Feasibility of an Intervention Delivered via Mobile Phone and Internet to Improve the Continuity of Care in Schizophrenia: A Randomized Controlled Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Study Design and Procedures
2.3. Measures
2.3.1. Sociodemographics and Clinical Variables
2.3.2. Psychopathology
2.3.3. Attitudes and Expectations
2.3.4. User Satisfaction and Program Utilization
2.3.5. Reasons for Declining Participation
2.4. Intervention
2.5. Statistical Analysis
3. Results
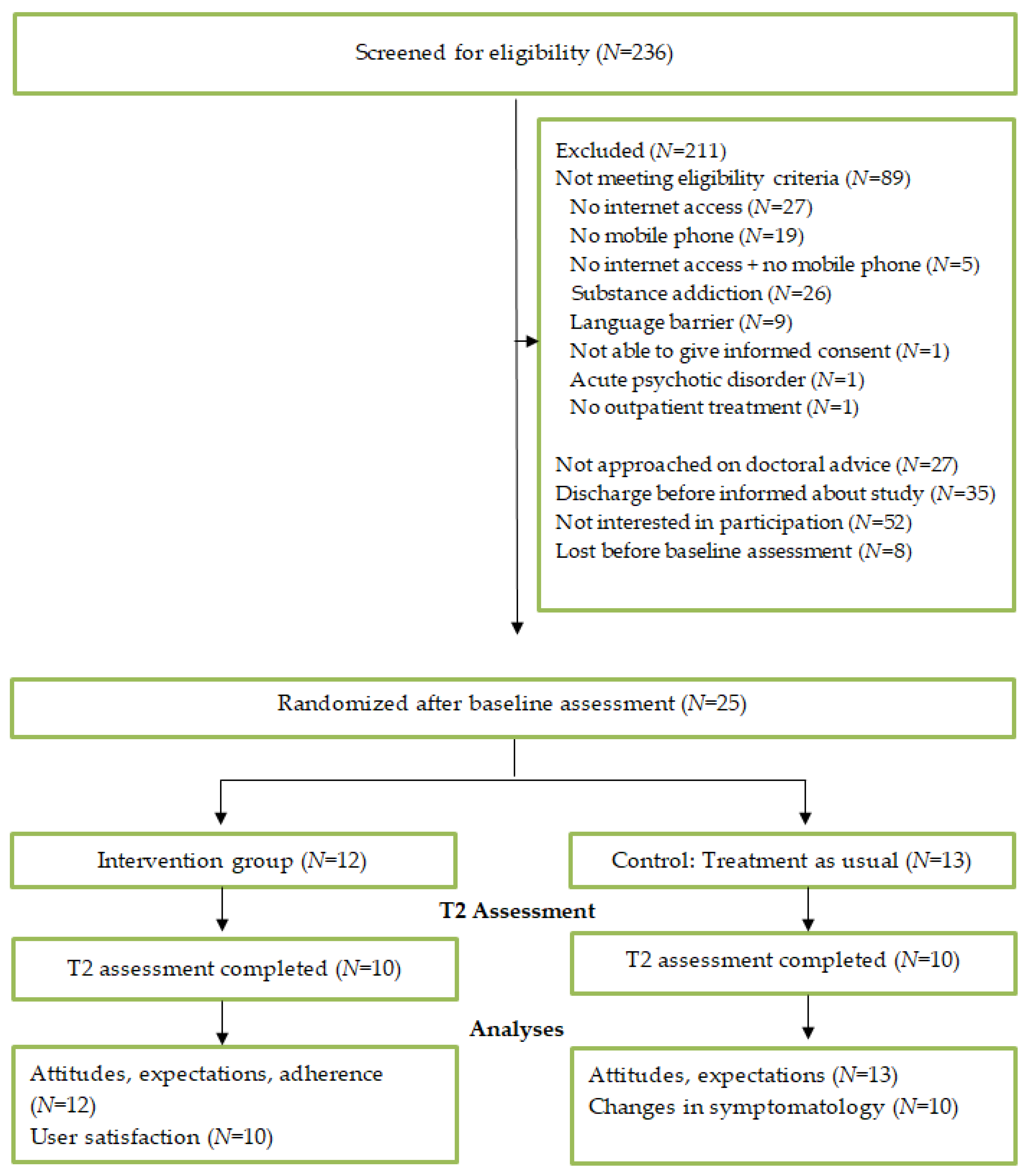
3.1. Recruitment and Participant Flow
3.2. Participants
3.3. Reasons for Declining Participation
3.4. Attitudes and Expectations
3.5. User Satisfaction
3.6. Adherence and Program Use
3.7. Healthcare Utilization during Study Participation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Glob. Health Metr. 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Caseiro, O.; Pérez-Iglesias, R.; Mata, I.; Martínez-Garcia, O.; Pelayo-Terán, J.M.; Tabares-Seisdedos, R.; de la Foz, V.O.G.; Vázquez-Barquero, J.L.; Crespo-Facorro, B. Predicting relapse after a first episode of non-affective psychosis: A three-year follow-up study. J. Psychiatr. Res. 2012, 46, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Woerner, M.G.; Alvir, J.M.J.; Bilder, R.; Goldman, R.; Geisler, S.; Koreen, A.; Sheitman, B.; Chakos, M.; Mayerhoff, D.; et al. Predictors of Relapse Following Response From a First Episode of Schizophrenia or Schizoaffective Disorder. Arch. Gen. Psychiatry 1999, 56, 241–247. [Google Scholar] [CrossRef]
- Alvarez-Jimenez, M.; Priede, A.; Hetrick, S.; Bendall, S.; Killackey, E.; Parker, A.; McGorry, P.; Gleeson, J.F. Risk factors for relapse following treatment for first episode psychosis: A systematic review and meta-analysis of longitudinal studies. Schizophr. Res. 2012, 139, 116–128. [Google Scholar] [CrossRef]
- Zipursky, R.B.; Menezes, N.M.; Streiner, D.L. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: A systematic review. Schizophr. Res. 2014, 152, 408–414. [Google Scholar] [CrossRef]
- Porcelli, S.; Bianchini, O.; de Girolamo, G.; Aguglia, E.; Crea, L.; Serretti, A. Clinical factors related to schizophrenia relapse. Int. J. Psychiatry Clin. Pr. 2016, 20, 54–69. [Google Scholar] [CrossRef]
- Barnes, T.R.; Drake, R.; Paton, C.; Cooper, S.J.; Deakin, B.; Ferrier, I.N.; Gregory, C.J.; Haddad, P.M.; Howes, O.D.; Jones, I.; et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2019, 34, 3–78. [Google Scholar] [CrossRef] [Green Version]
- National Collaborating Centre for Mental Health. Psychosis and Schizophrenia in Adults: Prevention and Management; National Institute for Health and Care Excellence (UK): London, UK, 2014. [Google Scholar]
- Andersson, G.; Titov, N.; Dear, B.; Rozental, A.; Carlbring, P. Internet-delivered psychological treatments: From innovation to implementation. World Psychiatry 2019, 18, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Andrews, G.; Basu, A.; Cuijpers, P.; Craske, M.; McEvoy, P.; English, C.; Newby, J. Computer therapy for the anxiety and depression disorders is effective, acceptable and practical health care: An updated meta-analysis. J. Anxiety Disord. 2018, 55, 70–78. [Google Scholar] [CrossRef]
- Hennemann, S.; Farnsteiner, S.; Sander, L. Internet- and mobile-based aftercare and relapse prevention in mental disorders: A systematic review and recommendations for future research. Internet Interv. 2018, 14, 1–17. [Google Scholar] [CrossRef]
- Schlosser, D.; Campellone, T.R.; Truong, B.; Etter, K.; Vergani, S.; Komaiko, K.; Vinogradov, S. Efficacy of PRIME, a Mobile App Intervention Designed to Improve Motivation in Young People With Schizophrenia. Schizophr. Bull. 2018, 44, 1010–1020. [Google Scholar] [CrossRef]
- Lado-Codesido, M.; Pérez, C.M.; Mateos, R.; Olivares, J.M.; Caballero, A.G. Improving emotion recognition in schizophrenia with “VOICES”: An on-line prosodic self-training. PLoS ONE 2019, 14, e0210816. [Google Scholar] [CrossRef] [PubMed]
- Španiel, F.; Hrdlička, J.; Novák, T.; Kožený, J.; Höschl, C.; Mohr, P.; Motlová, L.B. Effectiveness of the Information Technology-Aided Program of Relapse Prevention in Schizophrenia (ITAREPS). J. Psychiatr. Pr. 2012, 18, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, M.; Kannisto, K.A.; Vahlberg, T.; Hätönen, H.E.; Adams, C.; Oh, H.; Rotondi, A.; Ballesteros, S.; Schulz, P.; Lokman, S. Short Text Messages to Encourage Adherence to Medication and Follow-up for People With Psychosis (Mobile.Net): Randomized Controlled Trial in Finland. J. Med. Internet Res. 2017, 19, e245. [Google Scholar] [CrossRef] [Green Version]
- Krzystanek, M.; Borkowski, M.; Skałacka, K.; Krysta, K. A telemedicine platform to improve clinical parameters in paranoid schizophrenia patients: Results of a one-year randomized study. Schizophr. Res. 2018, 204, 389–396. [Google Scholar] [CrossRef]
- Alvarez-Jimenez, M.; Alcázar-Córcoles, M.; González-Blanch, C.; Bendall, S.; McGorry, P.; Gleeson, J.F. Online, social media and mobile technologies for psychosis treatment: A systematic review on novel user-led interventions. Schizophr. Res. 2014, 156, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Xiao, S.; He, H.; Caine, E.D.; Gloyd, S.; Simoni, J.; Hughes, J.P.; Nie, J.; Lin, M.; He, W.; et al. Lay health supporters aided by mobile text messaging to improve adherence, symptoms, and functioning among people with schizophrenia in a resource-poor community in rural China (LEAN): A randomized controlled trial. PLOS Med. 2019, 16, e1002785. [Google Scholar] [CrossRef] [PubMed]
- Westermann, S.; Rüegg, N.; Lüdtke, T.; Moritz, S.; Berger, T. Internet-based self-help for psychosis: Findings from a randomized controlled trial. J. Consult. Clin. Psychol. 2020, 88, 937–950. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Leucht, S.; Kane, J.M.; Kissling, W.; Hamann, J.; Etschel, E.; Engel, R. What does the PANSS mean? Schizophr. Res. 2005, 79, 231–238. [Google Scholar] [CrossRef]
- Wilhelm, M.; Feldhege, J.; Bauer, S.; Moessner, M. Einsatz internetbasierter Verlaufsmessung in der Psychotherapieforschung. Psychotherapeut 2020, 65, 505–511. [Google Scholar] [CrossRef]
- Firth, J.; Cotter, J.; Torous, J.; Bucci, S.; Firth, J.A.; Yung, A. Mobile Phone Ownership and Endorsement of “mHealth” Among People With Psychosis: A Meta-analysis of Cross-sectional Studies. Schizophr. Bull. 2015, 42, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.J.; Stewart, A.; Schrimsher, J.; Peeples, D.; Buckley, P.F. How connected are people with schizophrenia? Cell phone, computer, email, and social media use. Psychiatry Res. 2015, 225, 458–463. [Google Scholar] [CrossRef]
- Fernández-Sotos, P.; Fernández-Caballero, A.; González, P.; Aparicio, A.I.; Martínez-Gras, I.; Torio, I.; Dompablo, M.; García-Fernández, L.; Santos, J.L.; Rodriguez-Jimenez, R.; et al. Digital Technology for Internet Access by Patients With Early-Stage Schizophrenia in Spain: Multicenter Research Study. J. Med. Internet Res. 2019, 21, e11824. [Google Scholar] [CrossRef]
- Athanasopoulou, C.; Välimäki, M.; Koutra, K.; Löttyniemi, E.; Bertsias, A.; Basta, M.; Vgontzas, A.N.; Lionis, C. Internet use, eHealth literacy and attitudes toward computer/internet among people with schizophrenia spectrum disorders: A cross-sectional study in two distant European regions. BMC Med. Inf. Decis. Mak. 2017, 17, 136. [Google Scholar] [CrossRef] [Green Version]
- Valimaki, M.; Kuosmanen, L.; Hätönen, H.; Koivunen, M.; Pitkänen, A.; Athanasopoulou, C.; Anttila, M. Connectivity to computers and the Internet among patients with schizophrenia spectrum disorders: A cross-sectional study. Neuropsychiatr. Dis. Treat. 2017, ume 13, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Spanakis, P.; Peckham, E.; Mathers, A.; Shiers, D.; Gilbody, S. The digital divide: Amplifying health inequalities for people with severe mental illness in the time of COVID-19. Br. J. Psychiatry 2021, 219, 529–531. [Google Scholar] [CrossRef]
- Allan, S.; Mcleod, H.; Bradstreet, S.; Bell, I.; Whitehill, H.; Wilson-Kay, A.; Clark, A.; Matrunola, C.; Morton, E.; Farhall, J.; et al. Perspectives of Trial Staff on the Barriers to Recruitment in a Digital Intervention for Psychosis and How to Work Around Them: Qualitative Study Within a Trial. JMIR Hum. Factors 2021, 8, e24055. [Google Scholar] [CrossRef]
- Görgen, W.; Engler, U. Ambulante Psychotherapeutische Versorgung von Psychosekranken Menschen Sowie Älteren Menschen in Berlin; Verlagsgruppe Hüthig Jehle Rehm: Heidelberg, Germany, 2005. [Google Scholar]
- Wittmann, W.W.; Steffanowski, A.; Kriz, D.; Glahn, E.M.; Völkle, M.C. Qualitätsmonitoring in der Ambulanten Psychotherapie; Techniker Krankenkasse: Hamburg, Germany, 2011. [Google Scholar]
- Bauer, S.; Okon, E.; Meermann, R.; Kordy, H. Technology-enhanced maintenance of treatment gains in eating disorders: Efficacy of an intervention delivered via text messaging. J. Consult. Clin. Psychol. 2012, 80, 700–706. [Google Scholar] [CrossRef]
- Kordy, H.; Wolf, M.; Aulich, K.; Bürgy, M.; Hegerl, U.; Hüsing, J.; Puschner, B.; Rummel-Kluge, C.; Vedder, H.; Backenstrass, M. Internet-Delivered Disease Management for Recurrent Depression: A Multicenter Randomized Controlled Trial. Psychother. Psychosom. 2016, 85, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.; Ainsworth, J.; Sanders, C.; Stockton-Powdrell, C.; Machin, M.; Whelan, P.; Hopkins, R.; He, Z.; Applegate, E.; Drake, R.; et al. Smartphone-Enhanced Symptom Management In Psychosis: Open, Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e17019. [Google Scholar] [CrossRef]
- Jäger, M.; Riedel, M.; Messer, T.; Laux, G.; Pfeiffer, H.; Naber, D.; Schmidt, L.G.; Gaebel, W.; Huff, W.; Heuser, I.; et al. Psychopathological characteristics and treatment response of first episode compared with multiple episode schizophrenic disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 257, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Spellmann, I.; Riedel, M.; Schennach, R.; Seemüller, F.; Obermeier, M.; Musil, R.; Jäger, M.; Schmauß, M.; Laux, G.; Pfeiffer, H.; et al. One-year functional outcomes of naturalistically treated patients with schizophrenia. Psychiatry Res. 2012, 198, 378–385. [Google Scholar] [CrossRef]
- Kasenda, B.; von Elm, E.; You, J.; Blümle, A.; Tomonaga, Y.; Saccilotto, R.; Amstutz, A.; Bengough, T.; Meerpohl, J.J.; Stegert, M.; et al. Prevalence, Characteristics, and Publication of Discontinued Randomized Trials. JAMA 2014, 311, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Dwan, K.; Gamble, C.; Williamson, P.R.; Kirkham, J.; The Reporting Bias Group. Systematic Review of the Empirical Evidence of Study Publication Bias and Outcome Reporting Bias—An Updated Review. PLoS ONE 2013, 8, e0066844. [Google Scholar] [CrossRef] [Green Version]
- Ebert, D.D.; Zarski, A.-C.; Christensen, H.; Stikkelbroek, Y.; Cuijpers, P.; Berking, M.; Riper, H. Internet and Computer-Based Cognitive Behavioral Therapy for Anxiety and Depression in Youth: A Meta-Analysis of Randomized Controlled Outcome Trials. PLoS ONE 2015, 10, e0119895. [Google Scholar] [CrossRef]
| Intervention Group (N = 12) | Control Group (N = 13) | |
| Gender | ||
| Female | 66.7% (N = 8) | 61.5% (N = 8) |
| Male | 33.3% (N = 4) | 38.5% (N = 5) |
| Mean age (SD) | 34.3 (10.33) | 38.2 (14.07) |
| Mean illness duration in years (SD) | 7.6 (7.90) | 8.0 (7.36) |
| Number of previous inpatient treatments due to the schizophrenia spectrum disorder | 3.2 (3.83) | 2.7 (3.47) |
| PANSS total score M (SD) | ||
| Discharge from hospital (t1) | 48.42 (16.63) | 46.00 (12.49) |
| 6-months assessment (t2) | 44.30 (14.61) | 43.30 (11.48) |
| Would you use the following modules of HEINS? | Agreement |
| Weekly monitoring | 100.0% (N = 25) |
| Information materials | 80.0% (N = 20) |
| Personal crisis plan | 88.0% (N = 22) |
| Counseling via internet chat | 72.0% (N = 18) |
| Counseling via phone | 80.0% (N = 20) |
| Contact the hospital via HEINS in case of a crisis | 92.0% (N = 23) |
| How much do you agree? | |
| I think that the program HEINS provides a feeling of security to the participants. | 96.0% (N = 24) |
| I believe that participation in the HEINS program has a positive effect on one’s wellbeing. | 92.0% (N = 23) |
| I think it’s good that one is called by a doctor in case of alarming answers in the monitoring via mobile phone. | 92.0% (N = 23) |
| I think that I would benefit from the participation in HEINS. | 88.0% (N = 22) |
| I think participants feel supported by the program HEINS after discharge. | 88.0% (N = 22) |
| My motivation to participate in the HEINS program is high. | 88.0% (N = 22) |
| I think that the weekly contact via monitoring helps participants to take their medications as prescribed. | 80.0% (N = 20) |
| I think that participating in HEINS helps to cope with daily routine after discharge. | 80.0% (N = 20) |
| I feel secure about the data privacy in the HEINS program. | 75.0% (N = 18) |
| I am sufficiently supplied with aftercare support without the HEINS program. | 72.0% (N = 18) |
| I think it’s good that the hospital offers an aftercare program like HEINS | 80.0% (N = 20) |
| The effort to participate in the HEINS program seems low to me. | 84.0% (N = 21) |
| In general, I have a positive attitude towards communication technologies (e.g., computer, mobile phone, internet). | 84.0% (N = 21) |
| Agreement | Not Able to Evaluate | |
|---|---|---|
| I like the idea that the program contains information materials on schizophrenia. | 80.0% (N = 8) | 10.0% (N = 1) |
| The information materials were helpful for me. | 70.0% (N = 7) | 10.0% (N = 1) |
| I like the idea of the weekly monitoring via mobile phone | 100.0% (N = 10) | / |
| The monitoring feedback was appropriate | 80.0% (N = 8) | / |
| The monitoring feedback was helpful for me. | 60.0% (N = 6) | / |
| I like the idea that telephone and internet chat appointments are offered. | 90.0% (N = 9) | 10.0% (N = 1) |
| The utilization of telephone and internet chat appointments was helpful for me. | 30.0% (N = 3) | 50.0% (N = 5) |
| I like the idea that a personal crisis plan is prepared for every participant. | 70.0% (N = 7) | 30.0% (N = 3) |
| The personal crisis plan was helpful for me. | 60.0% (N = 6) | 20.0% (N = 2) |
| I felt supported by the HEINS program after discharge. | 90.0% (N = 9) | |
| Participating in the HEINS program helped me to cope better with my daily routine after discharge. | 70.0% (N = 7) | |
| Participating in the HEINS program had a positive effect on my wellbeing. | 80.0% (N = 8) | |
| Participating in the HEINS program gave me a feeling of security. | 60.0% (N = 6) | |
| Participating in the HEINS program supported me in dealing with my disorder. | 60.0% (N = 6) | |
| Participating in the HEINS program helped me to take my medication as prescribed. | 55.5% (N = 5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallinat, C.; Moessner, M.; Apondo, S.; Thomann, P.A.; Herpertz, S.C.; Bauer, S. Feasibility of an Intervention Delivered via Mobile Phone and Internet to Improve the Continuity of Care in Schizophrenia: A Randomized Controlled Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 12391. https://doi.org/10.3390/ijerph182312391
Gallinat C, Moessner M, Apondo S, Thomann PA, Herpertz SC, Bauer S. Feasibility of an Intervention Delivered via Mobile Phone and Internet to Improve the Continuity of Care in Schizophrenia: A Randomized Controlled Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(23):12391. https://doi.org/10.3390/ijerph182312391
Chicago/Turabian StyleGallinat, Christina, Markus Moessner, Sandra Apondo, Philipp A. Thomann, Sabine C. Herpertz, and Stephanie Bauer. 2021. "Feasibility of an Intervention Delivered via Mobile Phone and Internet to Improve the Continuity of Care in Schizophrenia: A Randomized Controlled Pilot Study" International Journal of Environmental Research and Public Health 18, no. 23: 12391. https://doi.org/10.3390/ijerph182312391
APA StyleGallinat, C., Moessner, M., Apondo, S., Thomann, P. A., Herpertz, S. C., & Bauer, S. (2021). Feasibility of an Intervention Delivered via Mobile Phone and Internet to Improve the Continuity of Care in Schizophrenia: A Randomized Controlled Pilot Study. International Journal of Environmental Research and Public Health, 18(23), 12391. https://doi.org/10.3390/ijerph182312391






