undocumented Immigrants and Racial Minorities in the US. Int. J. Environ. Res. Public Health 2021, 18, 12708. https://doi.org/10.3390/ijerph182312708
Hasan Bhuiyan MT, Mahmud Khan I, Rahman Jony SS, Robinson R, Nguyen U-SDT, Keellings D, Rahman MS, Haque U. The Disproportionate Impact of COVID-19 among Undocumented Immigrants and Racial Minorities in the US. International Journal of Environmental Research and Public Health. 2021; 18(23):12708. https://doi.org/10.3390/ijerph182312708
Chicago/Turabian StyleHasan Bhuiyan, Mohammad Tawhidul, Irtesam Mahmud Khan, Sheikh Saifur Rahman Jony, Renee Robinson, Uyen-Sa D. T. Nguyen, David Keellings, M. Sohel Rahman, and Ubydul Haque. 2021. "The Disproportionate Impact of COVID-19 among Undocumented Immigrants and Racial Minorities in the US" International Journal of Environmental Research and Public Health 18, no. 23: 12708. https://doi.org/10.3390/ijerph182312708
APA StyleHasan Bhuiyan, M. T., Mahmud Khan, I., Rahman Jony, S. S., Robinson, R., Nguyen, U.-S. D. T., Keellings, D., Rahman, M. S., & Haque, U. (2021). The Disproportionate Impact of COVID-19 among Undocumented Immigrants and Racial Minorities in the US. International Journal of Environmental Research and Public Health, 18(23), 12708. https://doi.org/10.3390/ijerph182312708









