Transparent Exopolymer Particles in Drinking Water Treatment—A Brief Review
Abstract
:1. Introduction
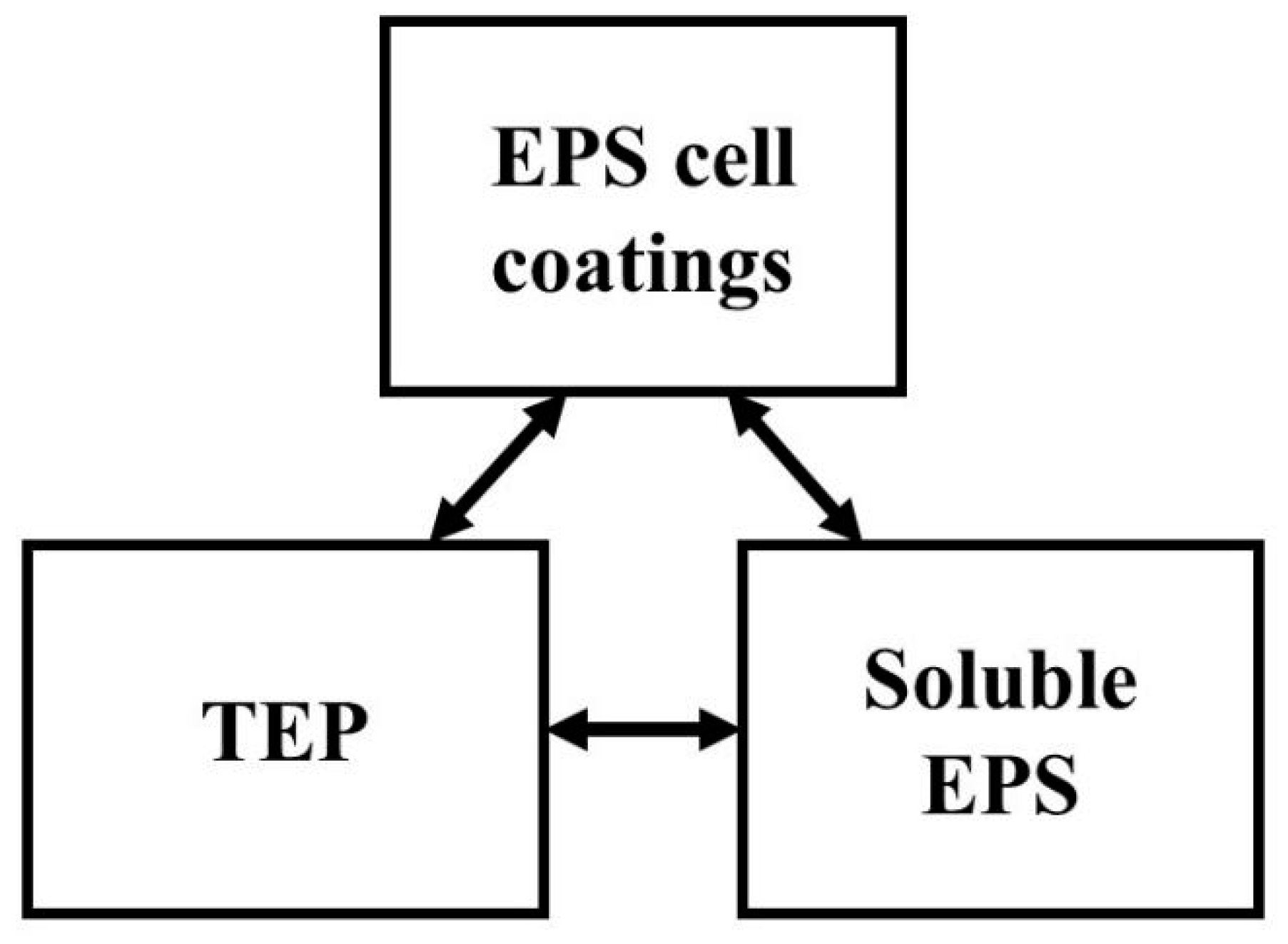
2. Definition of TEP in Water Treatment
3. TEP in Source Water Reservoir
4. TEPs in Water Treatment Process
4.1. Removal of TEP by Water Treatment Process
4.2. Role of TEP in the Water Treatment Process
4.3. TEP in Drinking Water
5. Membrane Contamination Caused by TEPs
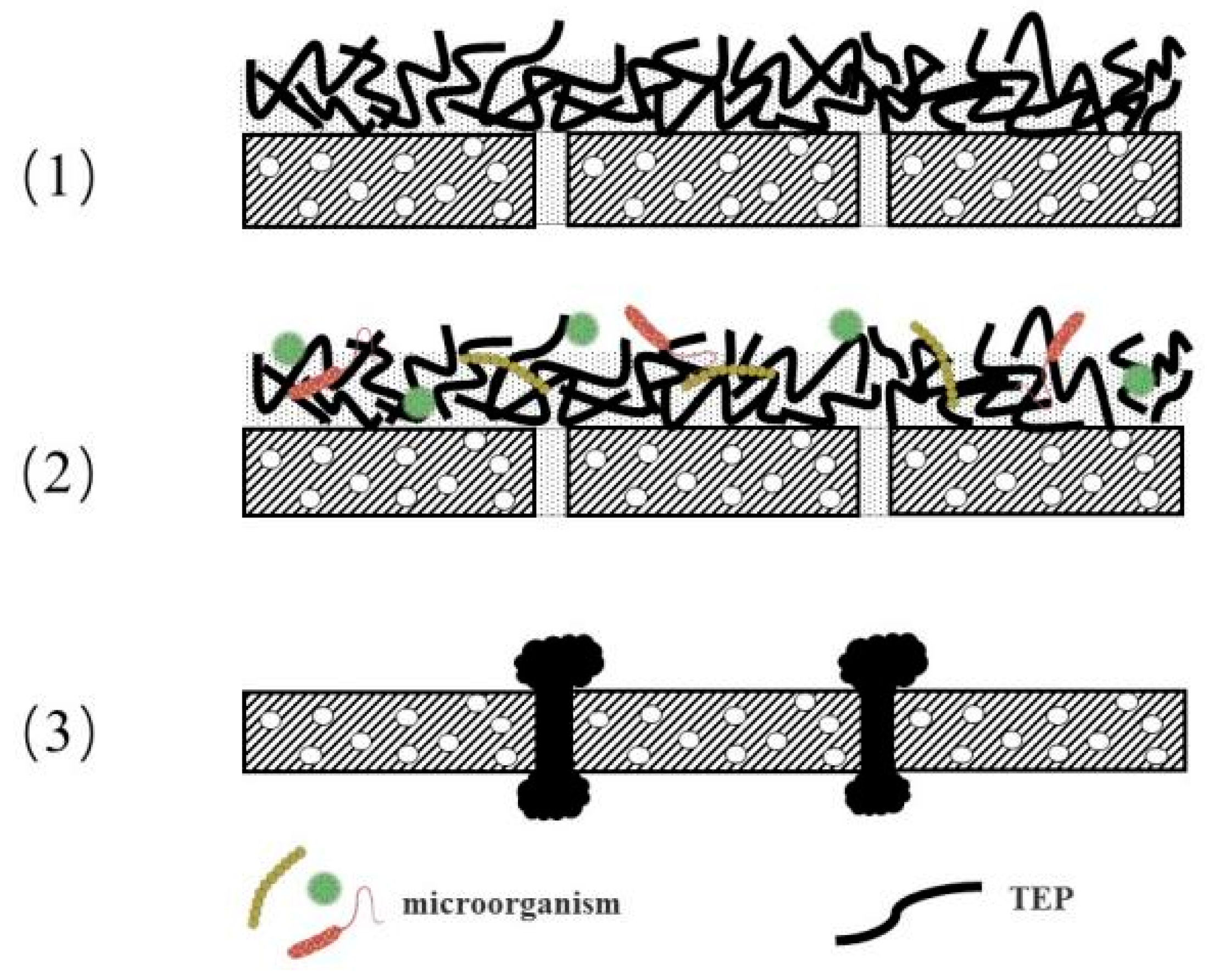
5.1. Mechanism of TEP-Induced Membrane Fouling
5.2. Effect of Alleviating TEP-induced Membrane Fouling
6. Conclusions and Prospects
- (1)
- It is difficult to separate AOM from organic matter and other sources in natural water. TEP can be used as an important characterization method to study AOM in natural water due to special chemical properties in TEP.
- (2)
- The seasonal water stratification has a significant impact on water quality and phytoplankton reproduction and may indirectly affect the formation of TEP in water. However, there is a lack of systematic investigation and research on the formation and the temporal and spatial distribution of TEP in a source water reservoir with a certain depth of water where stratification may occur.
- (3)
- The relationship between TEP and water treatment process is mutual. The impact of TEPs on the conventional water treatment process is an urgent problem to be addressed.
- (4)
- Mechanism of TEP-induced membrane fouling can be explained from three aspects: the formation of cake layer, provision of nutrients for microorganisms and a plug membrane channel. In addition to improving membrane materials, it is also a feasible way to reduce the generation probability of TEP by regulating the inlet water quality.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TEP | Transparent exopolymer particles |
| cTEP | Colloidal transparent exopolymer particles |
| pTEP | Particulate transparent exopolymer particles |
| AOM | Algal organic matter |
| NOM | Natural organic matter |
| DOM | Dissolved organic matter |
| POM | Particulate organic matter |
| EPS | Extracellular polymer |
| COD | Chemical oxygen demand |
| TOC | Total organic carbon |
| RO | Reverse osmosis |
| UF | Ultrafiltration |
| MF | Microfiltration |
References
- Li, L.; Gao, N.; Deng, Y.; Yao, J.; Zhang, K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Simon, A.P.; Bruce, J. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms. Water Res. 2008, 42, 3435–3445. [Google Scholar] [CrossRef]
- Pivokonský, M.; Načeradská, J.; Kopecká, I.; Baresova, M.; Jefferson, B.; Li, X.; Henderson, R.K. The impact of algogenic organic matter on water treatment plant operation and water quality: A review. Crit. Rev. Environ. Sci. Technol. 2015, 46, 291–335. [Google Scholar] [CrossRef]
- Ye, L.; Shi, X.; Wu, X.; Zhang, M.; Yu, Y.; Li, D.; Kong, F. Dynamics of dissolved organic carbon after a cyanobacterial bloom in hypereutrophic Lake Taihu (China). Limnologica 2011, 41, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, W.; Chen, J.; Zhang, B.; Zhao, L.; Jiang, X. Characteristics of Dissolved Organic Matter and Its Role in Lake Eutrophication at the Early Stage of Algal Blooms—A Case Study of Lake Taihu, China. Water 2020, 12, 2278. [Google Scholar] [CrossRef]
- United Nations. Goal 6: Ensure Access to Water and Sanitation for All. Available online: https://unric.org/en/sdg-6/ (accessed on 15 November 2021).
- Sadoff, C.W.; Borgomeo, E.; Uhlenbrook, S. Rethinking water for SDG 6. Nat. Sustain. 2020, 3, 346–347. [Google Scholar] [CrossRef]
- Alldredge, A.L.; Passow, U.; Logan, B.E. The abundance and significance of a class of large, transparent organic particles in the ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 1131–1140. [Google Scholar] [CrossRef]
- Berman, T.; Holenberg, M. Don’t fall foul of biofilm through high TEP levels. Filtr. Sep. 2005, 42, 30–32. [Google Scholar] [CrossRef]
- Berman, T.; Passow, U. Transparent Exopolymer Particles (TEP): An overlooked factor in the process of biofilm formation in aquatic environments. Nat. Preced. 2007, 1182, 1. [Google Scholar] [CrossRef]
- Passow, U. Transparent exopolymer particles (TEP) in aquatic environments. Prog. Oceanogr. 2002, 55, 287–333. [Google Scholar] [CrossRef] [Green Version]
- Berman, T.; Mizrahi, R.; Dosoretz, C.G. Transparent exopolymer particles (TEP): A critical factor in aquatic biofilm initiation and fouling on filtration membranes. Desalination 2011, 276, 184–190. [Google Scholar] [CrossRef]
- Meng, S.; Meng, X.; Fan, W.; Liang, D.; Wang, L.; Zhang, W.; Liu, Y. The role of transparent exopolymer particles (TEP) in membrane fouling: A critical review. Water Res. 2020, 181, 115930. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, M.; Yao, M.; Qiu, Z.; Hong, Y.; Lan, W.; Xia, H.; Jin, X. Membrane Fouling and Performance of Flat Ceramic Membranes in the Application of Drinking Water Purification. Water 2019, 11, 2606. [Google Scholar] [CrossRef] [Green Version]
- Emery, K.O.; Johns, I.A.; Honjo, S. Organic films on particulate matter in surface waters off eastern Asia. Sedimentology 1984, 31, 503–514. [Google Scholar] [CrossRef]
- Passow, U.; Alldredge, A.L. A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP). Limnol. Oceanogr. 1995, 40, 1326–1335. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Ekowati, Y.; Calix-Ponce, H.N.; Schippers, J.C.; Amy, G.L.; Kennedy, M.D. Improved method for measuring transparent exopolymer particles (TEP) and their precursors in fresh and saline water. Water Res. 2015, 70, 300–312. [Google Scholar] [CrossRef]
- Thornton, D.C.O. Diatom aggregation in the sea: Mechanisms and ecological implications. Eur. J. Phycol. 2002, 37, 149–161. [Google Scholar] [CrossRef]
- Passow, U. Formation of transparent exopolymer particles, TEP, from dissolved precursor material. Mar. Ecol. Prog. Ser. 2000, 192, 1–11. [Google Scholar] [CrossRef]
- Passow, U. Production of TEP by phytoplankton and bacteria. J. Phycol. 2002, 236, 1–12. [Google Scholar]
- Bittar, T.B.; Passow, U.; Hamaraty, L.; Bidle, K.D.; Harvey, E.L. An updated method for the calibration of transparent exo-polymer particle measurements. Limnol. Oceanogr. Methods 2018, 16, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Wetz, M.S.; Robbins, M.C.; Paerl, H.W. Transparent Exopolymer Particles (TEP) in a River-Dominated Estuary: Spatial–Temporal Distributions and an Assessment of Controls upon TEP Formation. Chesap. Sci. 2009, 32, 447–455. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Li, Q.; Yue, W.; Wang, Y.; Sun, F.; Peng, Y. Distribution characteristics of transparent exopolymer particles in the Pearl River estuary, China. J. Geophys. Res. 2012, 117, G00N17. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Liu, L.; Qin, B.; Cai, X.; Zhu, G.; Zhang, Y.; Gong, Z.; Tang, X. Abundance, characteristics, and size spectra of transparent exopolymer particles and Coomassie stainable particles during spring in a large shallow lake, Taihu, China. J. Great Lakes Res. 2016, 42, 455–463. [Google Scholar] [CrossRef]
- Berman, T.; Viner-Mozzini, Y. Abundance and characteristics of polysaccharide and proteinaceous particles in Lake Kinneret. Aquat. Microb. Ecol. 2001, 24, 255–264. [Google Scholar] [CrossRef] [Green Version]
- De Vicente, I.; Ortega-Retuerta, E.; Mazuecos, I.P.; Pace, M.L.; Cole, J.J.; Reche, I. Variation in transparent exopolymer particles in relation to biological and chemical factors in two contrasting lake districts. Aquat. Sci. 2010, 72, 443–453. [Google Scholar] [CrossRef]
- Brachvogel, T.; Schweitzer, B.; Simon, M. Dynamics and bacterial colonization of microaggregates in a large mesotrophic lake. Aquat. Microb. Ecol. 2001, 26, 23–35. [Google Scholar] [CrossRef]
- Nevel, S.; Hennebel, T.; Beuf, K.; Laing, G.; Verstraete, W.; Boon, N. Transparent exopolymer particle removal in different drinking water production centers. Water Res. 2012, 46, 3603–3611. [Google Scholar] [CrossRef]
- Discart, V.; Bilad, M.; Van Nevel, S.; Boon, N.; Cromphout, J.; Vankelecom, I. Role of transparent exopolymer particles on membrane fouling in a full-scale ultrafiltration plant: Feed parameter analysis and membrane autopsy. Bioresour. Technol. 2014, 173, 67–74. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Kennedy, M.D.; Amy, G.L.; Schippers, J.C. The fate of Transparent Exopolymer Particles (TEP) in integrated membrane systems: Removal through pre-treatment processes and deposition on reverse osmosis membranes. Water Res. 2009, 43, 5039–5052. [Google Scholar] [CrossRef]
- Kennedy, M.D.; Tobar, F.P.M.; Amy, G.; Schippers, J.C. Transparent exopolymer particle (TEP) fouling of ultrafiltration membrane systems. Desalin. Water Treat. 2009, 6, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, Z.; Liu, M.; He, J.; Shi, K.; Zhou, Y.; Wang, M.; Liu, X. Dissolved oxygen stratification and response to thermal structure and long-term climate change in a large and deep subtropical reservoir (Lake Qiandaohu, China). Water Res. 2015, 75, 249–258. [Google Scholar] [CrossRef] [PubMed]
- JianChao, S.; Yongrui, Y.; Fei, L.; TingLin, H.; Qitao, Y. Constraining release of pollutants from anoxic bottom sediment via water-lifting aeration in a source water reservoir, East China. J. Soils Sediments 2021, 21, 3300–3309. [Google Scholar] [CrossRef]
- Chin, W.-C.; Orellana, M.V.; Verdugo, P. Spontaneous assembly of marine dissolved organic matter into polymer gels. Nat. Cell Biol. 1998, 391, 568–572. [Google Scholar] [CrossRef]
- Şebnem, E. Effects of thermal stratification and mixing on reservoir water quality. Limnology 2008, 9, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.-C.; Huang, T.-L.; Wen, G.; Liu, F.; Qiu, X.-P.; Wang, B.-S. The Variation Characteristic of Sulfides and VOSc in a Source Water Reservoir and Its Control Using a Water-Lifting Aerator. Int. J. Environ. Res. Public Heal. 2016, 13, 427. [Google Scholar] [CrossRef] [Green Version]
- Bar-Zeev, E.; Belkin, N.; Liberman, B.; Berman, T.; Berman-Frank, I. Rapid sand filtration pretreatment for SWRO: Microbial maturation dynamics and filtration efficiency of organic matter. Desalination 2012, 286, 120–130. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Vigneswaran, S.; Rice, S.A. Microbial activity in biofilter used as a pretreatment for seawater desalination. Desalination 2013, 309, 254–260. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Schurer, R.; Kennedy, M.D.; Amy, G.L.; Schippers, J.C. The fate of transparent exopolymer particles (TEP) in seawater UF-RO system: A pilot plant study in Zeeland, The Netherlands. Desalin. Water Treat. 2010, 13, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lee, S.-T.; Sinha, S.; Leiknes, T.; Amy, G.L.; Ghaffour, N. Transparent exopolymer particles (TEP) removal efficiency by a combination of coagulation and ultrafiltration to minimize SWRO membrane fouling. Water Res. 2016, 102, 485–493. [Google Scholar] [CrossRef]
- De La Torre, T.; Lesjean, B.; Drews, A.; Kraume, M. Monitoring of transparent exopolymer particles (TEP) in a membrane bioreactor (MBR) and correlation with other fouling indicators. Water Sci. Technol. 2008, 58, 1903–1909. [Google Scholar] [CrossRef]
- Monnot, M.; Laborie, S.; Cabassud, C. Granular activated carbon filtration plus ultrafiltration as a pretreatment to seawater desalination lines: Impact on water quality and UF fouling. Desalination 2016, 383, 1–11. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Belkin, N.; Liberman, B.; Berman-Frank, I.; Berman, T. Bioflocculation: Chemical free, pre-treatment technology for the desalination industry. Water Res. 2013, 47, 3093–3102. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Berman-Frank, I.; Girshevitz, O.; Berman, T. Revised paradigm of aquatic biofilm formation facilitated by microgel transparent exopolymer particles. Proc. Natl. Acad. Sci. USA 2012, 109, 9119–9124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Qin, B.; Huang, Q. Advances in Ttransparent Exopolymer Particles(TEP) in Freshwaters. Adv. Earth Sci. 2014, 29, 1149–1157. [Google Scholar]
- Meng, S.J.; Liu, Y. Alginate blockfractions and their effects on membrane fouling. Water Res. 2013, 47, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Villacorte, L.O.; Ekowati, Y.; Winters, H.; Amy, G.L.; Schippers, J.C.; Kennedy, M.D. Characterisation of transparent exopolymer particles (TEP) produced during algal bloom: A membrane treatment perspective. Desalin. Water Treat. 2013, 51, 1021–1033. [Google Scholar] [CrossRef]
- Meng, S.; Fan, W.; Li, X.; Liu, Y.; Liang, D.; Liu, X. Intermolecular interactions of polysaccharides in membrane fouling during microfiltration. Water Res. 2018, 143, 38–46. [Google Scholar] [CrossRef]
- Meng, S.; Wang, R.; Zhang, M.; Liu, H. Insights into the Fouling Propensities of Natural Derived Alginate Blocks during the Microfiltration Process. Processes 2019, 7, 858. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J. A review of transparent exopolymer particles and their membrane fouling mechanisms. Environ. Chem. 2020, 39, 3038–3049. [Google Scholar]
- Zhang, Z.; Chen, M.; Li, J.; Zhao, B.; Wang, L. Significance of transparent exopolymer particles derived from aquatic algae in membrane fouling. Arab. J. Chem. 2020, 13, 4577–4585. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Mi, N.; Gao, Z.; Liu, T.; Lv, Y.; Wang, X.; Meng, X.; Yang, Y. Enhancement and Mitigation Mechanisms of Protein Fouling of Ultrafiltration Membranes under Different Ionic Strengths. Environ. Sci. Technol. 2015, 49, 6574–6580. [Google Scholar] [CrossRef]
- Liu, G.; Yu, S.; Yang, H.; Hu, J.; Zhang, Y.; He, B.; Li, L.; Liu, Z. Molecular Mechanisms of Ultrafiltration Membrane Fouling in Polymer-Flooding Wastewater Treatment: Role of Ions in Polymeric Fouling. Environ. Sci. Technol. 2016, 50, 1393–1402. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Kennedy, M.D.; Amy, G.L.; Schippers, J.C. Measuring transparent exopolymer particles (TEP) as indicator of the (bio)fouling potential of RO feed water. Desalin. Water Treat. 2009, 5, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Winters, H.; Liu, Y. Ultrafiltration behaviors of alginate blocks at various calcium concentrations. Water Res. 2015, 83, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, Y. Transparent exopolymer particles (TEP)-associated membrane fouling at different Na+ concentrations. Water Res. 2017, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Henthorne, L.; Boysen, B. State-of-the-art of reverse osmosis desalination pretreatment. Desalination 2015, 356, 129–139. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, L.; Yan, Y.; Yin, L.; Liu, Y.; Kang, Q. Investment and Cost Analysis of Large Scale Seawater Desalination Project. J. Salt Sci. Chem. Ind. 2021, 50, 6–9. [Google Scholar]
- Water Reuse Association. Seawater Desalination Costs, White Paper. January 2012. Available online: https://www.watereuse.org (accessed on 25 October 2021).
- Gao, C.; Zhou, Y.; Liu, L. Recent Development and Prospect of Seawater Reverse Osmosis Desalination Technology. J. Ocean. Technol. 2016, 35, 1–14. [Google Scholar]
- Lin, J.C.-T.; Wu, C.-Y.; Chu, Y.-L.; Huang, W.-J. Effects of high turbidity seawater on removal of boron and transparent exopolymer particles by chemical oxo-precipitation. J. Taiwan Inst. Chem. Eng. 2019, 94, 109–118. [Google Scholar] [CrossRef]
| Sample Type | pTEP | cTEP | Reference |
|---|---|---|---|
| μg Xeq·L−1 | μg Xeq·L−1 | ||
| Neuse River Estuary (Jan Apr), USA | 991~1712 | / | [22] |
| Neuse River Estuary (May–Aug), USA | 805~1801 | / | [22] |
| Neuse River Estuary (Aug), USA | >3500 | / | [22] |
| Pearl River Estuary (Jan), China | 88.7~1586.9 | / | [23] |
| Pearl River Estuary (Aug), China | 521.5~1727.4 | / | [23] |
| Lake Taihu, China | 0~5190 | / | [24] |
| Lake Kinneret, Israel | 759~2385 | / | [25] |
| Mediterranean lakes, Spain | 66~9038 | / | [26] |
| North temperate lakes, USA | 36~1462 | / | [26] |
| Quentar Reservoir, Spain | 1.9~335.2 | / | [27] |
| Surface water, Belgium | 14.8 ± 14 | 684 ± 94 | [28] |
| Ground water, Belgium | <5 | <50 | [28] |
| Secondary wastewater effluent, Belgium | 102 ± 20 | 1470 ± 189 | [28] |
| Surface water in a pond, Belgium | 2~143 | 5~137 | [29] |
| Meuse River (Jul), The Netherlands | ~105 | ~165 | [30] |
| Lake IJssel (Jun), The Netherlands | ~110 | ~500 | [30] |
| Gent-Terneuzen canal (Jul), Belgium | ~80 | ~330 | [30] |
| River Estuary (Jul), Belgium | ~230 | ~290 | [30] |
| Surface water, The Netherlands | 990 | / | [31] |
| Water Treatment Processes | Feed Water | Key Description | Reference |
|---|---|---|---|
| Prechlorination | Secondary wastewater effluent | Increased cTEP and pTEP concentrations with respectively 34 and 41% | [28] |
| Coagulation+Sedimentation | Surface water | A decrease of cTEP amount and an increase of pTEP weight, while total TEP concentrations did not change significantly | [28] |
| Coagulation+Flotation | Surface water | The pTEP amount stayed minimal and the cTEP concentration decreased by 70% | [28] |
| River water | Decreased the total TEP concentration further with 70% | [17] | |
| Filtration | Effluent after Coagulation+Sedimentation | A good option to remove these coagulated pTEP (decrease ~90%) but was a too rough method to abate the smaller cTEP (decrease ~5%) | [28] |
| Effluent after Coagulation+Flotation | The pTEP amount stayed minimal and the cTEP concentration increased | [28] | |
| In-line coagulation | The removal of TEP was 70% while the remaining fraction of TEP was totally removed by UF | [31] | |
| Coagulation Effluent | TEP concentrations in the input seawater were diminished by 27% (±19) after passing the stage of the sand/ mixed-bed filter | [37] | |
| Activated carbon | Filter Effluent | Decreased the cTEP concentration further with 50% | [28] |
| Biological activated carbon filter | Seawater | The AOC and TEP concentration in seawater was reduced significantly by 90% and 84%, respectively | [38] |
| Membrane Processes | Feed Water | Rejection Rates | Reference |
|---|---|---|---|
| Microfiltration (MF) | Canal water | 0% pTEP, cTEP ~70% | [30] |
| Estuary water | ~65% pTEP, ~50% cTEP | [30] | |
| Surface water | 95% pTEP, 97% cTEP ※ | [28] | |
| Ultrafiltration (UF) | Surface water | 100% pTEP, 17~67% cTEP | [30] |
| coagulation effluent | 100% pTEP | [31] | |
| Filtration effluent | 95% pTEP, 97% cTEP ※ | [28] | |
| coagulation effluent | ~100% pTEP, ~99% cTEP | [37] | |
| coagulation effluent | 26~29% total TEP | [38] | |
| Reverse osmosis (RO) | UF effluent | 100% | [30] |
| Surface water | 100% | [28] |
| Pretreatment Processes | Investment (Million ¥) | Notes |
|---|---|---|
| Micro-Flocculation + Multi-media Filtration | 7.0~10.0 | |
| Coagulation + Sedimentation + Filtration + UF | 10.0~13.0 | Includes sludge treatment systems |
| Micro-Flocculation + Filtration + UF | 11.0~13.0 | |
| Coagulation + Flotation + Filtration + UF | 10.0~14.0 | Includes sludge treatment systems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Yang, Y.; Yi, Q.; Zhang, J.; Wang, L. Transparent Exopolymer Particles in Drinking Water Treatment—A Brief Review. Int. J. Environ. Res. Public Health 2021, 18, 12344. https://doi.org/10.3390/ijerph182312344
Shi J, Yang Y, Yi Q, Zhang J, Wang L. Transparent Exopolymer Particles in Drinking Water Treatment—A Brief Review. International Journal of Environmental Research and Public Health. 2021; 18(23):12344. https://doi.org/10.3390/ijerph182312344
Chicago/Turabian StyleShi, Jianchao, Yongrui Yang, Qitao Yi, Jin Zhang, and Lianxiang Wang. 2021. "Transparent Exopolymer Particles in Drinking Water Treatment—A Brief Review" International Journal of Environmental Research and Public Health 18, no. 23: 12344. https://doi.org/10.3390/ijerph182312344
APA StyleShi, J., Yang, Y., Yi, Q., Zhang, J., & Wang, L. (2021). Transparent Exopolymer Particles in Drinking Water Treatment—A Brief Review. International Journal of Environmental Research and Public Health, 18(23), 12344. https://doi.org/10.3390/ijerph182312344





