A One Health Review of Community-Acquired Antimicrobial-Resistant Escherichia coli in India
Abstract
1. Introduction
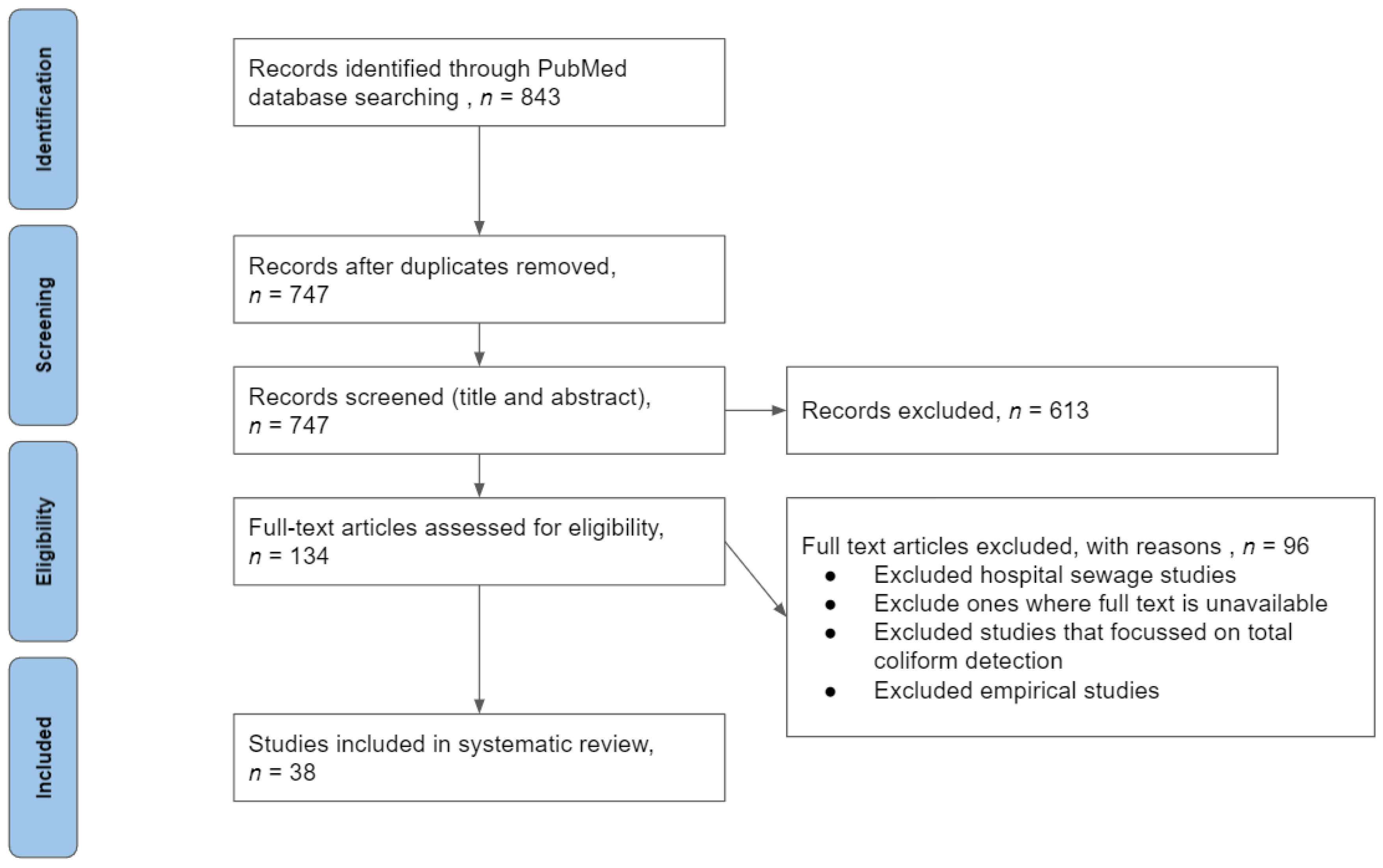
2. Materials and Methods
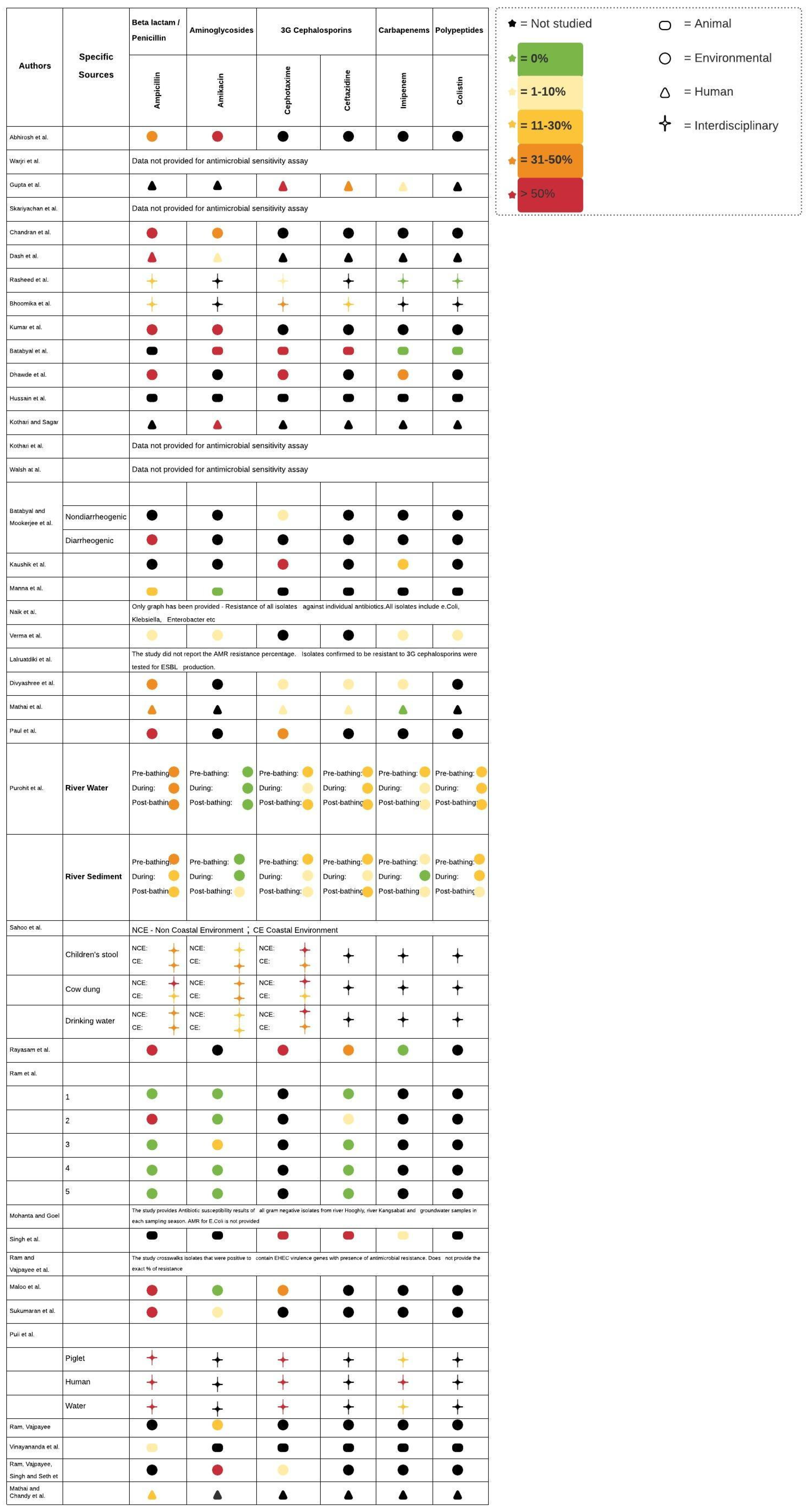
3. Results
3.1. AR-E in Humans
3.2. AR-E in Animals
3.3. AR-E in the Environment
3.4. AR-E in Interdisciplinary Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Humans (Enterobacteriaceae[MeSH Major Topic] OR (Enterobacteriaceae*[tiab] OR Gram negative bacteri*[tiab] OR E. coli [tiab]OR Escherischia*[tiab] OR Klebsiella*[tiab] OR Salmonella[tiab] OR Proteus[tiab] OR Enterobacter[tiab] OR Shigella[tiab] OR Citrobacter[tiab] OR Yersinia[tiab] OR gut flora[tiab] OR Morganella[tiab])) AND (Drug Resistance, Bacterial [MeSH Major Topic] OR Drug resistan*[tiab] OR Extended-spectrum beta-lactamase[tiab] OR ESBL[tiab] OR Cephalosporinase[tiab] OR beta-lactamase[tiab] OR Carbapenem[tiab] OR Cephalosporin[tiab] OR colistin[tiab] OR antibiotic*[tiab] OR antimicrob*[tiab]) AND (resistan*[tiab]) AND (human*[tiab] OR community acquired OR community-acquired) AND (india) |
| Animals (Enterobacteriaceae[MeSH Major Topic] OR (Enterobacteriaceae*[tiab] OR Gram negative bacteri*[tiab] OR E. coli [tiab]OR Escherischia*[tiab] OR Klebsiella*[tiab] OR Salmonella[tiab] OR Proteus[tiab] OR Enterobacter[tiab] OR Shigella[tiab] OR Citrobacter[tiab] OR Yersinia[tiab] OR gut flora[tiab] OR Morganella[tiab])) AND (Drug Resistance, Bacterial[MeSH Major Topic] OR Drug resistan*[tiab] OR Extended-spectrum beta-lactamase[tiab] OR ESBL[tiab] OR Cephalosporinase[tiab] OR beta-lactamase[tiab] OR Carbapenem[tiab] OR Cephalosporin[tiab] OR colistin[tiab] OR antibiotic*[tiab] OR antimicrob*[tiab]) AND (resistan*[tiab]) AND (livestock*[tiab] OR poultry[tiab] OR cattle[tiab] OR cows[tiab] OR pets[tiab] OR chickens[tiab]) AND (india) |
| Environment (Enterobacteriaceae[MeSH Major Topic] OR (Enterobacteriaceae*[tiab] OR Gram negative bacteri*[tiab] OR E. coli [tiab]OR Escherischia*[tiab] OR Klebsiella*[tiab] OR Salmonella[tiab] OR Proteus[tiab] OR Enterobacter[tiab] OR Shigella[tiab] OR Citrobacter[tiab] OR Yersinia[tiab] OR gut flora[tiab] OR Morganella[tiab])) AND (Drug Resistance, Bacterial[MeSH Major Topic] OR Drug resistan*[tiab] OR Extended-spectrum beta-lactamase[tiab] OR ESBL[tiab] OR Cephalosporinase[tiab] OR beta-lactamase[tiab] OR Carbapenem[tiab] OR Cephalosporin[tiab] OR colistin[tiab] OR antibiotic*[tiab] OR antimicrob*[tiab]) AND (resistan*[tiab]) AND (environment*[tiab] OR water) AND (india) |
| Author | Location | Type of Study | Sample Size and Source | No. of Isolates * | Key Takeaways |
|---|---|---|---|---|---|
| Manna et al. [8] | Kolkatta, West Bengal | Animal | Fecal samples from cows (n = 177) 7 | 14 strains of E. coli serotype O157 | 14 strains of E. coli serotype O157 were detected and isolated with shiga toxin genes found in all the isolates. |
| Rasheed et al. [11] | Hyderabad | Interdisciplinary | Vegetables and food of animal origin (n = 150) 2 | 99 | E. coli with highest drug resistance were detected in raw chicken (23.3%), followed by vegetable salad (20%). |
| Skariyachan et al. [16] | River Cauvery | Environmental | River water samples from 10 sites | 283 | 97% of E. coli isolated (n = 273) were completely resistant to all 30 antimicrobials tested. River Cauvery is a major source for potable water in Karnataka, India. |
| Mathai et al. [25] | Vellore, India | Human | Urine culture of pregnant women *; sample size unspecified | 58 | Multiple integrons per isolate were detected with class 1 being more prevalent than class 2. 45% of the isolates were resistant to more than two groups of antimicrobials with a significant association with the presence of integrons. |
| Kothari and Sagar [26] | Delhi | Human | Urine samples (n = 531) | 361 | E. coli was the most common pathogen associated with CA-UTI in women with the highest level of resistance to amoxicillin. |
| Dash et al. [27] | Southern Odisha | Human | Adult urine samples (n = 1670) | 397 | E. coli showed highest resistance to ampicillin and least resistance to amikacin. |
| Kothari et al. [28] | Delhi | Human | Stool samples of neonates (n = 75) | 219 | ESBL production increased threefold from days 1 to 60 in neonates with gene transmission, likely from mother to infant. |
| Batabyal et al. [29] | West Bengal | Animal | Bovine milk (n = 182) | 22 | 12.1% of the milk samples contained E. coli. More than 50% of isolates were ESBL producers; all were resistant to cefotaxime. |
| Lalruatdiki et al. [30] | Assam and Meghalaya | Animal | Fecal samples (n = 228) | 867 | Pig population was confirmed to carry multidrug-resistant and ESBL-producing E. coli with at least one of the three genes–blaCTX-M, blaTEM, and blaCMY. |
| Singh et al. [31] | Mumbai | Animal | Fish and shellfish (n = 50) | 475 | More than 70% of the isolates were ESBL producers, which highlights the risk of AMR dissemination via the food chain. |
| Vinayananda et al. [32] | Southern India | Animal | Eggs (n = 840) | 24 | Overall, E. coli occurred in 28.6% of the table eggs, with the highest level recorded in free-range eggs when compared to processed and unprocessed ones 11. |
| Ram et al. [33] | River Ganga in Kanpur city | Environmental | Water samples from five sites | 75 | 24% of the isolates exhibited AMR. |
| Ram et al. [34] | River Gomti, Lucknow | Environmental | Water samples from six sites | 81 | Water samples from five out of the six sites were contaminated with E. coli. 18 isolates were confirmed to be positive for virulence determinants of EHEC ###, 100% of which were resistant to at least one antimicrobial. |
| Ram et al. [35] | River Saryu (Ghaghra) | Environmental | Water samples from three sites | 42 | Resistance to multiple antimicrobials was observed. 50.0% and 33.3% of E. coli isolates were resistant to tetracycline and neomycin, respectively. |
| Ram et al. [36] | River Gomti | Environmental | Water samples from six sites | 90 | More than half of the total E. coli isolates were resistant to three or more antimicrobials. |
| Maloo et al. [37] | Mumbai | Environmental | Water samples from five recreational beaches | 65 | 95% of these isolates were multidrug-resistant. The findings also suggest a potential high-risk source of contamination of coastal waters. |
| Verma et al. [38] | Rajasthan | Environmental * | Vegetables/fruits (n = 520) | 73 | The overall prevalence rate ** of E. coli was observed to be 14%. Four of the 73 E. coli isolates were shiga toxin producers with the stx1/stx2/eae genes. |
| Kaushik et al. [40] | Yamuna River | Environmental | Water samples | 141 | The study reported a high incidence of multidrug-resistant bacteria that correlated with the prevalence of integrons (Class 1 class 2 integrons detected in 32% of the isolates). |
| Sahoo et al. [41] | Odisha, India | Interdisciplinary | Child stool samples, cow dung, and drinking water (n = 1251) 9 | 696 | 90% of the E. coli isolates were resistant to at least one antimicrobial. |
| Warjri et al. [42] | Mizoram | Human | Human feces (n = 180) 1 | 333 | 22% of the E. coli isolated were confirmed ESBL-producers. 10% of the E. coli isolated were confirmed to harbor either blaCTX-M-1 or blaSHV genes. |
| Bhoomika et al. [43] | Tribal districts of Chhattisgarh | Interdisciplinary | Human urine and stool samples (n = 60) and food of animal origin (n = 270) 3 (n = 330) | 191 | More than 50% of the samples were positive for E. coli; 74 E. coli isolates (from human and animal samples) were resistant to two or more antimicrobials. |
| Hussain et al. [44] | Karnataka, Telangana, Andhra Pradesh, and Maharashtra | Animal | Chicken samples (n = 120) 5 | 168 | E. coli contamination was lower in free-range chicken (15%) when compared to broiler chickens (78%). Moreover, E. coli from free-range chickens were resistant to fewer antimicrobial agents. |
| Sukumaran et al. [46] | Cochin Estuary, Vembanadu Lake | Environmental | Water samples from five water stations | 75 | More than half of the isolates were MDR. Two E. coli isolates were resistant to more than seven antimicrobials and both contained class 1 integrons. |
| Abhirosh et al. [47] | Vembanadu Lake | Environmental | Lake water samples from 10 locations | 33 | Multidrug resistance (i.e., resistance to three or more drugs) in E. coli was 100%. |
| Mohanta and Goel [48] | Kolkatta and West Bengal | Environmental | Water samples | 163 ## | Prevalence of AMR was highest post-monsoon, followed by winter and summer, respectively. |
| Gupta et al. [49] | Chandigarh, India | Human | Stool samples (n = 207) | 131 | ~70% of stool samples carried AR-E, and resistance to cephalosporins was common. Among the cephalosporin-resistant E. coli isolates, TEM was the most prevalent ESBL-encoding gene, followed by OXA. |
| Chandran et al. [50] | Cochin Estuary, Vembanadu Lake | Environmental | Water samples from five stations | 81 | >95% of E. coli isolates exhibited multidrug resistance. |
| Kumar et al. [51] | Mathura | Environmental | Water samples (n = 100) 4 | 1.08 log10 CFU mL−1 to 6.34 log10 CFU mL−1 # | Multidrug resistance was observed in both drinking water and river water samples. Resistance was highest against tetracycline, while most isolates were susceptible to chloramphenicol. |
| Batabyal and Mookerjee et al. [52] | Kolkatta | Environmental | Water samples (n = 411) | 88 E. coli of 25 serotypes | Approximately 20% of potable water harbored E. coli, with highest levels in pond water. |
| Dhawde et al. [53] | River Mula-Mutha | Environmental | River water samples across eight sites | 219 | 28% of the E. coli were resistant to more than six antimicrobials, with 12% being resistant to all eight antimicrobials; 10% of the isolates were ESBL producers. |
| Walsh et al. [54] | Delhi | Environmental | Water samples (n = 291) 6 | N/A | The blaNDM-1 gene was found in both seepage and tap water samples with serious implications for people using these sources for water consumption. This study looked for NDM-1 β-lactamase-producing bacteria in environmental samples and did not isolate E. coli. |
| Naik et al. [55] | Mumbai | Animal | Fish (n = 15) | N/A | MAR was detected with high prevalence of AMR genes in fish. |
| Divyashree et al. [56] | Mangalore | Environmental | Untreated wastewater (n = 45) | 31 | 17.3% of the total isolates (n = 179) were confirmed to be E. coli, an indicator of fecal contamination of seafood. |
| Rayasam et al. [57] | Alibag | Environmental | Water samples from a drinking water distribution system (n = 147) | 104 | 100% of the E. coli isolates were resistant to ampicillin and 26% to two or more antimicrobials. 43% of the E. coli isolates belonged to STs archived as ExPEC or IPEC strains ###. |
| Puii et al. [58] | North East India | Interdisciplinary | Water sources and fecal samples from piglets on pig farms (n = 219) 10 | 496 | 67.9% of the total E. coli isolates were ESBL producers, of which the majority came from piglets followed by humans. This study took a One Health approach to detecting E. coli. |
| Mathai and Chandy et al. [59] | Vellore, India | Human | Urine cultures of women with UTI symptoms | 1095 | Among the commensal isolates, 42% were resistant to at least one antimicrobial. 8.4% of the isolates were resistant to the three most prescribed antimicrobials in the treatment of UTI, namely, ampicillin, co-trimoxazole, and nalidixic acid. This study suggested a direct link between antimicrobial use and AMR. |
| Paul et al. [60] | City of Silchar, Assam | Environmental | Human sewage from 19 sites | 108 | This study revealed that the majority of resistant strains belonged to just three sequence types (STs), namely, ST167, ST410, and ST648. |
| Purohit et al. [61] | River Kshipra | Environmental | Water and sediment samples (n = 216) 8 | 807 (river water) and 353 (river sediment) | The total count of E. coli was significantly higher during and post-bathing events. AMR was higher in river water versus sediment. |
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance; Independent Review of Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 17 November 2021).
- World Health Organization. Antimicrobial Resistance in the South-East Asia. Available online: https://www.who.int/southeastasia/health-topics/antimicrobial-resistance (accessed on 2 November 2021).
- Centers for Disease Control and Prevention. A One Health Challenge the Interconnected Threat of Antibiotic Resistance. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/One-Health-Challenge-508.pdf (accessed on 25 March 2021).
- Anderson, M.A.; Whitlock, J.E.; Harwood, V.J. Diversity and distribution of Escherichia coli genotypes and antibiotic resistance phenotypes in feces of humans, cattle, and horses. Appl. Environ. Microbiol. 2006, 72, 6914–6922. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, N.; Porrero, M.C.; Mentaberre, G.; Serrano, E.; Mateos, A.; Domínguez, L.; Lavín, S. Antimicrobial resistance in indicator Escherichia coli isolates from free-ranging livestock and sympatric wild ungulates in a natural environment (Northeastern Spain). Appl. Environ. Microbiol. 2013, 79, 6184–6186. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.-G.; Sadowsky, M.; Byappanahalli, M.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H.; Suzuki, T.A.; Phifer-Rixey, M.; Nachman, M.W. Transmission modes of the mammalian gut microbiota. Science 2018, 362, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Brahmane, M.P.; Manna, C.; Batabyal, K.; Das, R. Occurrence, virulence characteristics and antimicrobial resistance of Escherichia coli O157 in slaughtered cattle and diarrhoeic calves in West Bengal, India. Lett. Appl. Microbiol. 2006, 43, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.O.A.; Dantas, G.; Church, G.M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 2009, 325, 1128–1131. [Google Scholar] [CrossRef]
- Szmolka, A.; Nagy, B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front. Microbiol. 2013, 4, 258. [Google Scholar] [CrossRef]
- Rasheed, M.U.; Thajuddin, N.; Ahamed, P.; Teklemariam, Z.; Jamil, K. Antimicrobial Drug Resistance in Strains of Escherichia coli Isolated from Food Sources; Revista Do Instituto de Medicina Tropical de São Paulo: São Paulo, Brazil, 2014; Volume 56, pp. 341–346. Available online: https://www-ncbi-nlm-nih-gov.libproxy.berkeley.edu/pmc/articles/PMC4131821/ (accessed on 15 November 2021).
- Vasco, G.; Spindel, T.; Carrera, S.; Grigg, A.; Trueba, G. The Role of Aerobic Respiration in the Life Cycle of Escherichia coli: Public Health Implications. Av. Cienc. Ing. 2015, 7, B7–B9. Available online: https://revistas.usfq.edu.ec/index.php/avances/article/view/248/249 (accessed on 15 November 2021).
- Hu, Y.; Yang, X.; Li, J.; Lv, N.; Liu, F.; Wu, J.; Lin, I.Y.; Wu, N.; Weimer, B.C.; Gao, G.F. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl. Environ. Microbiol. 2016, 82, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Stewardson, A.J.; Marimuthu, K.; Sengupta, S.; Allignol, A.; El-Bouseary, M.; Carvalho, M.J.; Hassan, B.; Delgado-Ramirez, M.A.; Arora, A.; Bagga, R. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): A multinational prospective cohort study. Lancet Infect. Dis. 2019, 19, 601–610. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Skariyachan, S.; Mahajanakatti, A.B.; Grandhi, N.J.; Prasanna, A.; Sen, B.; Sharma, N.; Vasist, K.S.; Narayanappa, R. Environmental monitoring of bacterial contamination and antibiotic resistance patterns of the fecal coliforms isolated from Cauvery River, a major drinking water source in Karnataka, India. Environ. Monit. Assess. 2015, 187, 279. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Desphande, J. Poultry farming, climate change, and drivers of antimicrobial resistance in India. Lancet Planet. Health 2019, 3, e494–e495. [Google Scholar] [CrossRef]
- Taneja, N.; Sharma, M. Antimicrobial resistance in the environment: The Indian scenario. Indian J. Med. Res. 2019, 149, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Nirwan, P.; Jain, S.; Kapil, A. Nosocomial outbreak of septicaemia in neonatal intensive care unit due to extended spectrum β-lactamase producing Klebsiella pneumoniae showing multiple mechanisms of drug resistance. Indian J. Med. Microbiol. 2010, 28, 380–384. [Google Scholar] [CrossRef]
- Banerjee, T.; Mishra, A.; Das, A.; Sharma, S.; Barman, H.; Yadav, G. High prevalence and endemicity of multidrug resistant Acinetobacter spp. In intensive care unit of a tertiary care Hospital, Varanasi, India. J. Pathog. 2018, 2018, 9129803. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Datta, P.; Gupta, V.; Kumari, P.; Kaur, G.; Chander, J. Characterization of Bacteriological Isolates from Patients and Environment Samples of Burn Ward: A Study from a Tertiary Care Hospital of India. Infect. Disord. Drug Targets 2020, 21, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.E. Problems and dilemmas of antimicrobial resistance. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1992, 12, 86S–93S. [Google Scholar]
- Matheu, J.; Aidara-Kane, A.; Andremont, A. The ESBL Tricycle AMR Surveillance Project: A Simple, One Health Approach to Global Surveillance. AMR Control. 2017. Available online: http://resistancecontrol.info/2017/the-esbl-tricycle-amr-surveillance-project-a-simple-one-health-approach-to-global-surveillance/ (accessed on 15 November 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Mathai, E.; Grape, M.; Kronvall, G. Integrons and multidrug resistance among Escherichia coli causing community-acquired urinary tract infection in southern India. Apmis 2004, 112, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Sagar, V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: A multicenter study. J. Infect. Dev. Ctries. 2008, 2, 354–358. [Google Scholar] [CrossRef]
- Dash, M.; Padhi, S.; Mohanty, I.; Panda, P.; Parida, B. Antimicrobial resistance in pathogens causing urinary tract infections in a rural community of Odisha, India. J. Fam. Community Med. 2013, 20, 20–26. [Google Scholar] [CrossRef]
- Kothari, C.; Gaind, R.; Singh, L.C.; Sinha, A.; Kumari, V.; Arya, S.; Chellani, H.; Saxena, S.; Deb, M. Community acquisition of β-lactamase producing Enterobacteriaceae in neonatal gut. BMC Microbiol. 2013, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, K.; Banerjee, A.; Pal, S.; Dey, S.; Joardar, S.N.; Samanta, I.; Isore, D.P.; Singh, A. Detection, characterization, and antibiogram of extended-spectrum beta-lactamase Escherichia coli isolated from bovine milk samples in West Bengal, India. Vet. World 2018, 11, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Lalruatdiki, A.; Dutta, T.; Roychoudhury, P.; Subudhi, P. Extended-spectrum β-lactamases producing multidrug resistance Escherichia coli, Salmonella and Klebsiella pneumoniae in pig population of Assam and Meghalaya, India. Vet. World 2018, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Nayak, B.B.; Kumar, S.H. High Prevalence of Multiple Antibiotic-Resistant, Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli in Fresh Seafood Sold in Retail Markets of Mumbai, India. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Vinayananda, C.; Fairoze, N.; Madhavaprasad, C.B.; Byregowda, S.M.; Nagaraj, C.S.; Bagalkot, P.; Karabasanavar, N. Studies on occurrence, characterisation and decontamination of emerging pathogenic Escherichia coli (STEC, ETEC and EIEC) in table eggs. Br. Poult. Sci. 2017, 58, 664–672. [Google Scholar] [CrossRef]
- Ram, S.; Vajpayee, P.; Tripathi, U.; Singh, R.; Seth, P.; Shanker, R. Determination of antimicrobial resistance and virulence gene signatures in surface water isolates of Escherichia coli. J. Appl. Microbiol. 2008, 105, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Vajpayee, P.; Shanker, R. Prevalence of multi-antimicrobial-agent resistant, shiga toxin and enterotoxin producing Escherichia coli in surface waters of river Ganga. Environ. Sci. Technol. 2007, 41, 7383–7388. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Vajpayee, P.; Shanker, R. Contamination of potable water distribution systems by multiantimicrobial-resistant enterohemorrhagic Escherichia coli. Environ. Health Perspect. 2008, 116, 448–452. [Google Scholar] [CrossRef]
- Ram, S.; Vajpayee, P.; Singh, R.L.; Shanker, R. Surface water of a perennial river exhibits multi-antimicrobial resistant shiga toxin and enterotoxin producing Escherichia coli. Ecotoxicol. Environ. Saf. 2009, 72, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Maloo, A.; Fulke, A.B.; Mulani, N.; Sukumaran, S.; Ram, A. Pathogenic multiple antimicrobial resistant Escherichia coli serotypes in recreational waters of Mumbai, India: A potential public health risk. Environ. Sci. Pollut. Res. 2017, 24, 11504–11517. [Google Scholar] [CrossRef]
- Verma, P.; Saharan, V.; Nimesh, S.; Singh, A. Phenotypic and virulence traits of Escherichia coli and Salmonella strains isolated from vegetables and fruits from India. J. Appl. Microbiol. 2018, 125, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, P.; Boerlin, P.; Frey, J. Target genes for virulence assessment of Escherichia coli isolates from water, food and the environment. FEMS Microbiol. Rev. 2000, 24, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Khare, N.; Kumar, S.; Gulati, P. High prevalence of antibiotic resistance and integrons in Escherichia coli isolated from urban river water, India. Microb. Drug Resist. 2019, 25, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.C.; Tamhankar, A.J.; Sahoo, S.; Sahu, P.S.; Klintz, S.R.; Lundborg, C.S. Geographical variation in antibiotic-resistant Escherichia coli isolates from stool, cow-dung and drinking water. Int. J. Environ. Res. Public Health 2012, 9, 746–759. [Google Scholar] [CrossRef]
- Warjri, I.; Dutta, T.; Lalzampuia, H.; Chandra, R. Detection and characterization of extended-spectrum β-lactamases (blaCTX-M-1 and blaSHV) producing Escherichia coli, Salmonella spp. And Klebsiella pneumoniae isolated from humans in Mizoram. Vet. World 2015, 8, 599. [Google Scholar] [CrossRef]
- Bhoomika, S.S.; Patyal, A.; Gade, N.E. Occurrence and characteristics of extended-spectrum β-lactamases producing Escherichia coli in foods of animal origin and human clinical samples in Chhattisgarh, India. Vet. World 2016, 9, 996. [Google Scholar] [CrossRef]
- Hussain, A.; Shaik, S.; Ranjan, A.; Nandanwar, N.; Tiwari, S.K.; Majid, M.; Baddam, R.; Qureshi, I.A.; Semmler, T.; Wieler, L.H.; et al. Risk of transmission of antimicrobial resistant Escherichia coli from commercial broiler and free-range retail chicken in India. Front. Microbiol. 2017, 8, 2120. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.; Fairbrother, J.M.; Bekal, S.; Sanschagrin, F.; Levesque, R.C.; Brousseau, R.; Masson, L.; Larivière, S.; Harel, J. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149: K91 isolates obtained over a 23-year period from pigs. Antimicrob. Agents Chemother. 2003, 47, 3214–3221. [Google Scholar] [CrossRef]
- Sukumaran, D.P.; Durairaj, S.; Abdulla, M.H. Antibiotic resistance of Escherichia coli serotypes from Cochin estuary. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 124879. Available online: https://downloads.hindawi.com/journals/ipid/2012/124879.pdf (accessed on 15 November 2021). [CrossRef] [PubMed][Green Version]
- Abhirosh, C.; Sherin, V.; Thomas, A.; Hatha, A.; Mazumder, A. Potential public health significance of faecal contamination and multidrug-resistant Escherichia coli and Salmonella serotypes in a lake in India. Public Health 2011, 125, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.; Goel, S. Prevalence of antibiotic-resistant bacteria in three different aquatic environments over three seasons. Environ. Monit. Assess. 2014, 186, 5089–5100. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Gupta, M.; Didwal, G.; Bansal, S.; Kaushal, K.; Batra, N.; Gautam, V. Antibiotic-resistant Enterobacteriaceae in healthy gut flora: A report from north Indian semiurban community. Indian J. Med Res. 2019, 149, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Chandran, A.; Mohamed Hatha, A.A.; Varghese, S.; Sheeja, K.M. Prevalence of multiple drug resistant Escherichia coli serotypes in a tropical estuary, India. Microbes Environ. 2008, 23, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, A.K.; Gupta, M.K.; Rahal, A. Multidrug resistant pathogenic Escherichia coli status in water sources and Yamuna River in and around Mathura, India. Pakistan Pak. J. Biol. Sci. 2014, 17, 540–544. [Google Scholar] [CrossRef]
- Batabyal, P.; Mookerjee, S.; Sur, D.; Palit, A. Diarrheogenic Escherechia coli in potable water sources of West Bengal, India. Acta Trop. 2013, 127, 153–157. [Google Scholar] [CrossRef]
- Dhawde, R.; Macaden, R.; Saranath, D.; Nilgiriwala, K.; Ghadge, A.; Birdi, T. Antibiotic resistance characterization of environmental E. coli isolated from River Mula-Mutha, Pune District, India. Int. J. Environ. Res. Public Health 2018, 15, 1247. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Naik, O.A.; Shashidhar, R.; Rath, D.; Bandekar, J.R.; Rath, A. Characterization of multiple antibiotic resistance of culturable microorganisms and metagenomic analysis of total microbial diversity of marine fish sold in retail shops in Mumbai, India. Environ. Sci. Pollut. Res. 2018, 25, 6228–6239. [Google Scholar] [CrossRef]
- Divyashree, M.; Kumar, D.V.; Ballamoole, K.K.; Shetty, V.; Chakraborty, A.; Karunasagar, I. Occurrence of antibiotic resistance among gram-negative bacteria isolated from effluents of fish processing plants in and around Mangalore. Int. J. Environ. Health Res. 2019, 30, 653–660. [Google Scholar]
- Rayasam, S.; Ray, I.; Smith, K.R.; Riley, L.W. Extraintestinal pathogenic escherichia coli and antimicrobial drug resistance in a maharashtrian drinking water system. Am. J. Trop. Med. Hyg. 2019, 100, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Puii, L.; Dutta, T.; Roychoudhury, P.; Kylla, H.; Chakraborty, S.; Mandakini, R.; Kawlni, L.; Samanta, I.; Bandopaddhay, S.; Singh, S. Extended spectrum beta-lactamase producing Shiga-toxin producing-Escherichia coli in piglets, humans and water sources in North East region of India. Lett. Appl. Microbiol. 2019, 69, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Mathai, E.; Chandy, S.; Thomas, K.; Antoniswamy, B.; Joseph, I.; Mathai, M.; Sorensen, T.L.; Holloway, K. Antimicrobial resistance surveillance among commensal Escherichia coli in rural and urban areas in Southern India. Trop. Med. Int. Health 2008, 13, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Babenko, D.; Toleman, M.A. Human carriage of cefotaxime-resistant Escherichia coli in North-East India: An analysis of STs and associated resistance mechanisms. J. Antimicrob. Chemother. 2020, 75, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.; Diwan, V.; Parashar, V.; Tamhankar, A.J.; Lundborg, C.S. Mass bathing events in River Kshipra, Central India-influence on the water quality and the antibiotic susceptibility pattern of commensal E. coli. PLoS ONE 2020, 15, e0229664. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajagopal, K.; Chandy, S.J.; Graham, J.P. A One Health Review of Community-Acquired Antimicrobial-Resistant Escherichia coli in India. Int. J. Environ. Res. Public Health 2021, 18, 12089. https://doi.org/10.3390/ijerph182212089
Rajagopal K, Chandy SJ, Graham JP. A One Health Review of Community-Acquired Antimicrobial-Resistant Escherichia coli in India. International Journal of Environmental Research and Public Health. 2021; 18(22):12089. https://doi.org/10.3390/ijerph182212089
Chicago/Turabian StyleRajagopal, Keerthana, Sujith J. Chandy, and Jay P. Graham. 2021. "A One Health Review of Community-Acquired Antimicrobial-Resistant Escherichia coli in India" International Journal of Environmental Research and Public Health 18, no. 22: 12089. https://doi.org/10.3390/ijerph182212089
APA StyleRajagopal, K., Chandy, S. J., & Graham, J. P. (2021). A One Health Review of Community-Acquired Antimicrobial-Resistant Escherichia coli in India. International Journal of Environmental Research and Public Health, 18(22), 12089. https://doi.org/10.3390/ijerph182212089







