Differential Cutaneous Thermal Sensitivity in Humans: Method of Limit vs. Method of Sensation Magnitude
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Statistical Analyses
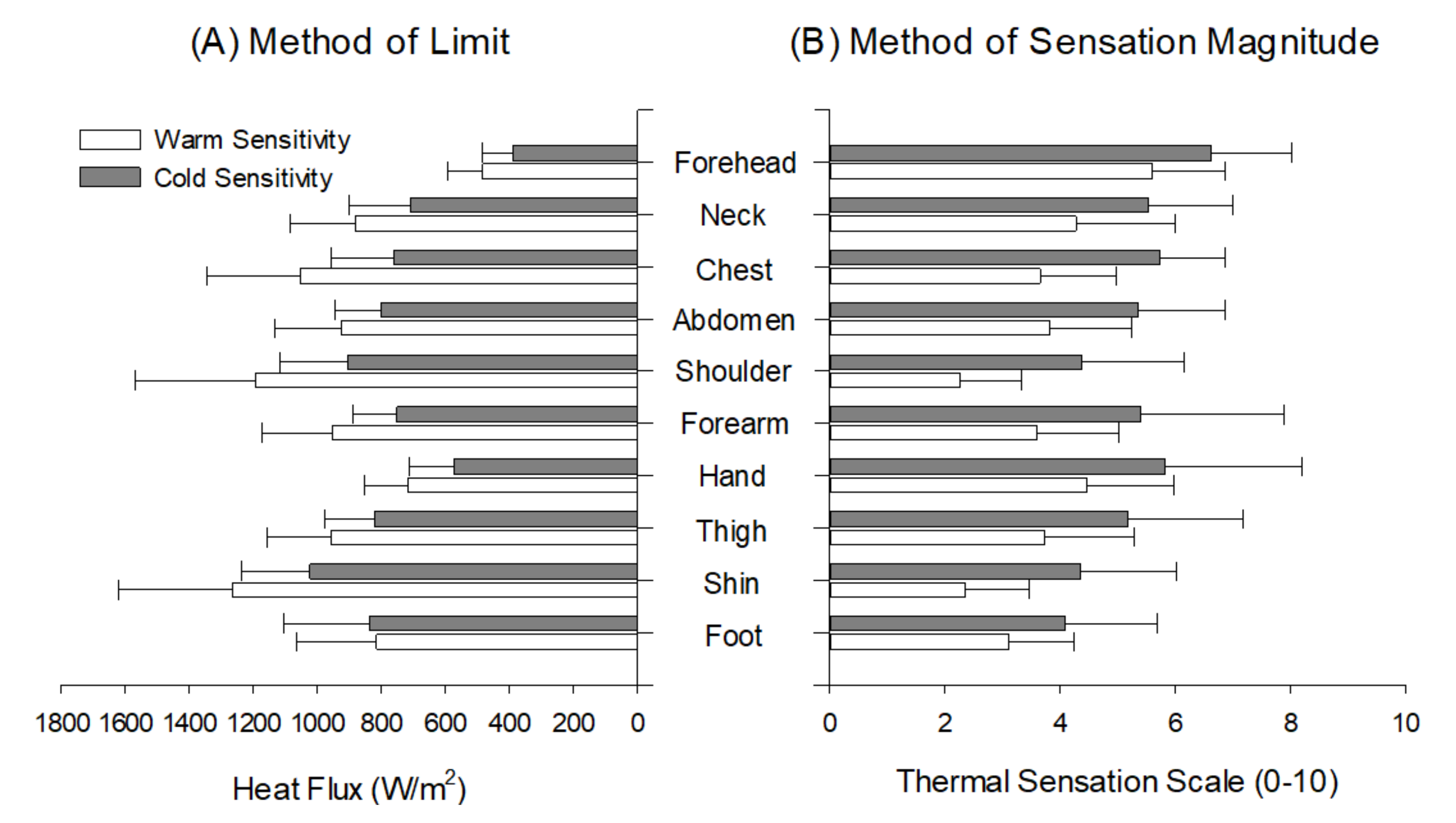
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filingeri, D.; Zhang, H.; Arens, E.A. Characteristics of the local cutaneous sensory thermoneutral zone. J. Neurophysiol. 2017, 117, 1797–1806. [Google Scholar] [CrossRef]
- Hensel, H. Thermoreception and temperature regulation. Monogr. Physiol. Soc. 1981, 38, 1–321. [Google Scholar]
- Nakamura, M.; Yoda, T.; Crawshaw, L.I.; Kasuga, M.; Uchida, Y.; Tokizawa, K.; Nagashima, K.; Kanosue, K. Relative importance of different surface regions for thermal comfort in humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 113, 63–76. [Google Scholar] [CrossRef]
- Nakamura, M.; Yoda, T.; Crawshaw, L.I.; Yasuhara, S.; Saito, Y.; Kasuga, M.; Nagashima, K.; Kanosue, K. Regional differences in temperature sensation and thermal comfort in humans. J. Appl. Physiol. 2008, 105, 1897–1906. [Google Scholar] [CrossRef]
- Coull, N.A.; West, A.M.; Hodder, S.G.; Wheeler, P.; Havenith, G. Body mapping of regional sweat distribution in young and older males. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 121, 109–125. [Google Scholar] [CrossRef]
- Flouris, A.D. Functional architecture of behavioural thermoregulation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 111, 1–8. [Google Scholar] [CrossRef]
- Lue, Y.-J.; Shih, Y.-C.; Lu, Y.-M.; Liu, Y.-F. Method of Limit and Method of Level for Thermal and Pain Detection Assessment. Int. J. Phys. Ther. Rehabil. 2017, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D.; Sprecher, E. Thermal testing: Normative data and repeatability for various test algorithms. J. Neurol. Sci. 1994, 125, 39–45. [Google Scholar] [CrossRef]
- Moloney, N.A.; Hall, T.; Doody, C.M. Reliability of thermal quantitative sensory testing: A systematic review. J. Rehabil. Res. Dev. 2012, 49, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Kemler, M.A.; Reulen, J.P.; van Kleef, M.; Barendse, G.A.; Wildenberg, F.A.V.D.; Spaans, F. Thermal thresholds in complex regional pain syndrome type I: Sensitivity and repeatability of the methods of limits and levels. Clin. Neurophysiol. 2000, 111, 1561–1568. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Ochoa, J.L. Differential Effect of Compression-Ischaemia Block on Warm Sensation and Heat-Induced Pain. Brain 1991, 114, 907–913. [Google Scholar] [CrossRef][Green Version]
- Levy, D.; Abraham, R.; Reid, G. A comparison of two methods for measuring thermal thresholds in diabetic neuropathy. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1072–1077. [Google Scholar] [CrossRef]
- Muijser, H.; Hooisma, J.; Hoogendijk, E.M.G.; Twisk, D. Vibration sensitivity as a parameter for detecting peripheral neuropathy, Results in healthy workers. Int. Arch. Occup. Environ. Health 1986, 58, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Gerr, F.E.; Letz, R. Reliability of a widely used test of peripheral cutaneous vibration sensitivity and a comparison of two testing protocols. Occup. Environ. Med. 1988, 45, 635–639. [Google Scholar] [CrossRef]
- Gerrett, N.; Ouzzahra, Y.; Coleby, S.; Hobbs, S.; Redortier, B.; Voelcker, T.; Havenith, G. Thermal sensitivity to warmth during rest and exercise: A sex comparison. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 114, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Ouzzahra, Y.; Havenith, G.; Redortier, B. Regional distribution of thermal sensitivity to cold at rest and during mild exercise in males. J. Therm. Biol. 2012, 37, 517–523. [Google Scholar] [CrossRef]
- Gerrett, N.; Ouzzahra, Y.; Redortier, B.; Voelcker, T.; Havenith, G. Female thermal sensitivity to hot and cold during rest and exercise. Physiol. Behav. 2015, 152, 11–19. [Google Scholar] [CrossRef]
- Temel, M.; Johnson, A.A.; Havenith, G.; Arnold, J.T.; West, A.M.; Lloyd, A.B. An examination of five theoretical foundations associated with localized thermosensory testing. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 121, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. Measures of Reliability in Sports Medicine and Science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-L.; Hsieh, C.-L.; Lin, J.-H.; Chen, H.-M. Optimal scoring methods of hand-strength tests in patients with stroke. Int. J. Rehabil. Res. 2011, 34, 178–180. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Adair, R.K. A model of the detection of warmth and cold by cutaneous sensors through effects on voltage-gated membrane channels. Proc. Natl. Acad. Sci. USA 1999, 96, 11825–11829. [Google Scholar] [CrossRef]
- Darian-Smith, I.; Johnson, K.O.; Dykes, R. “Cold” fiber population innervating palmar and digital skin of the monkey: Responses to cooling pulses. J. Neurophysiol. 1973, 36, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Darian-Smith, I.; Johnson, K.O.; LaMotte, C.; Shigenaga, Y.; Kenins, P.; Champness, P. Warm fibers innervating palmar and digital skin of the monkey: Responses to thermal stimuli. J. Neurophysiol. 1979, 42, 1297–1315. [Google Scholar] [CrossRef]
- Ciuha, U.; Mekjavic, I.B. Regional thermal comfort zone in males and females. Physiol. Behav. 2016, 161, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.M.; Raja, S.N.; Bulcao, C.F.; Goldstein, D.S. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J. Appl. Physiol. 1999, 86, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.E.; Mekjavic, I. Estimation of regional cutaneous cold sensitivity by analysis of the gasping response. J. Appl. Physiol. 1991, 71, 1933–1940. [Google Scholar] [CrossRef]
- Essick, G.; Guest, S.; Martinez, E.; Chen, C.; McGlone, F. Site-dependent and subject-related variations in perioral thermal sensitivity. Somatosens. Mot. Res. 2004, 21, 159–175. [Google Scholar] [CrossRef]
- Dyck, P.J.; Zimmerman, I.; Gillen, D.A.; Johnson, D.; Karnes, J.L.; O’Brien, P.C. Cool, warm, and heat-pain detection thresholds: Testing methods and inferences about anatomic distribution of receptors. Neurology 1993, 43, 1500. [Google Scholar] [CrossRef]
- Wang, H.; Kim, M.; Normoyle, K.P.; Llano, D. Thermal Regulation of the Brain-An Anatomical and Physiological Review for Clinical Neuroscientists. Front. Neurosci. 2015, 9, 528. [Google Scholar] [CrossRef]
- Brajkovic, D.; Ducharme, M.B. Facial cold-induced vasodilation and skin temperature during exposure to cold wind. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 96, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Seo, Y.; Quinn, T.; Yorio, P.; Roberge, R. Intersegmental differences in facial warmth sensitivity during rest, passive heat and exercise. Int. J. Hyperth. 2019, 36, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.; Lee, G.; Joester, J.; Lynch, M.; Barnes, E.H.; Wrigley, P.J.; Ng, K. Thermal quantitative sensory testing: A study of 101 control subjects. J. Clin. Neurosci. 2015, 22, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Gerrett, N.; Ichinose-Kuwahara, T.; Umino, Y.; Kiuchi, S.; Amano, T.; Ueda, H.; Havenith, G.; Kondo, N. Sex differences in age-related changes on peripheral warm and cold innocuous thermal sensitivity. Physiol. Behav. 2016, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]

| MLI | MSM | |||
|---|---|---|---|---|
| Warm | Cold | Warm | Cold | |
| Mean ± SD | 924.7 ± 324.3 * | 757.7 ± 243.2 * | 3.6 ± 1.6 * | 5.2 ± 1.8 * |
| 95% CI (upper-lower) | 879.5–969.9 | 723.8–791.7 | 3.4–3.9 | 4.9–5.5 |
| Standard error | 22.93 | 17.20 | 0.11 | 0.13 |
| Smallest real difference | 63.56 | 47.68 | 0.32 | 0.37 |
| SRD % | 6.87 | 6.29 | 8.66 | 7.09 |
| Intra-class correlation | 0.826 ** | 0.839 ** | 0.906 * | 0.878 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.; Kim, J.-H. Differential Cutaneous Thermal Sensitivity in Humans: Method of Limit vs. Method of Sensation Magnitude. Int. J. Environ. Res. Public Health 2021, 18, 12576. https://doi.org/10.3390/ijerph182312576
Seo Y, Kim J-H. Differential Cutaneous Thermal Sensitivity in Humans: Method of Limit vs. Method of Sensation Magnitude. International Journal of Environmental Research and Public Health. 2021; 18(23):12576. https://doi.org/10.3390/ijerph182312576
Chicago/Turabian StyleSeo, Yongsuk, and Jung-Hyun Kim. 2021. "Differential Cutaneous Thermal Sensitivity in Humans: Method of Limit vs. Method of Sensation Magnitude" International Journal of Environmental Research and Public Health 18, no. 23: 12576. https://doi.org/10.3390/ijerph182312576
APA StyleSeo, Y., & Kim, J.-H. (2021). Differential Cutaneous Thermal Sensitivity in Humans: Method of Limit vs. Method of Sensation Magnitude. International Journal of Environmental Research and Public Health, 18(23), 12576. https://doi.org/10.3390/ijerph182312576







