A Single Bout of High-Intensity Cardiovascular Exercise Does Not Enhance Motor Performance and Learning of a Visuomotor Force Modulation Task, but Triggers Ipsilateral Task-Related EEG Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cardiovascular Fitness Test
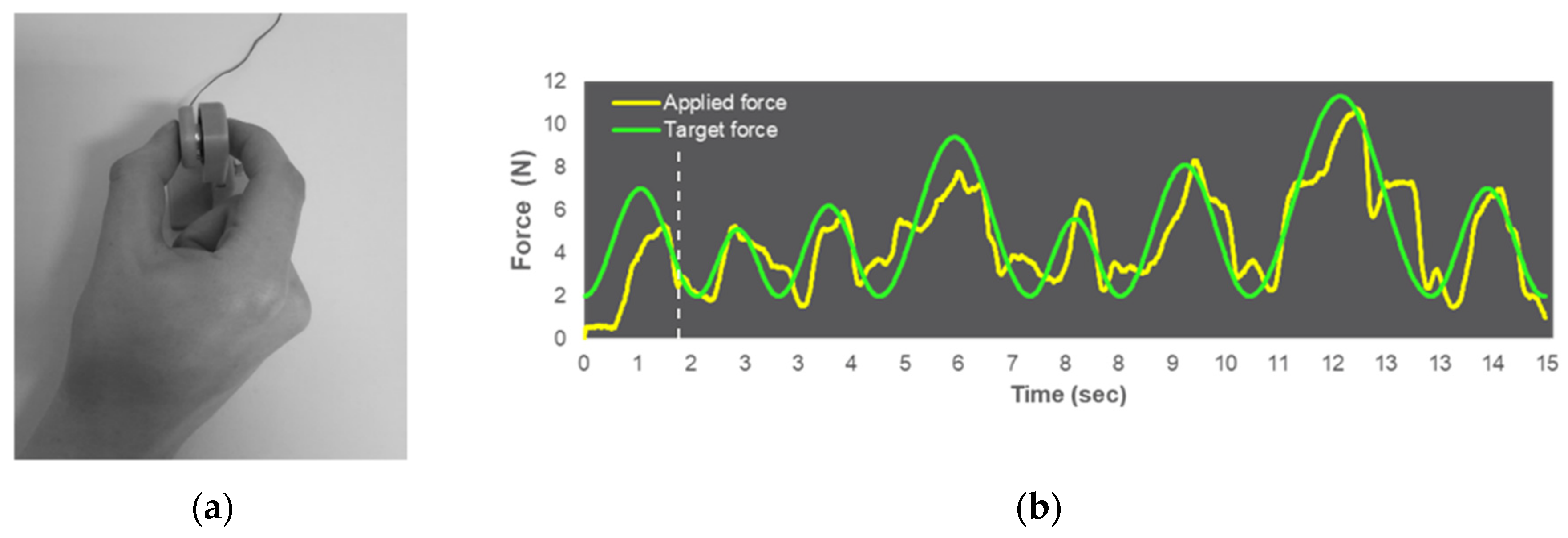
2.3. Visuomotor Force-Matching Task (FM Task)
2.4. EEG Recording
2.5. Acute Exercise Session
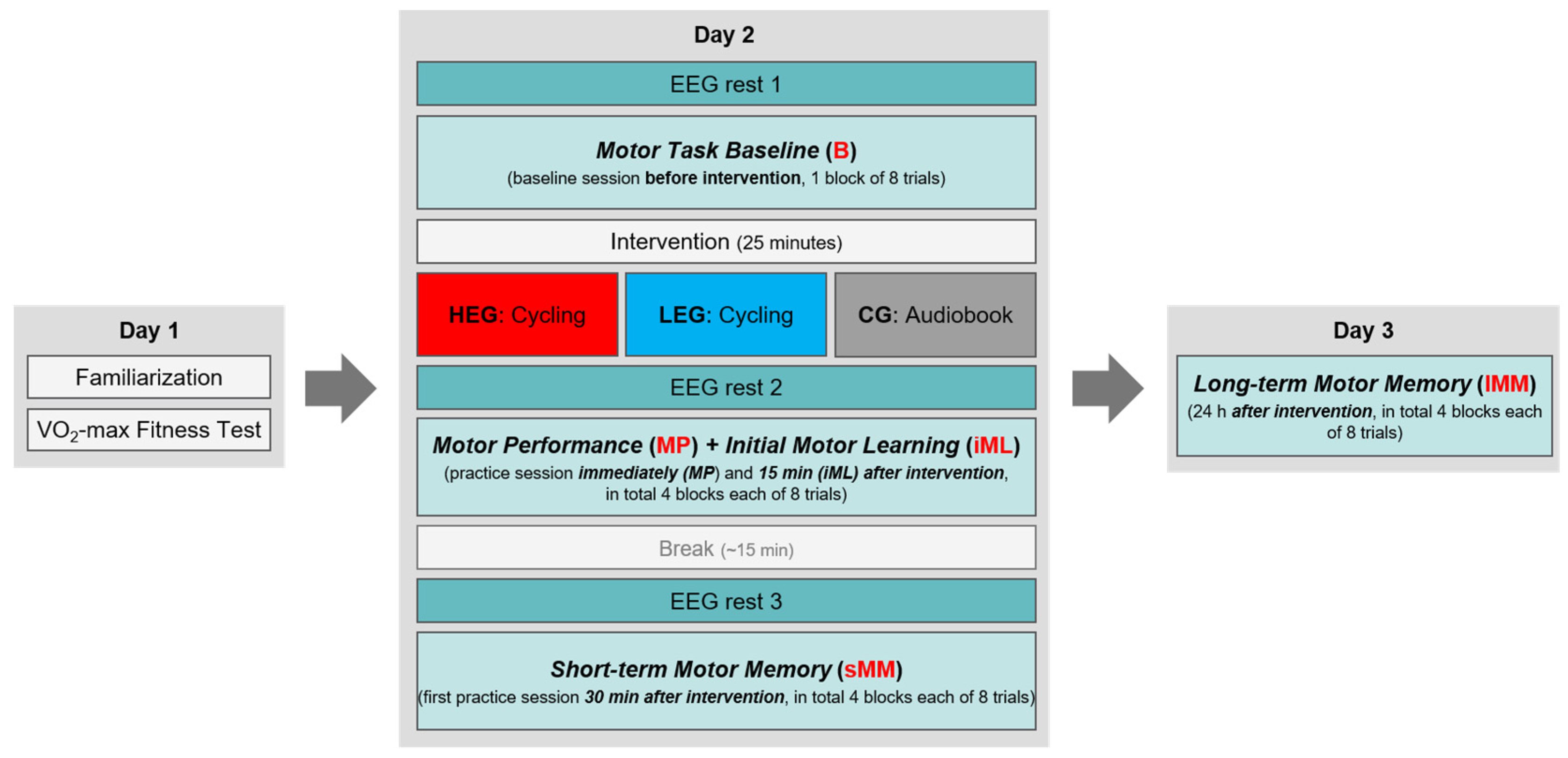
2.6. Procedure
2.7. Data Analysis
2.7.1. FM Task Data
2.7.2. EEG Data
2.8. Statistical Analyses
3. Results
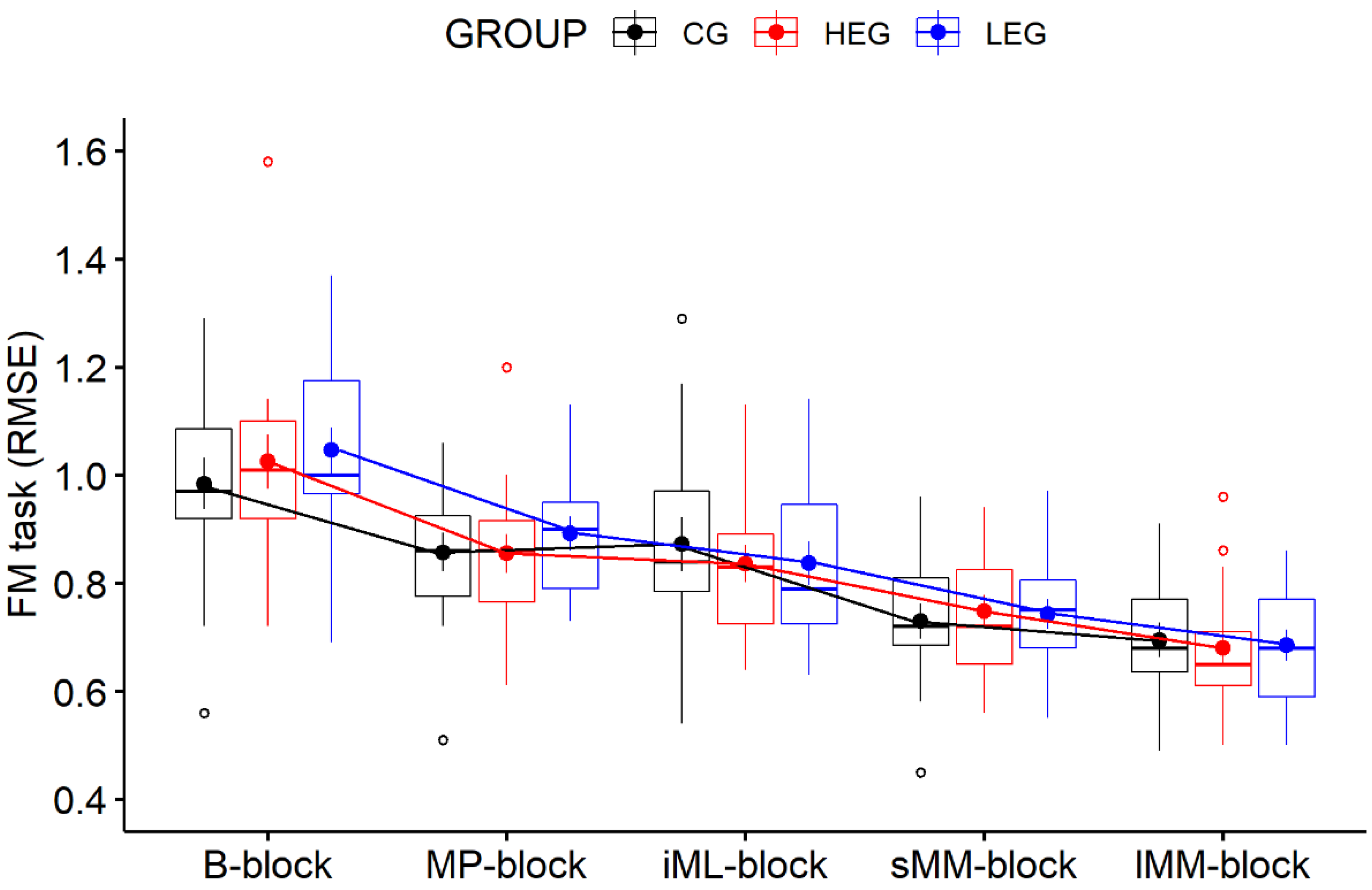
3.1. Behavioral Data—FM Task
3.2. EEG Data—Task-Related Power
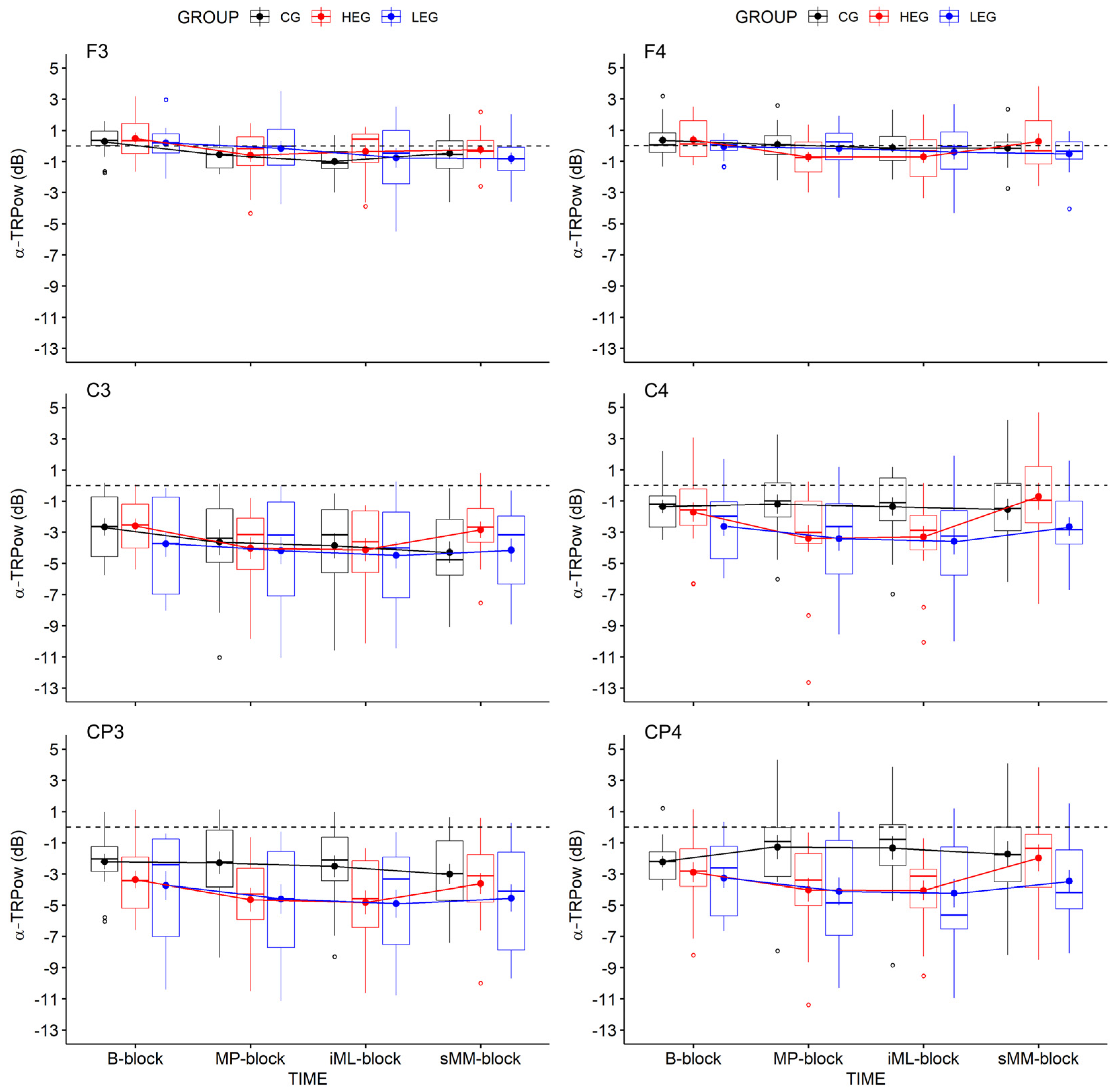
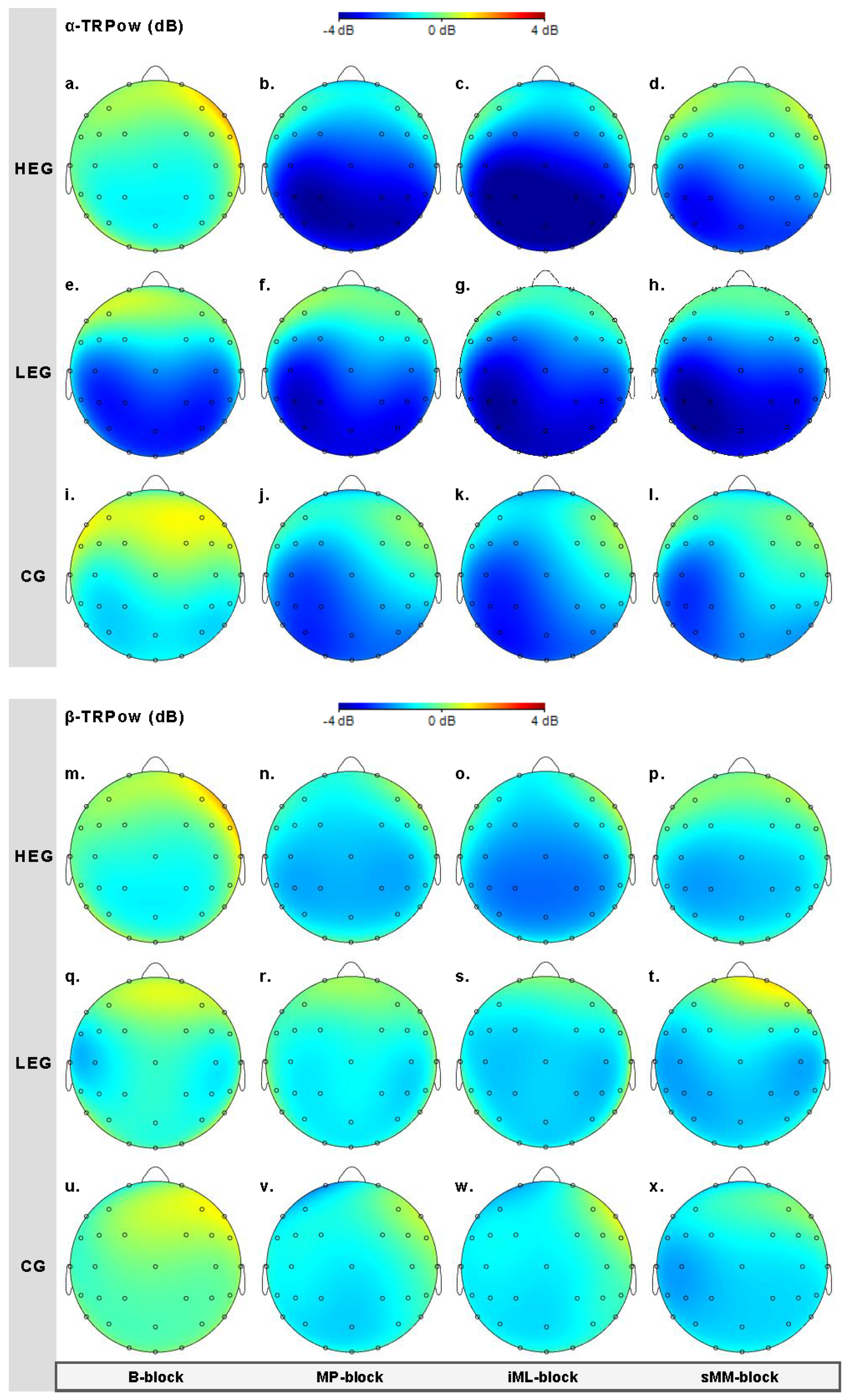
3.2.1. Changes in TRPow in the Alpha Frequency Band (α-TRPow)
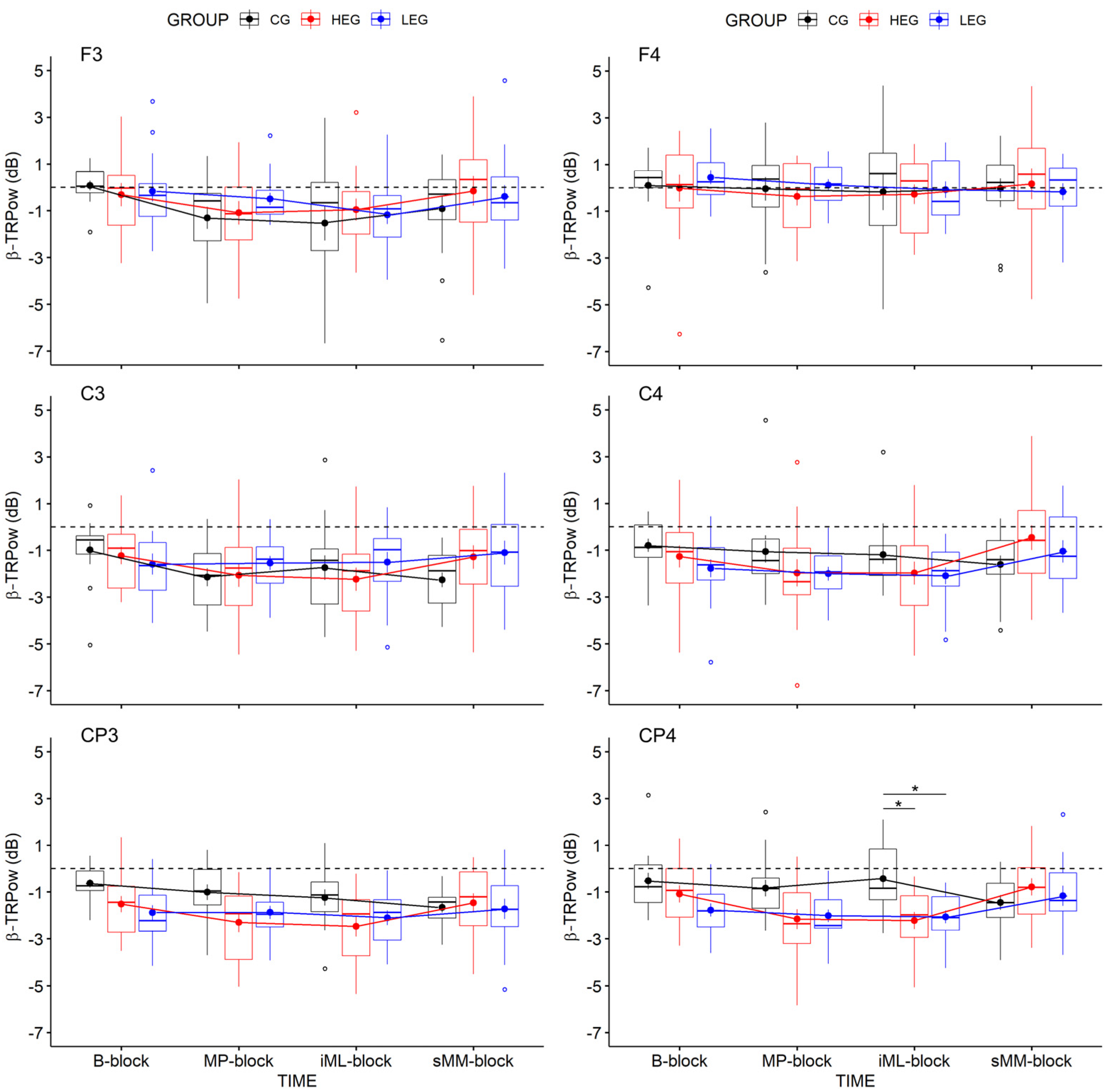
3.2.2. Changes in TRPow in the Beta Frequency Band (β-TRPow)
4. Discussion
4.1. Effects on Motor Performance and Learning
4.2. Effects on Task-Related Power during Motor Performance and Learning
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, N.; Taubert, M. Exercise-induced improvement in motor learning. In The Exercise Effect on Mental Health; CRC Press: Boca Raton, FL, USA, 2018; pp. 188–224. ISBN 1315113902. [Google Scholar]
- Roig, M.; Thomas, R.; Mang, C.S.; Snow, N.J.; Ostadan, F.; Boyd, L.A.; Lundbye-Jensen, J. Time-Dependent Effects of Cardiovascular Exercise on Memory. Exerc. Sport Sci. Rev. 2016, 44, 81–88. [Google Scholar] [CrossRef]
- Taubert, M.; Villringer, A.; Lehmann, N. Endurance Exercise as an “Endogenous” Neuro-enhancement Strategy to Facilitate Motor Learning. Front. Hum. Neurosci. 2015, 9, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, P.; Cheng, F.-H.; Steib, S. Effects of acute cardiovascular exercise on motor memory encoding and consolidation: A systematic review with meta-analysis. Neurosci. Biobehav. Rev. 2020, 116, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Mang, C.S.; Snow, N.J.; Campbell, K.L.; Ross, C.J.D.; Boyd, L.A. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J. Appl. Physiol. 2014, 117, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, F.; Desormeau, B.; Boudrias, M.-H.; Roig, M. Acute cardiovascular exercise promotes functional changes in cortico-motor networks during the early stages of motor memory consolidation. Neuroimage 2018, 174, 380–392. [Google Scholar] [CrossRef]
- Roig, M.; Skriver, K.; Lundbye-Jensen, J.; Kiens, B.; Nielsen, J.B. A single bout of exercise improves motor memory. PLoS ONE 2012, 7, e44594. [Google Scholar] [CrossRef] [Green Version]
- Skriver, K.; Roig, M.; Lundbye-Jensen, J.; Pingel, J.; Helge, J.W.; Kiens, B.; Nielsen, J.B. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn. Mem. 2014, 116, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Stavrinos, E.L.; Coxon, J.P. High-intensity Interval Exercise Promotes Motor Cortex Disinhibition and Early Motor Skill Consolidation. J. Cogn. Neurosci. 2017, 29, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Johnsen, L.K.; Geertsen, S.S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute Exercise and Motor Memory Consolidation: The Role of Exercise Intensity. PLoS ONE 2016, 11, e0159589. [Google Scholar] [CrossRef] [PubMed]
- Mellow, M.L.; Goldsworthy, M.R.; Coussens, S.; Smith, A.E. Acute aerobic exercise and neuroplasticity of the motor cortex: A systematic review. J. Sci. Med. Sport 2020, 23, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.M.; Staines, W.R. The effects of acute aerobic exercise on the primary motor cortex. J. Mot. Behav. 2015, 47, 328–339. [Google Scholar] [CrossRef]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef] [Green Version]
- El-Sayes, J.; Harasym, D.; Turco, C.V.; Locke, M.B.; Nelson, A.J. Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neuroscientist 2019, 25, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Bellec, P.; Amsel, R.; Penhune, V.; Monchi, O.; Carrier, J.; Lehéricy, S.; Benali, H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009, 199, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef]
- Halsband, U.; Lange, R.K. Motor learning in man: A review of functional and clinical studies. J. Physiol. Paris 2006, 99, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Rottschy, C.; Miall, R.C.; Eickhoff, S.B. A quantitative meta-analysis and review of motor learning in the human brain. NeuroImage 2013, 67, 283–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karni, A.; Meyer, G.; Jezzard, P.; Adams, M.M.; Turner, R.; Ungerleider, L.G. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 1995, 377, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Lohse, K.R.; Wadden, K.; Boyd, L.A.; Hodges, N.J. Motor skill acquisition across short and long time scales: A meta-analysis of neuroimaging data. Neuropsychologia 2014, 59, 130–141. [Google Scholar] [CrossRef]
- Seidler, R.D. Neural correlates of motor learning, transfer of learning, and learning to learn. Exerc. Sport Sci. Rev. 2010, 38, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, L. Imaging Brain Plasticity during Motor Skill Learning. Neurobiol. Learn. Mem. 2002, 78, 553–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayan, E.; Cohen, L.G. Neuroplasticity subserving motor skill learning. Neuron 2011, 72, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Crone, N.E.; Miglioretti, D.L.; Gordon, B.; Sieracki, J.M.; Wilson, M.T.; Uematsu, S.; Lesser, R.P. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain 1998, 121 Pt 12, 2271–2299. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C. Movement and ERD/ERS. In The Bereitschaftspotential: Movement-Related Cortical Potentials; Jahanshahi, M., Hallett, M., Eds.; Springer: Boston, MA, USA, 2003; pp. 191–206. ISBN 978-1-4613-4958-7. [Google Scholar]
- Neuper, C.; Pfurtscheller, G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef]
- Neuper, C.; Wörtz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. In Event-Related Dynamics of Brain Oscillations, 1st ed.; Klimesch, W., Neuper, C., Eds.; Elsevier textbooks: Amsterdam, The Netherlands, 2006; pp. 211–222. ISBN 9780444521835. [Google Scholar]
- Steriade, M.; Llinás, R.R. The functional states of the thalamus and the associated neuronal interplay. Physiol. Rev. 1988, 68, 649–742. [Google Scholar] [CrossRef] [PubMed]
- Denker, M.; Roux, S.; Lindén, H.; Diesmann, M.; Riehle, A.; Grün, S. The local field potential reflects surplus spike synchrony. Cereb. Cortex 2011, 21, 2681–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, S.D.; Stanford, I.M.; Yamawaki, N.; McAllister, C.J.; Rönnqvist, K.C.; Woodhall, G.L.; Furlong, P.L. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage 2011, 56, 1506–1510. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.R.; Baker, S.N. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J. Physiol. 2003, 546, 931–942. [Google Scholar] [CrossRef]
- Jensen, O.; Goel, P.; Kopell, N.; Pohja, M.; Hari, R.; Ermentrout, B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. Neuroimage 2005, 26, 347–355. [Google Scholar] [CrossRef]
- Floyer-Lea, A.; Wylezinska, M.; Kincses, T.; Matthews, P.M. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 2006, 95, 1639–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagg, C.J. Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage 2014, 86, 19–27. [Google Scholar] [CrossRef]
- Houweling, S.; Daffertshofer, A.; van Dijk, B.W.; Beek, P.J. Neural changes induced by learning a challenging perceptual-motor task. Neuroimage 2008, 41, 1395–1407. [Google Scholar] [CrossRef]
- Moisello, C.; Meziane, H.B.; Kelly, S.; Perfetti, B.; Kvint, S.; Voutsinas, N.; Blanco, D.; Quartarone, A.; Tononi, G.; Ghilardi, M.F. Neural activations during visual sequence learning leave a trace in post-training spontaneous EEG. PLoS ONE 2013, 8, e65882. [Google Scholar] [CrossRef] [PubMed]
- Pollok, B.; Latz, D.; Krause, V.; Butz, M.; Schnitzler, A. Changes of motor-cortical oscillations associated with motor learning. Neuroscience 2014, 275, 47–53. [Google Scholar] [CrossRef]
- Zhuang, P.; Toro, C.; Grafman, J.; Manganotti, P.; Leocani, L.; Hallett, M. Event-related desynchronization (ERD) in the alpha frequency during development of implicit and explicit learning. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 374–381. [Google Scholar] [CrossRef]
- Veldman, M.P.; Maurits, N.M.; Nijland, M.A.M.; Wolters, N.E.; Mizelle, J.C.; Hortobágyi, T. Spectral and temporal electroencephalography measures reveal distinct neural networks for the acquisition, consolidation, and interlimb transfer of motor skills in healthy young adults. Clin. Neurophysiol. 2018, 129, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.N.; Krause, V.; Südmeyer, M.; Hartmann, C.J.; Pollok, B. The significance of brain oscillations in motor sequence learning: Insights from Parkinson’s disease. Neuroimage Clin. 2018, 20, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Hübner, L.; Godde, B.; Voelcker-Rehage, C. Acute Exercise as an Intervention to Trigger Motor Performance and EEG Beta Activity in Older Adults. Neural Plast. 2018, 2018, 4756785. [Google Scholar] [CrossRef] [Green Version]
- Hübner, L.; Godde, B.; Voelcker-Rehage, C. Older adults reveal enhanced task-related beta power decreases during a force modulation task. Behav. Brain Res. 2018, 345, 104–113. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Vieluf, S.; Mahmoodi, J.; Godde, B.; Reuter, E.-M.; Voelcker-Rehage, C. The Influence of Age and Work-Related Expertise on Fine Motor Control. GeroPsych 2012, 25, 199–206. [Google Scholar] [CrossRef]
- Buchfuhrer, M.J.; Hansen, J.E.; Robinson, T.E.; Sue, D.Y.; Wasserman, K.; Whipp, B.J. Optimizing the exercise protocol for cardiopulmonary assessment. J. Appl. Physiol. 1983, 55, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Zwingmann, K.; Hübner, L.; Verwey, W.B.; Barnhoorn, J.S.; Godde, B.; Voelcker-Rehage, C. Regular participation in leisure time activities and high cardiovascular fitness improve motor sequence learning in older adults. Psychol. Res. 2021, 85, 1488–1502. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Fagergren, A.; Jonsson, T.; Westling, G.; Johansson, R.S.; Forssberg, H. Cortical activity in precision- versus power-grip tasks: An fMRI study. J. Neurophysiol. 2000, 83, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Kuhtz-Buschbeck, J.P.; Ehrsson, H.H.; Forssberg, H. Human brain activity in the control of fine static precision grip forces: An fMRI study. Eur. J. Neurosci. 2001, 14, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; The MIT Press: Cambridge, MA, USA, 2014; ISBN 9780262019873. [Google Scholar]
- Manganotti, P.; Gerloff, C.; Toro, C.; Katsuta, H.; Sadato, N.; Zhuang, P.; Leocani, L.; Hallett, M. Task-related coherence and task-related spectral power changes during sequential finger movements. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 50–62. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V.; Wagenmakers, E.-J. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 2020, 23, 788–799. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V.; Wagenmakers, E.-J. Author Correction: Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 2020, 23, 1453. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Wagenmakers, E.-J.; Marsman, M.; Jamil, T.; Ly, A.; Verhagen, J.; Love, J.; Selker, R.; Gronau, Q.F.; Šmíra, M.; Epskamp, S.; et al. Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon. Bull. Rev. 2018, 25, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Dienes, Z. Using Bayes to get the most out of non-significant results. Front. Psychol. 2014, 5, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffreys, H. Theory of Probability; Oxford University Press: Oxford, UK, 1961. [Google Scholar]
- Westfall, P. A Bayesian perspective on the Bonferroni adjustment. Biometrika 1997, 84, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Snow, N.J.; Mang, C.S.; Roig, M.; McDonnell, M.N.; Campbell, K.L.; Boyd, L.A. The Effect of an Acute Bout of Moderate-Intensity Aerobic Exercise on Motor Learning of a Continuous Tracking Task. PLoS ONE 2016, 11, e0150039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statton, M.A.; Encarnacion, M.; Celnik, P.; Bastian, A.J. A Single Bout of Moderate Aerobic Exercise Improves Motor Skill Acquisition. PLoS ONE 2015, 10, e0141393. [Google Scholar] [CrossRef] [PubMed]
- Wanner, P.; Müller, T.; Cristini, J.; Pfeifer, K.; Steib, S. Exercise Intensity Does not Modulate the Effect of Acute Exercise on Learning a Complex Whole-Body Task. Neuroscience 2020, 426, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Pollok, B.; Krause, V.; Butz, M.; Schnitzler, A. Modality specific functional interaction in sensorimotor synchronization. Hum. Brain Mapp. 2009, 30, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Klostermann, F.; Nikulin, V.V.; Kühn, A.A.; Marzinzik, F.; Wahl, M.; Pogosyan, A.; Kupsch, A.; Schneider, G.-H.; Brown, P.; Curio, G. Task-related differential dynamics of EEG alpha- and beta-band synchronization in cortico-basal motor structures. Eur. J. Neurosci. 2007, 25, 1604–1615. [Google Scholar] [CrossRef]
- Andres, F.G.; Mima, T.; Schulman, A.E.; Dichgans, J.; Hallett, M.; Gerloff, C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 1999, 122, 855–870. [Google Scholar] [CrossRef] [Green Version]
- Espenhahn, S.; van Wijk, B.C.M.; Rossiter, H.E.; de Berker, A.O.; Redman, N.D.; Rondina, J.; Diedrichsen, J.; Ward, N.S. Cortical beta oscillations are associated with motor performance following visuomotor learning. Neuroimage 2019, 195, 340–353. [Google Scholar] [CrossRef]
- Boonstra, T.W.; Daffertshofer, A.; Breakspear, M.; Beek, P.J. Multivariate time-frequency analysis of electromagnetic brain activity during bimanual motor learning. Neuroimage 2007, 36, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chettouf, S.; Rueda-Delgado, L.M.; de Vries, R.; Ritter, P.; Daffertshofer, A. Are unimanual movements bilateral? Neurosci. Biobehav. Rev. 2020, 113, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Bundy, D.T.; Leuthardt, E.C. The Cortical Physiology of Ipsilateral Limb Movements. Trends Neurosci. 2019, 42, 825–839. [Google Scholar] [CrossRef]
- Rudisch, J.; Müller, K.; Kutz, D.F.; Brich, L.; Sleimen-Malkoun, R.; Voelcker-Rehage, C. How Age, Cognitive Function and Gender Affect Bimanual Force Control. Front. Physiol. 2020, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.; Wiestler, T.; Diedrichsen, J. Cooperation Not Competition: Bihemispheric tDCS and fMRI Show Role for Ipsilateral Hemisphere in Motor Learning. J. Neurosci. 2017, 37, 7500–7512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lulic, T.; El-Sayes, J.; Fassett, H.J.; Nelson, A.J. Physical activity levels determine exercise-induced changes in brain excitability. PLoS ONE 2017, 12, e0173672. [Google Scholar] [CrossRef] [PubMed]
- El-Sayes, J.; Turco, C.V.; Skelly, L.E.; Nicolini, C.; Fahnestock, M.; Gibala, M.J.; Nelson, A.J. The Effects of Biological Sex and Ovarian Hormones on Exercise-Induced Neuroplasticity. Neuroscience 2019, 410, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, T.P.; Fried, P.J.; Macone, J.; Stillman, A.; Gomes-Osman, J.; Costa-Miserachs, D.; Tormos Muñoz, J.M.; Santarnecchi, E.; Pascual-Leone, A. Light aerobic exercise modulates executive function and cortical excitability. Eur. J. Neurosci. 2020, 51, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Beck, M.M.; Lind, R.R.; Korsgaard Johnsen, L.; Geertsen, S.S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute Exercise and Motor Memory Consolidation: The Role of Exercise Timing. Neural Plast. 2016, 2016, 6205452. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Uris, B.; Busquets, A.; Lopez-Alonso, V.; Fernandez-Del-Olmo, M.; Angulo-Barroso, R. Enhancing consolidation of a rotational visuomotor adaptation task through acute exercise. PLoS ONE 2017, 12, e0175296. [Google Scholar] [CrossRef] [Green Version]
- Tomporowski, P.D.; Pendleton, D.M. Effects of the Timing of Acute Exercise and Movement Complexity on Young Adults’ Psychomotor Learning. J. Sport Exerc. Psychol. 2018, 40, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hübner, L.; Voelcker-Rehage, C. Does physical activity benefit motor performance and learning of upper extremity tasks in older adults?—A systematic review. Eur. Rev. Aging Phys. Act. 2017, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimesch, W. The frequency architecture of brain and brain body oscillations: An analysis. Eur. J. Neurosci. 2018, 48, 2431–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Dal Maso, F.; Roig, M.; Mitsis, G.D.; Boudrias, M.-H. Unfolding the Effects of Acute Cardiovascular Exercise on Neural Correlates of Motor Learning Using Convolutional Neural Networks. Front. Neurosci. 2019, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
| HEG (n = 15, 8 ♀) | LEG (n = 15, 9 ♀) | CG (n = 15, 10 ♀) | F-Statistics | Bayes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | p | η2 | BFincl | |
| Age | 23.60 | 3.96 | 23.80 | 4.96 | 22.13 | 2.90 | 0.77 | 0.472 | 0.03 | 0.280 |
| Education | 16.01 | 3.65 | 15.83 | 3.26 | 15.48 | 2.00 | 0.13 | 0.883 | 0.01 | 0.179 |
| Subj. health | 4.13 | 1.06 | 4.20 | 0.68 | 4.47 | 0.64 | 0.70 | 0.501 | 0.03 | 0.268 |
| Subj. hand usage | 17.07 | 2.74 | 17.20 | 2.54 | 19.60 | 3.18 | 3.81 | 0.030 | 0.15 | 2.184 |
| MVC (N) | 64.70 | 16.39 | 61.51 | 20.39 | 64.35 | 16.35 | 0.14 | 0.866 | 0.01 | 0.375 |
| Max. watt | 225.20 | 53.39 | 207.73 | 83.46 | NA | NA | 0.01 | 0.926 | <0.01 | 0.411 |
| VO2-max | 2.73 | 0.75 | 2.84 | 0.92 | NA | NA | 0.13 | 0.726 | 0.01 | 0.440 |
| HEG | LEG | |
|---|---|---|
| Warm-up | 5 min at 40% max watt | 25 min at 20% max watt |
| Exercise | 3 × 3 min at 90% max watt interspersed with 2 × 2 min at 60% max watt | |
| Cool-down | 5 min at 20% max watt | |
| HR (bpm) | 90%: 175.91 ± 12.97 60%: 168.65 ± 15.96 | 20%: 104.20 ± 10.22 |
| Load (watt) | 90%: 208.32 ± 47.63 60%: 138.88 ± 31.75 | 20%: 44.76 ± 11.95 |
| HEG | LEG | CG | Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | t | df | p | d | BF10 | |
| HR 1 | 82.00 | 15.03 | 78.07 | 11.59 | NA | NA | 0.80 | 28 | 0.429 | 0.293 | 0.440 |
| HR 2 | 102.53 | 15.70 | 77.00 | 11.51 | NA | NA | 5.08 | 28 | <0.001 | 1.855 | 760.419 |
| HR 3 | 92.47 | 14.92 | 73.40 | 8.81 | NA | NA | 4.26 | 28 | <0.001 | 1.566 | 113.161 |
| HR 4 | 93.93 | 14.37 | 73.53 | 9.10 | NA | NA | 4.65 | 28 | <0.001 | 1.696 | 273.584 |
| F | df | p | η2 | BFincl | |||||||
| Fatigue 1 | 1.33 | 0.98 | 1.07 | 1.28 | 0.93 | 0.80 | 0.58 | 2, 42 | 0.565 | 0.027 | 0.246 |
| Fatigue 2 | 2.01 | 1.16 | 1.60 | 1.18 | 2.00 | 1.20 | 0.67 | 2, 42 | 0.509 | 0.032 | 0.265 |
| Fatigue 3 | 3.67 | 1.35 | 2.87 | 1.77 | 3.067 | 1.49 | 1.09 | 2, 42 | 0.345 | 0.049 | 0.351 |
| Fatigue 4 | 1.07 | 1.03 | 0.68 | 0.90 | 0.68 | 0.62 | 1.06 | 2, 42 | 0.345 | 0.048 | 0.315 |
| Fatigue 5 | 4.13 | 1.55 | 3.73 | 1.71 | 3.33 | 1.54 | 0.93 | 2, 42 | 0.401 | 0.043 | 0.344 |
| α-TRPow | TIME | GROUP | TIME × GROUP | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrode | F | df | p | η2p | η2G | BFincl | F | df | p | η2p | η2G | BFincl | F | df | p | η2p | η2G | BFincl |
| F3 | 6.05 | 3, 126 | 0.004 | 0.126 | 0.068 | 45.499 | 0.25 | 2, 42 | 0.784 | 0.012 | 0.006 | 0.163 | 0.63 | 6, 126 | 0.631 | 0.029 | 0.015 | 0.069 |
| C3 | 5.70 | 3, 126 | 0.006 | 0.119 | 0.025 | 20.799 | 0.25 | 2, 42 | 0.784 | 0.012 | 0.013 | 0.386 | 1.61 | 6, 126 | 0.186 | 0.071 | 0.014 | 0.299 |
| CP3 | 3.89 | 3, 126 | 0.022 | 0.085 | 0.016 | 2.629 | 2.28 | 2, 42 | 0.115 | 0.098 | 0.082 | 1.014 | 1.46 | 6, 126 | 0.220 | 0.065 | 0.012 | 0.262 |
| F4 | 2.27 | 3, 126 | 0.110 | 0.051 | 0.029 | 0.451 | 0.51 | 2, 42 | 0.603 | 0.024 | 0.011 | 0.168 | 1.24 | 6, 126 | 0.300 | 0.056 | 0.032 | 0.206 |
| C4 | 3.52 | 3, 126 | 0.043 | 0.077 | 0.034 | 1.624 | 2.61 | 2, 42 | 0.086 | 0.110 | 0.068 | 1.009 | 1.95 | 6, 126 | 0.122 | 0.085 | 0.037 | 0.710 |
| CP4 | 1.90 | 3, 126 | 0.156 | 0.043 | 0.014 | .245 | 3.27 | 2, 42 | 0.048 | 0.135 | 0.097 | 1.719 | 2.17 | 6, 126 | 0.078 | 0.094 | 0.031 | 1.045 |
| β-TRPow | ||||||||||||||||||
| Electrode | F | df | p | η2p | η2G | BFincl | F | df | p | η2p | η2G | BFincl | F | df | p | η2p | η2G | BFincl |
| F3 | 4.09 | 3, 126 | 0.021 | 0.089 | 0.040 | 4.170 | 0.53 | 2, 42 | 0.592 | 0.025 | 0.014 | 0.236 | 0.612 | 6, 126 | 0.649 | 0.028 | 0.012 | 0.066 |
| C3 | 1.26 | 3, 126 | 0.287 | 0.029 | 0.014 | 0.125 | 0.02 | 2, 42 | 0.983 | 0.001 | < 0.001 | 0.144 | 1.82 | 6, 126 | 0.135 | 0.080 | 0.039 | 0.581 |
| CP3 | 2.48 | 3, 126 | 0.090 | 0.056 | 0.027 | 0.494 | 3.19 | 2, 42 | 0.051 | 0.132 | 0.075 | 1.370 | 2.01 | 6, 126 | 0.100 | 0.087 | 0.042 | 0.792 |
| F4 | 0.90 | 3, 126 | 0.443 | 0.021 | 0.010 | 0.089 | 0.09 | 2, 42 | 0.912 | 0.004 | 0.002 | 0.166 | 0.29 | 6, 126 | 0.920 | 0.014 | 0.006 | 0.036 |
| C4 | 3.04 | 3, 126 | 0.053 | 0.067 | 0.031 | 0.905 | 0.77 | 2, 42 | 0.468 | 0.036 | 0.020 | 0.258 | 2.33 | 6, 126 | 0.062 | 0.100 | 0.047 | 1.445 |
| CP4 | 2.86 | 3, 126 | 0.061 | 0.064 | 0.034 | 0.609 | 4.15 | 2, 42 | 0.023 | 0.165 | 0.086 | 2.241 | 3.90 | 6, 126 | 0.005 | 0.157 | 0.088 | 31.463 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pixa, N.H.; Hübner, L.; Kutz, D.F.; Voelcker-Rehage, C. A Single Bout of High-Intensity Cardiovascular Exercise Does Not Enhance Motor Performance and Learning of a Visuomotor Force Modulation Task, but Triggers Ipsilateral Task-Related EEG Activity. Int. J. Environ. Res. Public Health 2021, 18, 12512. https://doi.org/10.3390/ijerph182312512
Pixa NH, Hübner L, Kutz DF, Voelcker-Rehage C. A Single Bout of High-Intensity Cardiovascular Exercise Does Not Enhance Motor Performance and Learning of a Visuomotor Force Modulation Task, but Triggers Ipsilateral Task-Related EEG Activity. International Journal of Environmental Research and Public Health. 2021; 18(23):12512. https://doi.org/10.3390/ijerph182312512
Chicago/Turabian StylePixa, Nils Henrik, Lena Hübner, Dieter F. Kutz, and Claudia Voelcker-Rehage. 2021. "A Single Bout of High-Intensity Cardiovascular Exercise Does Not Enhance Motor Performance and Learning of a Visuomotor Force Modulation Task, but Triggers Ipsilateral Task-Related EEG Activity" International Journal of Environmental Research and Public Health 18, no. 23: 12512. https://doi.org/10.3390/ijerph182312512






