The Impact of Clinical, Biochemical, and Echocardiographic Parameters on the Quality of Life in Patients with Heart Failure with Reduced Ejection Fraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Quality of Life Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Natriuretic Peptides
4.2. Left Ventricular Ejection Fraction
4.3. Iron Metabolism
4.4. Other Biochemical Parameters
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Thomas, L.F.; Shengshou, H.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C., Jr.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003, 289, 194–202. [Google Scholar] [CrossRef]
- Bleumink, G.S.; Knetsch, A.M.; Sturkenboom, M.C.J.M.; Straus, S.M.J.M.; Hofman, A.; Deckers, J.W.; Witteman, J.C.M.; Stricker, B.H. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur. Heart J. 2004, 25, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Ceia, F.; Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; de Sousa, A.; Oliveira, A.; EPICA Investigators. Prevalence of chronic heart failure in Southwestern Europe: The EPICA study. Eur. J. Heart Fail. 2002, 4, 531–539. [Google Scholar] [CrossRef]
- Fang, J.; Mensah, G.A.; Croft, J.B.; Keenan, N.L. Heart failure-related hospitalization in the U.S., 1979 to 2004. J. Am. Coll. Cardiol. 2008, 52, 428–434. [Google Scholar] [CrossRef]
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissela, B.; et al. Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008, 117, e25–e146. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 8, e137–e161. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Abraham, W.T.; Albert, N.M.; Greenberg, B.H.; O’Connor, C.M.; She, L.; Gattis Stough, W.; Yancy, C.W.; Young, J.B.; Fonarow, G.C. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. J. Am. Med. Assoc. 2006, 296, 2217–2226. [Google Scholar] [CrossRef]
- Piepenburg, S.M.; Faller, H.; Störk, S.; Ertl, G.; Angermann, C.E. Symptom patterns and clinical outcomes in women versus men with systolic heart failure and depression. Clin. Res. Cardiol. 2019, 108, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Uchmanowicz, I.; Gobbens, R.J. The relationship between frailty, anxiety and depression, and health-related quality of life in elderly patients with heart failure. Clin. Interv. Aging 2015, 10, 1595–1600. [Google Scholar] [CrossRef]
- Zhang, J.; Hobkirk, J.; Carroll, S.; Pellicori, P.; Clark, A.L. Cleland JGF. Exploring quality of life in patients with and without heart failure. Int. J. Cardiol. 2016, 202, 676–684. [Google Scholar] [CrossRef]
- Kraai, I.H.; Vermeulen, K.M.; Luttik, M.L.A.; Hoekstra, T.; Jaarsma, H.L.H. Preferences of heart failure patients in daily clinical practice: Quality of life or longevity? Eur. J. Heart Fail. 2013, 15, 1113–1121. [Google Scholar] [CrossRef]
- Whoqol Group. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 79. [Google Scholar] [CrossRef]
- Kaul, P.; Reed, S.D.; Hernandez, A.F.; Howlett, J.G.; Ezekowitz, J.A.; Li, Y.; Zheng, Y.; Rouleau, J.L.; Starling, R.C.; O’Connor, C.M.; et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013, 1, 523–530. [Google Scholar] [CrossRef]
- Dijkstra, A.; Hakverdioglu, G.; Muszalik, M.; Andela, R.; Korhan, E.A.; Kędziora-Kornatowska, K. Health Related Quality of Life and Care Dependency among Elderly Hospital Patients: An International Comparison. Tohoku J. Exp. Med. 2015, 235, 193–200. [Google Scholar] [CrossRef]
- Knurowski, T.; Lazić, D.; van Dijk, J.P.; Madarasova Geckova, A.; Tobiasz-Adamczyk, B.; van den Heuvel, W.J.A. Survey of health status and quality of life of the elderly in Poland and Croatia. Croat. Med. J. 2004, 45, 750–756. [Google Scholar] [PubMed]
- Kozhekenova, L.G.; Lanzoni, M.; Rakhypbekov, T.K.; Mussakhanova, A.K.; Zurikanov, K.S.; Castaldi, S. Health-related quality of life in Kazakh heart failure patients evaluated by the Minnesota living with heart failure questionnaire and comparison with a published large international sample. Ann. Ig. 2014, 26, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, M.; Bielecki, W.; Drygas, W. Sociodemographic and clinical determinants of quality of life in urban population of Poland. Cent. Eur. J. Public Health 2004, 12, 63–68. [Google Scholar]
- Apers, S.; Kovacs, A.H.; Luyckx, K.; Thomet, C.; Budts, W.; Enomoto, J.; Sluman, M.A.; Wang, J.-K.; Jackson, J.L.; Khairy, P.; et al. Quality of Life of Adults With Congenital Heart Disease in 15 Countries: Evaluating Country-Specific Characteristics. J. Am. Coll. Cardiol. 2016, 67, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, R.; Georgiou, D.; Cianci, G.; Purcaro, A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999, 99, 1173–1182. [Google Scholar] [CrossRef]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.P.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of heart failure patients: Practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 157. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Sagar, V.A.; Davies, E.J.; Briscoe, S.; Coats, A.J.S.; Dalal, H.; Lough, F.; Rees, K.; Singh, S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst. Rev. 2014, 2014, CD003331. [Google Scholar] [CrossRef] [PubMed]
- Cowger, J.A.; Naka, Y.; Aaronson, K.D.; Horstmanshof, D.; Gulati, S.; Rinde-Hoffman, D.; Pinney, S.; Adatya, S.; Farrar, D.J.; Jorde, U.P. Quality of life and functional capacity outcomes in the MOMENTUM 3 trial at 6 months: A call for new metrics for left ventricular assist device patients. J. Heart Lung Transplant. 2018, 37, 15–24. [Google Scholar] [CrossRef]
- Grady, K.L.; Wissman, S.; Naftel, D.C.; Myers, S.; Gelijins, A.; Moskowitz, A.; Pagani, F.D.; Young, J.B.; Spertus, J.A.; Kirklin, J.K. Age and gender differences and factors related to change in health-related quality of life from before to 6 months after left ventricular assist device implantation: Findings from Interagency Registry for Mechanically Assisted Circulatory Support. J. Heart Lung Transplant. 2016, 35, 777–788. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, X.; Cai, H.; Wang, Z.; Xu, H.; Chen, S.; Chen, J.; Xu, X.; Xu, H.; Mao, W. Catheter ablation versus medical rate control for persistent atrial fibrillation in patients with heart failure: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4377. [Google Scholar] [CrossRef]
- Crocker, T.F.; Smith, J.K.; Skevington, S.M. Family and professionals underestimate quality of life across diverse cultures and health conditions: Systematic review. J. Clin. Epidemiol. 2015, 68, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, J.; Estep, J.D.; Selzman, C.H.; Rogers, J.G.; Spertus, J.A.; Shah, K.B.; Chuang, J.; Farrar, D.J.; Starling, R.C. Patient-Reported Health-Related Quality of Life Is a Predictor of Outcomes in Ambulatory Heart Failure Patients Treated With Left Ventricular Assist Device Compared With Medical Management: Results From the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management). Circ. Heart Fail. 2017, 10, e003910. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/westernpacific/health-topics/obesity (accessed on 7 February 2021).
- Otterstad, J.E. Measuring left ventricular volume and ejection fraction with the biplane Simpson’s method. Heart 2002, 88, 559–560. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Agewall, S.; Borggrefe, M.; Calvert, M.; Caro, J.J.; Cowie, M.R.; Ford, I.; Paty, J.A.; Riley, J.P.; Swedberg, K.; et al. The importance of patient-reported outcomes: A call for their comprehensive integration in cardiovascular clinical trials. Eur. Heart J. 2014, 35, 2001–2009. [Google Scholar] [CrossRef]
- Skevington, S.M. Social Comparisons in Cross-cultural Quality of Life Assessment. Int. J. Ment. Health 1994, 23, 29–47. [Google Scholar] [CrossRef]
- Perera, H.N.; Izadikhah, Z.; O’Connor, P.; McIlveen, P. Resolving Dimensionality Problems With WHOQOL-BREF Item Responses. Assessment 2018, 25, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Jaracz, K.; Kalfoss, M.; Górna, K.; Baczyk, G. Quality of life in Polish respondents: Psychometric properties of the Polish WHOQOL-Bref. Scand. J. Caring Sci. 2006, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Freedland, K.E.; Carney, R.M.; Rich, M.W. Effect of depression on prognosis in heart failure. Heart Fail. Clin. 2011, 7, 11–21. [Google Scholar] [CrossRef]
- Mbakwem, A.; Aina, F.; Amadi, C. Expert Opinion-Depression in Patients with Heart Failure: Is Enough Being Done? Card. Fail. Rev. 2016, 2, 110–112. [Google Scholar] [CrossRef]
- Junger, J.; Schellberg, D.; Muller-Tasch, T.; Raupp, G.; Zugck, C.; Haunstetter, A.; Zipfel, S.; Herzog, W.; Haass, M. Depression increasingly predicts mortality in the course of congestive heart failure. Eur. J. Heart Fail. 2005, 7, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Green, C.P.; Porter, C.B.; Bresnahan, D.R.; Spertus, J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: A new health status measure for heart failure. J. Am. Coll. Cardiol. 2000, 35, 1245–1255. [Google Scholar] [CrossRef]
- Rector, T.S.; Cohn, J.N. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: Reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am. Heart J. 1992, 124, 1017–1025. [Google Scholar] [CrossRef]
- Rector, T.S.; Kubo, S.H.; Cohn, J.N. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am. J. Cardiol. 1993, 71, 1106–1107. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Lucas, R.; Cowie, M.R. Assessing health-related quality of life in heart failure patients attending an outpatient clinic: A pragmatic approach. ESC Heart Fail. 2019, 6, 3–9. [Google Scholar] [CrossRef]
- Faxén, U.L.; Hage, C.; Donal, E.; Daubert, J.C.; Linde, C.; Lund, L.H. Patient reported outcome in HFpEF: Sex-specific differences in quality of life and association with outcome. Int. J. Cardiol. 2018, 267, 128–132. [Google Scholar] [CrossRef]
- Dewan, P.; Rorth, R.; Jhund, P.S.; Shen, L.; Raparelli, V.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Kober, L.; et al. Differential Impact of Heart Failure with Reduced Ejection Fraction on Men and Women. J. Am. Coll. Cardiol. 2019, 73, 29–40. [Google Scholar] [CrossRef]
- Melendo-Viu, M.; Abu-Assi, E.; Manzano-Fernández, S.; Flores-Blanco, P.J.; Cambronero-Sánchez, F.; Dobarro Pérez, D.; Cespón Fernández, M.; Sánchez Galian, M.J.; Gómez Molina, M.; Caneiro-Queija, B.; et al. Incidence, prognosis and predictors of heart failure after acute myocardial infarction. REC Cardio Clin. 2020, 55, 8–14. [Google Scholar] [CrossRef]
- Omar, H.R.; Guglin, M. Post-discharge rise in BNP and rehospitalization for heart failure. Herz 2019, 44, 450–454. [Google Scholar] [CrossRef] [PubMed]
- York, M.K.; Gupta, D.K.; Reynolds, C.F.; Farber-Eger, E.; Wells, Q.S.; Bachmann, K.N.; Xu, M.; Harrell, F.E.; Wang, T.J. B-Type Natriuretic Peptide Levels and Mortality in Patients With and Without Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 2079–2088. [Google Scholar] [CrossRef]
- Maries, L.; Manitiu, I. Diagnostic and prognostic values of B-type natriuretic peptides (BNP) and N-terminal fragment brain natriuretic peptides (NT-pro-BNP). Cardiovasc. J. Afr. 2013, 24, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Gheorghiade, M.; Reid, K.J.; Dunlay, S.M.; Chan, P.S.; Hauptman, P.J.; Zannad, F.; Konstam, M.A.; Spertus, J.A. Identifying Patients Hospitalized With Heart Failure at Risk for Unfavorable Future Quality of Life. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 389–398. [Google Scholar] [CrossRef]
- Hoekstra, T.; Lesman-Leegte, I.; van Veldhuisen, D.J.; Sanderman, R.; Jaarsma, T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. Eur. J. Heart Fail. 2011, 13, 1013–1018. [Google Scholar] [CrossRef]
- Karlström, P.; Johansson, P.; Dahlström, U.; Boman, K.; Alehagen, U. Can BNP-guided therapy improve health-related quality of life, and do responders to BNP-guided heart failure treatment have improved health-related quality of life? Results from the UPSTEP study. BMC Cardiovasc. Disord. 2016, 16, 39. [Google Scholar] [CrossRef][Green Version]
- Mark, D.B.; Cowper, P.A.; Anstrom, K.J.; Sheng, S.; Daniels, M.R.; Knight, J.D.; Baloch, K.N.; Davidson-Ray, L.; Fiuzat, M.; Januzzi, J.L., Jr.; et al. Economic and Quality-of-Life Outcomes of Natriuretic Peptide–Guided Therapy for Heart Failure. J. Am. Coll. Cardiol. 2018, 72, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Califf, R.M.; Nohria, A.; Bhapkar, M.; Bowers, M.; Mancini, D.M.; Fiuzat, M.; Stevenson, L.W.; O’Connor, C.M. The STARBRITE trial: A randomized, pilot study of B-type natriuretic peptide-guided therapy in patients with advanced heart failure. J. Card. Fail. 2011, 17, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Pina, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Warraich, H.J.; Kitzman, D.W.; Whellan, D.J.; Duncan, P.W.; Mentz, R.J.; Pastva, A.M.; Nelson, M.B.; Upadhya, B.; Reeves, G.R. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults ≥60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circ. Heart Fail. 2018, 11, e005254. [Google Scholar] [CrossRef]
- Lewis, E.F.; Lamas, G.A.; O’Meara, E.; Granger, C.B.; Dunlap, M.E.; McKelvie, R.S.; Probstfield, J.L.; Young, J.B.; Michelson, E.L.; Halling, K.; et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur. J. Heart Fail. 2007, 9, 83–91. [Google Scholar] [CrossRef]
- Staniūtė, M.; Vaskelyte, J.; Rumbinaite, E.; Kaminskaite, B.; Samsanaviciene, S.; Plungiene, S.; Brozaitiene, J.; Bunevicius, R. Impact of left ventricular function on health-related quality of life in coronary artery disease patients. Medicina 2015, 51, 233–239. [Google Scholar] [CrossRef]
- Cvetinovic, N.; Loncar, G.; Isakovic, A.M.; von Haehling, S.; Doehner, W.; Lainscak, M.; Farkas, J. Micronutrient Depletion in Heart Failure: Common, Clinically Relevant and Treatable. Int. J. Mol. Sci. 2019, 20, 5627. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Carbonara, R.; Ruggieri, R.; Passantino, A.; Scrutinio, D. Iron Deficiency: A New Target for Patients With Heart Failure. Front. Cardiovasc. Med. 2021, 8, 709872. [Google Scholar] [CrossRef]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rozentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Heart J. 2013, 165, 575–582.e3. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E. Iron regulation and erythropoiesis. Curr. Opin. Hematol. 2008, 15, 169–175. [Google Scholar] [CrossRef]
- Ingwall, J.S. Energy metabolism in heart failure and remodelling. Cardiovasc. Res. 2009, 81, 412–419. [Google Scholar] [CrossRef]
- Dziegala, M.; Josiak, K.; Kasztura, M.; Kobak, K.; von Haehling, S.; Banasiak, W.; Anker, S.D.; Ponikowski, P.; Jankowska, E. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J. Cachexia Sarcopenia Muscle 2018, 9, 802–815. [Google Scholar] [CrossRef]
- Loncar, G.; Obradovic, D.; Thiele, H.; von Haehling, S.; Mitja Lainscak, M. Iron deficiency in heart failure. ESC Heart Fail. 2021, 8, 2368–2379. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Jankowska, E.A.; von Haehling, S.; Anker, S.D.; Macdougall, I.C.; Ponikowski, P. Iron deficiency and heart failure: Diagnostic dilemmas and therapeutic perspectives. Eur. Heart J. 2013, 34, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Kasztura, M.; Sokolski, M.; Bronisz, M.; Nawrocka, S.; Oleskowska-Florek, W.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Banasiak, W.; et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur. Heart J. 2014, 35, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef]
- Parikh, A.; Natarajan, S.; Lipsitz, S.R.; Katz, S.D. Iron Deficiency in Community-Dwelling U.S. Adults with Self-Reported Heart Failure in NHANES III: Prevalence and Associations with Anemia and Inflammation. Circ. Heart Fail. 2011, 4, 599–606. [Google Scholar] [CrossRef]
- Comin-Colet, J.; Enjuanes, C.; Gonzalez, G.; Torrens, A.; Cladellas, M.; Merono, O.; Ribas, N.; Ruiz, S.; Gomez, M.; Verdu, J.M.; et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur. J. Heart Fail. 2013, 15, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Ebner, N.; Jankowska, E.A.; Ponikowski, P.; Lainscak, M.; Elsner, S.; Sliziuk, V.; Steinbeck, L.; Kube, J.; Bekfani, T.; Scherbakov, N.; et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the Studies Investigating Co-morbidities Aggravating Heart Failure. Int. J. Cardiol. 2016, 205, 6–12. [Google Scholar] [CrossRef]
- Enjuanes, C.; Bruguera, J.; Grau, M.; Cladellas, M.; Gonzalez, G.; Merono, O.; Moliner-Borja, P.; Verdu, J.M.; Farre, N.; Comin-Colet, J. Iron Status in Chronic Heart Failure: Impact on Symptoms, Functional Class and Submaximal Exercise Capacity. Rev. Esp. Cardiol. 2016, 69, 247–255. [Google Scholar] [CrossRef]
- Kasner, M.; Aleksandrov, A.S.; Westermann, D.; Lassner, D.; Gross, M.; von Haehling, S.; Anker, S.D.; Schultheiss, H.-P.; Tschope, C. Functional iron deficiency and diastolic function in heart failure with preserved ejection fraction. Int. J. Cardiol. 2013, 168, 4652–4657. [Google Scholar] [CrossRef]
- Rangel, I.; Goncalves, A.; de Sousa, C.; Leite, S.; Campelo, M.; Martins, E.; Amorim, S.; Moura, B.; Silva Cardoso, J.; Maciel, M.J. Iron deficiency status irrespective of anemia: A predictor of unfavorable outcome in chronic heart failure patients. Cardiology 2014, 128, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.J.; Yeo, P.S.D.; Ching-Chiew Wong, R.; Ong, H.Y.; Leong, K.T.G.; Jaufeerally, F.; Sim, D.; Santhanakrishnan, R.; Lim, S.L.; Chan, M.M.Y.; et al. Iron deficiency in a multi-ethnic Asian population with and without heart failure: Prevalence, clinical correlates, functional significance and prognosis. Eur. J. Heart Fail. 2014, 16, 1125–1132. [Google Scholar] [CrossRef]
- Schou, M.; Bosselmann, H.; Gaborit, F.; Iversen, K.; Goetze, J.P.; Soletomas, G.; Rasmussen, J.; Kistorp, C.; Kober, L.; Gustafsson, F.; et al. Iron deficiency: Prevalence and relation to cardiovascular biomarkers in heart failure outpatients. Int. J. Cardiol. 2015, 195, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J.; Comin-Colet, J.; Minana, G.; Nunez, E.; Santas, E.; Mollar, A.; Valero, E.; Garcia-Blas, S.; Cardells, I.; Bodi, V.; et al. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur. J. Heart Fail. 2016, 18, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Arzt, M.; Doehner, W.; Edelmann, F.; Evertz, R.; Ebner, N.; Herrmann-Lingen, C.; Garfias Macedo, T.; Koziolek, M.; Noutsias, M.; et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: Evidence from clinical trials. Eur. J. Heart Fail. 2021, 23, 92–113. [Google Scholar] [CrossRef]
- Nanas, J.N.; Matsouka, C.; Karageorgopoulos, D.; Leonti, A.; Tsolakis, E.; Drakos, S.G.; Tsagalou, E.P.; Maroulidis, G.D.; Alexopoulos, G.P.; Kanakakis, J.E.; et al. Etiology of anemia in patients with advanced heart failure. J. Am. Coll. Cardiol. 2006, 48, 2485–2489. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Damy, T.; Terbah, M.; Kerebel, S.; Baguet, J.-P.; Hanon, O.; Zannad, F.; Laperche, T.; Leclercq, C.; Concas, V.; et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur. J. Heart Fail. 2014, 16, 984–991. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.; Rigby, A.S.; Witte, K.K.A.; Nikitin, N.P.; Tin, L.; Goode, K.; Bhandari, S.; Clark, A.L.; Cleland, J.G.F. Anemia, renal dysfunction, and their interaction in patients with chronic heart failure. Am. J. Cardiol. 2006, 98, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Belmar-Vega, L.; de Francisco, A.; Fiestas, Z.A.; Soto, M.S.; Kislikova, M.; Mozas, M.S.; Unzueta, M.G.; Rodriguez, M.A. Iron deficiency in patients with congestive heart failure: A medical practice that requires greater attention. Nefrologia 2016, 36, 249–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Przybylowski, P.; Wasilewski, G.; Golabek, K.; Bachorzewska-Gajewska, H.; Dobrzycki, S.; Koc-Zorawska, E.; Malyszko, J. Absolute and Functional Iron Deficiency Is a Common Finding in Patients With Heart Failure and After Heart Transplantation. Transpl. Proc. 2016, 48, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladella, M.; Ponikowski, P.; Banasiak, W.; van Veldhuisen, D.J.; van der Meer, P.; Jankowska, E.A.; Comin-Colet, J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter European study. Int. J. Cardiol. 2014, 174, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Gu, H.Q.; Wang, W.; Ma, L.; Li, W. Health-related quality of life in blood pressure control and blood lipid-lowering therapies: Results from the CHIEF randomized controlled trial. Hypertens. Res. 2019, 42, 1561–1571. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Jia, W.; Cao, W.; Han, K.; Liu, M.; He, Y. Relationships of Lipids Profile with Health-Related Quality of Life in Chinese Centenarians. J. Nutr. Health Aging 2020, 24, 404–411. [Google Scholar] [CrossRef]
| Parameter (n = 152) | Value |
|---|---|
| Age [years] | 57 (48–62) |
| Men | 124 (81.6%) |
| Somatic QoL domain score [0–100] | 50 (42.9–57.1) |
| Psychological QoL domain score [0–100] | 66.7 (58.3–70.8) |
| Social QoL domain score [0–100] | 75.0 (66.7–91.7) |
| Environmental QoL domain score [0–100] | 71.9 (62.5–81.2) |
| Total QoL [0–400] | 265.2 (239.7–285.7) |
| BMI [kg/m2] | 28.1 (24.9–32.1) |
| IHD etiology | 73 (48.0%) |
| HF exacerbation | 40 (26.3%) |
| SBP on admission [mmHg] | 112.3 ± 18.9 |
| DBP on admission [mmHg] | 70 (68–80) |
| HR on discharge [beats per minute] | 72.0 (65–80) |
| Comorbidities | |
| DM | 42 (27.6%) |
| COPD | 12 (7.9%) |
| CKD | 24 (15.8%) |
| Hypertension | 80 (52.6%) |
| AF | 62 (40.8%) |
| NYHA class | |
| I | 3 (2.0%) |
| II | 62 (40.8%) |
| III | 71 (46.7%) |
| IV | 16 (10.5%) |
| I–II | 65 (42.8%) |
| III–IV | 87 (57.2%) |
| Biochemical parameters | |
| BNP [pg/mL] | 509.8 (213.1–869.7) |
| TSH [mIU/L] | 1.82 (0.98–3.12) |
| Uric acid [µmol/L] | 454 (358–554) |
| Creatinine [µmol/L] | 95.0 (80.0–114.1) |
| eGFR [mL/min] | 72.5 ± 23.7 |
| Na+ [mmol/L] | 140.0 (138.0–142.0) |
| K+ [mmol/L] | 4.30 ± 0.42 |
| hsCRP [mg/L] | 4.0 (4.0–6.0) |
| Fasting glucose [mmol/L] | 5.8 (5.3–6.7) |
| Serum protein [g/L] | 71.5 (67.2–75.6) |
| Serum albumin [g/L] | 40.3 (37.8–43.4) |
| CholT [mmol/L] | 4.14 (3.37–4.95) |
| TG [mmol/L] | 1.35 (1.05–1.80) |
| LDL [mmol/L] | 2.48 (1.87–3.25) |
| HDL [mmol/L] | 1.13 (0.93–1.41) |
| Hgb [mmol/L] | 8.9 (8.3–9.6) |
| Serum iron [µmol/L] | 15.8 ± 7.1 |
| TIBC [µmol/L] | 63.0 (56.1–70.2) |
| Transferrin saturation [%] | 25.4 ± 12.1 |
| Ferritin [ng/mL] | 129.9 (61.8–218.3) |
| GNRI score | 113.3 ± 12.2 |
| High nutritional risk (GNRI <82) | 0 |
| Intermediate nutritional risk (GNRI ≥82 and <92) | 4 (3.7%) |
| Low nutritional risk (GNRI ≥92 and ≤98) | 5 (4.6%) |
| No nutritional risk (GNRI >98) | 99 (91.7%) |
| Echocardiographic parameters | |
| LVEF [%] | 22 (20–30) |
| Medications (n) | |
| Loop diuretics [%] | 143 (94.1%) |
| Thiazides [%] | 23 (15.1%) |
| β-blocker [%] | 147 (96.7%) |
| ACEI/ARB [%] | 98 (64.5%) |
| ARNI [%] | 37 (24.3%) |
| MRA [%] | 132 (86.8%) |
| Ca-blocker [%] | 7 (4.6%) |
| Statin [%] | 96 (63.2%) |
| Characteristics | Group 1 The Highest Quality of Life Group (Somatic Domain Score > 55) (n = 47) | Group 3 The Lowest Quality of Life Group (Somatic Domain Score < 50) (n = 58) | p |
|---|---|---|---|
| Age [years] | 56.0 (46–62) | 56.5 (49–62) | 0.60 |
| Men | 39 (83.0%) | 47 (81.0%) | 0.99 |
| Somatic QoL domain score [0–100] | 64.3 (57.1–67.9) | 42.9 (35.7–46.4) | <0.0001 |
| Psychological QoL domain score [0–100] | 66.7 (62.5–79.2) | 62.5 (58.3–70.8) | 0.004 |
| Social QoL domain score [0–100] | 83.3 (75.0–100) | 66.6 (58.3–83.3) | <0.0001 |
| Environmental QoL domain score [0–100] | 75.0 (65.6–87.5) | 68.8 (59.4–75.0) | 0.001 |
| Total QoL [0–400] | 288.2 (267.6–317.3) | 244.6 (219.5–263.1) | <0.0001 |
| BMI [kg/m2] | 28.7 (26.0–32.3) | 27.1 (23.8–31.7) | 0.08 |
| IHD etiology | 18 (38.3%) | 31 (53.4%) | 0.12 |
| HF exacerbation | 7 (14.9%) | 17 (28.3%) | 0.16 |
| SBP on admission [mmHg] | 115.0 ± 17.9 | 112.5 ± 19.6 | 0.50 |
| DBP on admission [mmHg] | 70 (68–80) | 70(68–80) | 0.81 |
| HR on discharge [beats per minute] | 70 (65–80) | 72.5 (66–80) | 0.29 |
| Comorbidities | |||
| DM | 14 (29.8%) | 20 (34.5%) | 0.66 |
| COPD | 1 (2.1%) | 8 (13.8%) | 0.08 |
| CKD | 7 (14.8%) | 11 (19.0%) | 0.74 |
| Hypertension | 20 (42.6%) | 28 (48.3%) | 0.56 |
| AF | 15 (31.9%) | 22 (37.9%) | |
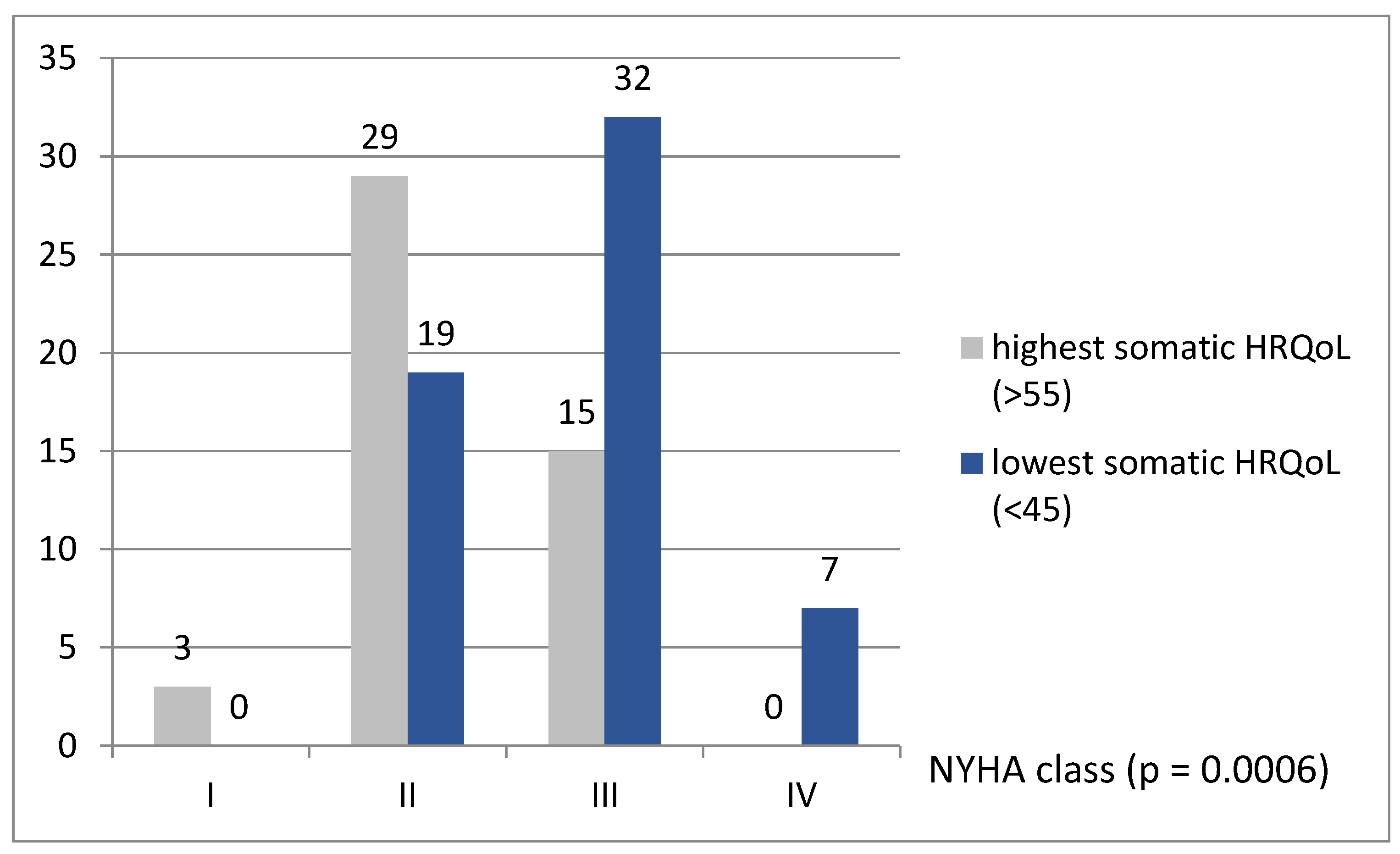
| NYHA class | |||
| I | 3 (6.4%) | 0 | 0.17 |
| II | 29(61.7%) | 19 (32.8%) | 0.003 |
| III | 15 (31.9%) | 32 (55.1%) | 0.02 |
| IV | 0 | 7 (12.1%) | 0.04 |
| I–II | 32 (68.1%) | 19 (32.8%) | 0.0003 |
| III–IV | 15 (31.9%) | 39 (57.3%) | |
| Biochemical parameters | |||
| BNP level [pg/mL] | 326.2 (162.2–687.9) | 578.3 (209.6–1124) | 0.06 |
| TSH [mIU/L] | 1.50 (0.98–3.07) | 1.52 (0.96–2.73) | 0.99 |
| Uricacid [µmol/L] | 424 (318–534) | 492 (383–559) | 0.03 |
| Creatinine [µmol/L] | 89.0 (79.0–107.0) | 100.2 (83.0–123.0) | 0.08 |
| eGFR | 77.3 ± 23.2 | 68.7 ± 21.2 | 0.052 |
| Na+ [mmol/L] | 140.0 (138.0–141.0) | 139.5 (137.0–142.0) | 0.44 |
| K+ [mmol/L] | 4.45 ± 0.39 | 4.21 ± 0.43 | 0.004 |
| hsCRP [mg/L] | 4.0 (2.6–5.6) | 4.0 (3.2–6.6) | 0.61 |
| Fastingglucose [mmol/L] | 5.8 (5.3–7.0) | 6.1 (5.3–6.7) | 0.92 |
| Serum protein [g/L] | 73.1 (69.5–77.9) | 71.8 (67.6–75.5) | 0.21 |
| Serum albumin [g/L] | 41.8 (40.0–44.0) | 40.0 (38.0–43.4) | 0.15 |
| CholT [mmol/L] | 4.61 (3.82–5.42) | 4.14 (3.20–4.79) | 0.01 |
| TG [mmol/L] | 1.48 (1.27–2.13) | 1.18 (0.91–1.57) | 0.006 |
| LDL [mmol/L] | 2.99 (2.38–3.60) | 2.40 (1.80–2.92) | 0.001 |
| HDL [mmol/L] | 1.15 (1.00–1.44) | 1.20 (0.92–1.54) | 0.96 |
| Hgb [mmol/L] | 9.2 (8.6–9.5) | 8.8 (8.2–9.7) | 0.24 |
| Serum iron [µmol/L] | 17.6 ± 7.0 | 15.1 ± 6.8 | 0.08 |
| TIBC [µmol/L] | 57.7 (52.7–68.6) | 64.9 (58.5–68.2) | 0.02 |
| Transferrinsaturation [%] | 29.7 ± 12.5 | 23.7 ± 11.1 | 0.01 |
| Ferritin [ng/mL] | 162.8 (76.2–255.5) | 126.0 (60.3–209.4) | 0.17 |
| Nutritional parameters | |||
| GNRI | 117.8 ± 12.4 | 112.3 ± 11.4 | 0.045 |
| Echocardiographic parameters | |||
| LVEF [%] | 25 (20–30) | 20 (15–25) | 0.003 |
| Medications (n) | |||
| Loopdiuretics | 42 (89.4%) | 56 (96.6%) | 0.29 |
| Thiazides | 4 (8.5%) | 12 (20.7%) | 0.16 |
| β-blocker | 45 (95.7%) | 58 (100%) | 0.90 |
| ACEI/ARB | 28 (59.6%) | 41 (70.7%) | 0.23 |
| ARNI | 13 (28.3%) | 13 (22.4%) | 0.49 |
| MRA | 38 (80.9%) | 52 (89.6%) | 0.45 |
| Ca-blocker | 2 (4.3%) | 1 (1.7%) | 0.85 |
| Statin | 29 (61.7%) | 38 (65.5%) | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałużna-Oleksy, M.; Sawczak, F.; Kukfisz, A.; Krysztofiak, H.; Szczechla, M.; Wleklik, M.; Przytarska, K.; Dudek, M.; Nowak, A.; Straburzyńska-Migaj, E.; et al. The Impact of Clinical, Biochemical, and Echocardiographic Parameters on the Quality of Life in Patients with Heart Failure with Reduced Ejection Fraction. Int. J. Environ. Res. Public Health 2021, 18, 12448. https://doi.org/10.3390/ijerph182312448
Kałużna-Oleksy M, Sawczak F, Kukfisz A, Krysztofiak H, Szczechla M, Wleklik M, Przytarska K, Dudek M, Nowak A, Straburzyńska-Migaj E, et al. The Impact of Clinical, Biochemical, and Echocardiographic Parameters on the Quality of Life in Patients with Heart Failure with Reduced Ejection Fraction. International Journal of Environmental Research and Public Health. 2021; 18(23):12448. https://doi.org/10.3390/ijerph182312448
Chicago/Turabian StyleKałużna-Oleksy, Marta, Filip Sawczak, Agata Kukfisz, Helena Krysztofiak, Magdalena Szczechla, Marta Wleklik, Katarzyna Przytarska, Magdalena Dudek, Alicja Nowak, Ewa Straburzyńska-Migaj, and et al. 2021. "The Impact of Clinical, Biochemical, and Echocardiographic Parameters on the Quality of Life in Patients with Heart Failure with Reduced Ejection Fraction" International Journal of Environmental Research and Public Health 18, no. 23: 12448. https://doi.org/10.3390/ijerph182312448
APA StyleKałużna-Oleksy, M., Sawczak, F., Kukfisz, A., Krysztofiak, H., Szczechla, M., Wleklik, M., Przytarska, K., Dudek, M., Nowak, A., Straburzyńska-Migaj, E., & Uchmanowicz, B. (2021). The Impact of Clinical, Biochemical, and Echocardiographic Parameters on the Quality of Life in Patients with Heart Failure with Reduced Ejection Fraction. International Journal of Environmental Research and Public Health, 18(23), 12448. https://doi.org/10.3390/ijerph182312448







