Transcriptome Profiles of Leaves and Roots of Goldenrain Tree (Koelreuteria paniculata Laxm.) in Response to Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Physiological Index of Cadmium Stress
2.3. RNA-Seq and Annotation
2.4. Differentially Expressed Genes
2.5. Reverse-Transcription Quantitative PCR Validation
3. Results
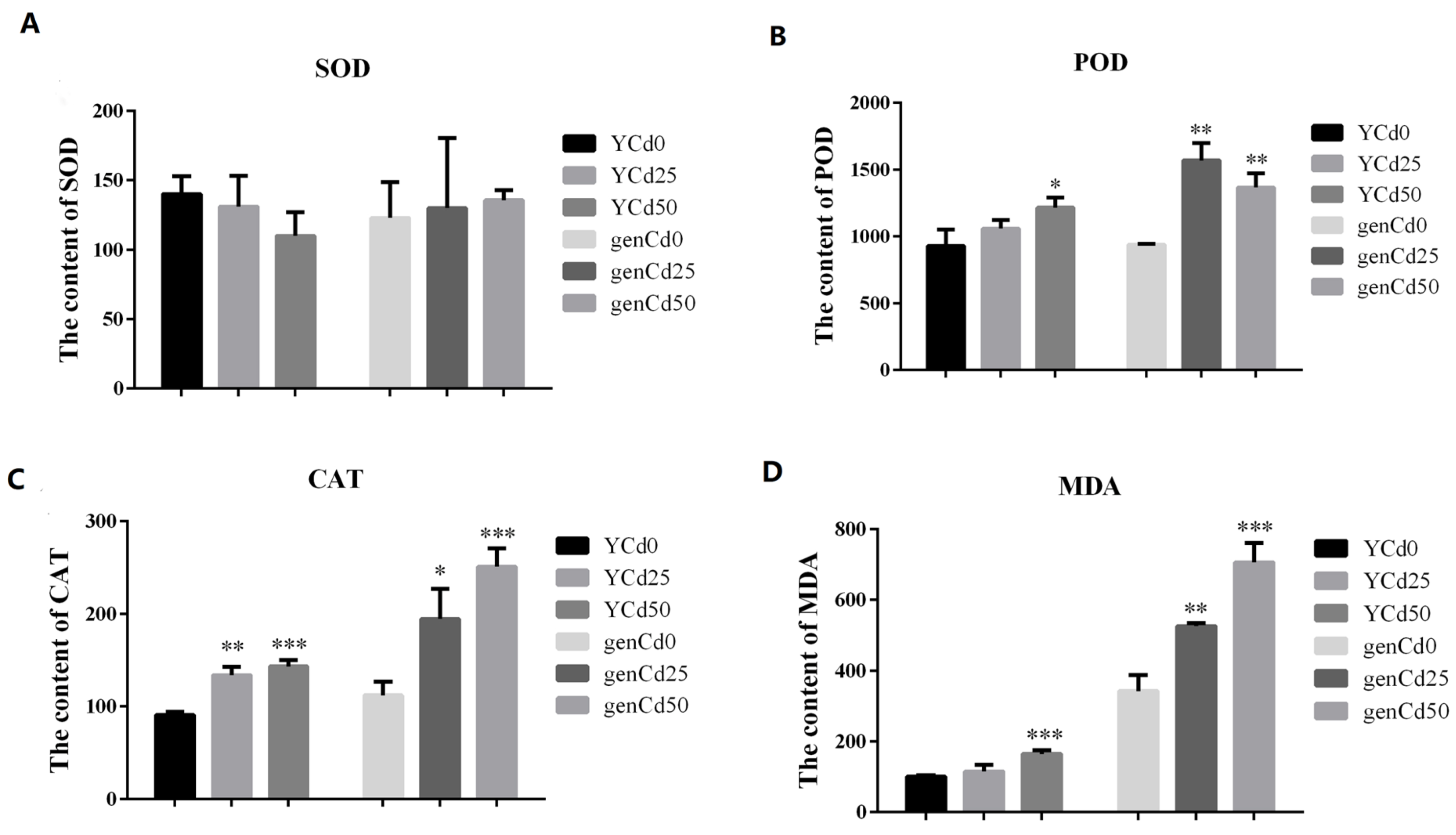
3.1. Growth, Antioxidant Activity, and MDA Content in Goldenrain Tree under Cadmium Stress
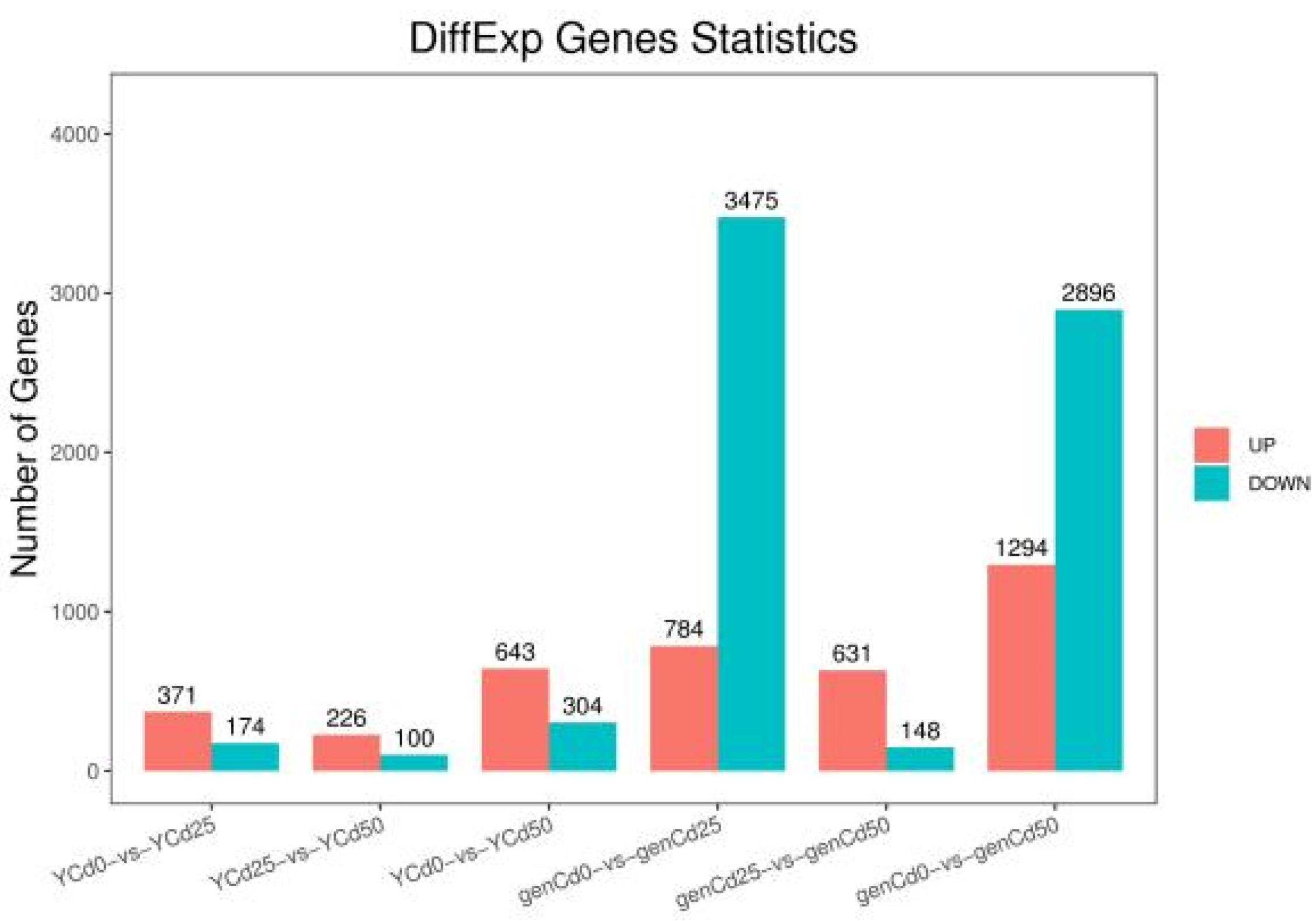
3.2. Transcriptomic Analysis of Goldenrain Tree Leaves and Roots under Cadmium Stress
3.3. GO Enrichment Analysis of Differentially Expressed Genes in Leaves and Roots
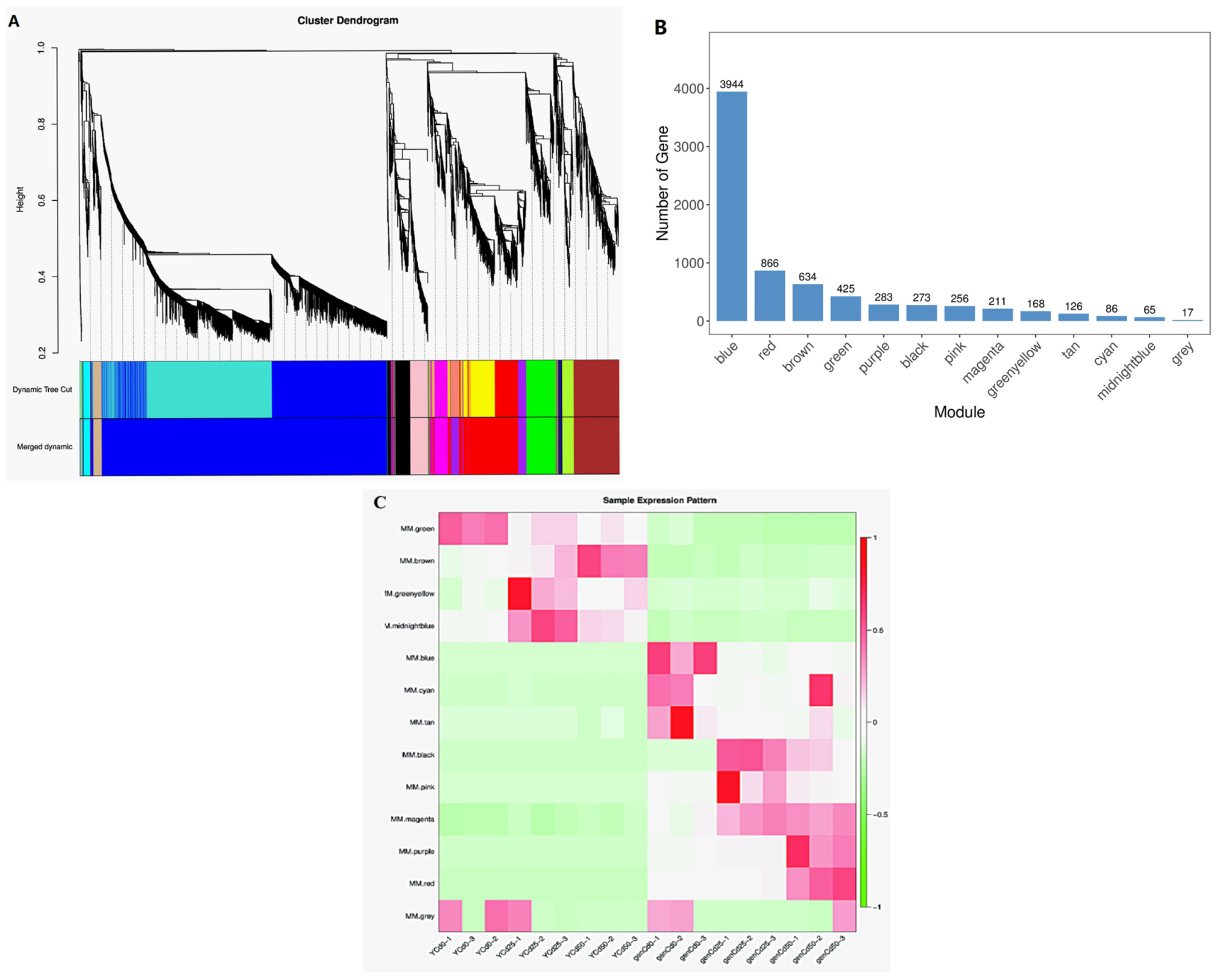
3.4. Identification of Co-Expression Modules
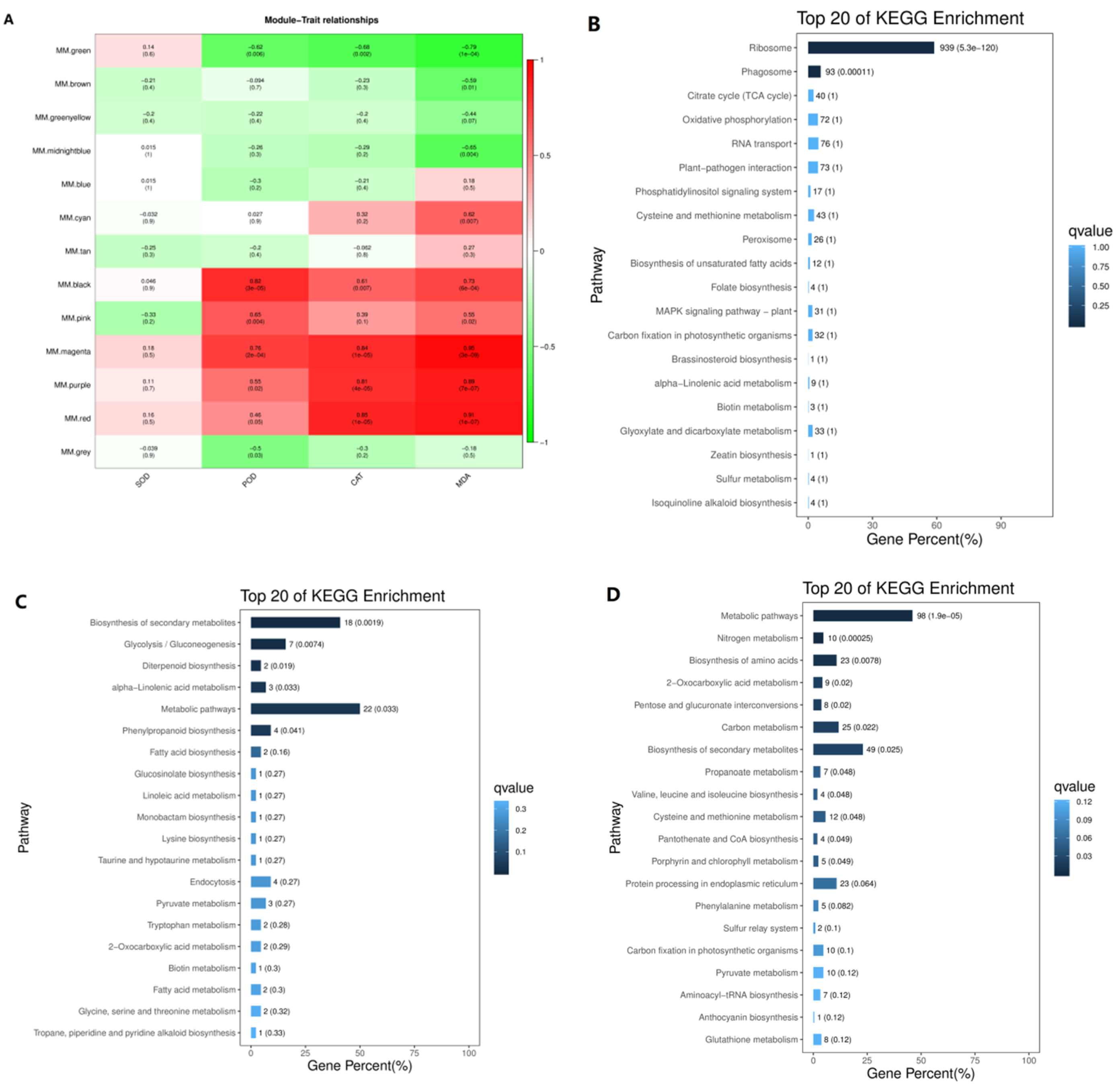
3.5. Transcription Regulatory Modules Associated with Antioxidant Enzymes and Malondialdehyde
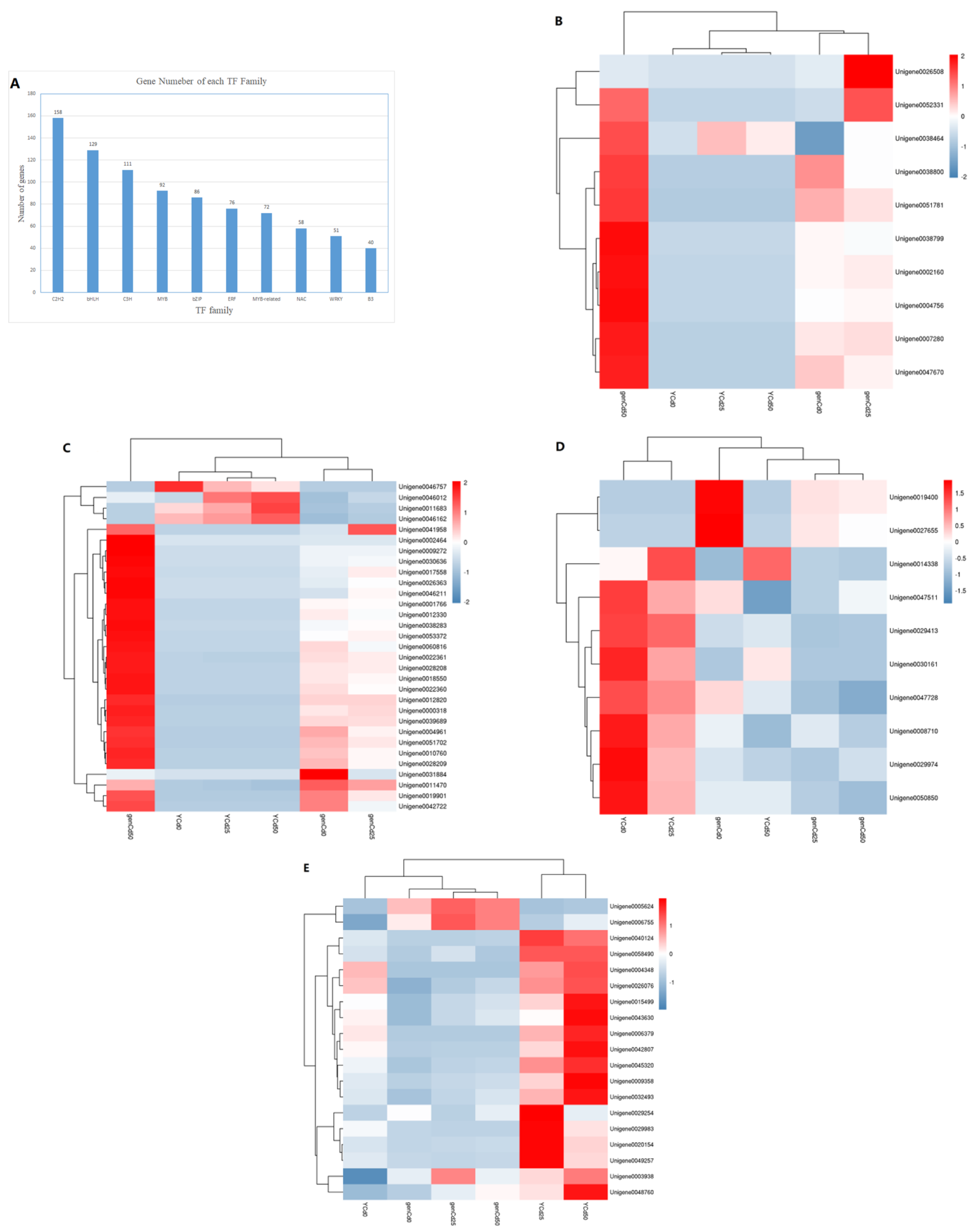
3.6. Transcription Factor Responses to Cadmium Stress
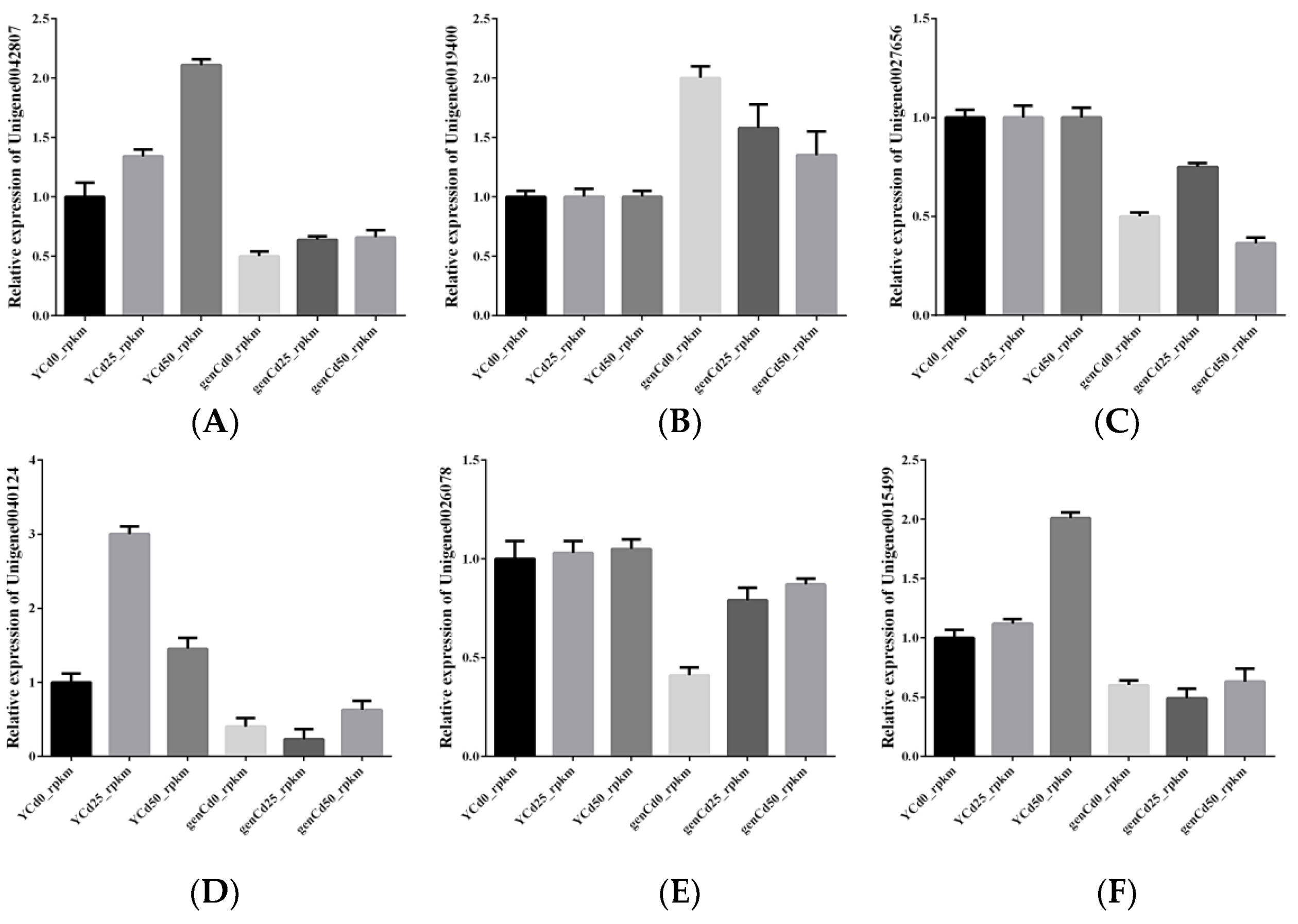
3.7. Validation of RNA-Seq by RT-qPCR
4. Discussion
4.1. Antioxidant Enzyme Activities and Malondialdehyde Content Increased Dramatically under Cadmium Stress
4.2. Differentially Expressed Genes Responded Rapidly to Cadmium Stress
4.3. Transcription Factors Were Important in the Response to Cadmium Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Guo, J.J.; He, C.T.; Shen, C.; Huang, Y.Y.; Chen, J.X.; Guo, J.H.; Yuan, J.G.; Yang, Z.Y. Comparative Transcriptome Analysis between Low- and High-Cadmium-Accumulating Genotypes of Pakchoi (Brassica chinensis L.) in Response to Cadmium Stress. Environ. Sci. Technol. 2016, 50, 6485–6494. [Google Scholar] [CrossRef]
- Locosselli, G.M.; Chacon-Madrid, K.; Arruda, M.A.; de Camargo, E.P.; Moreira, T.C.; de Andre, C.D.; de Andre, P.A.; Singer, J.M.; Saldiva, P.H.; Buckeridge, M.S. Tree rings reveal the reduction of Cd, Cu, Ni and Pb pollution in the central region of São Paulo, Brazil. Environ. Pollut. 2018, 242, 320–328. [Google Scholar] [CrossRef]
- Mao, P.; Zhuang, P.; Li, F.; McBride, M.B.; Ren, W.D.; Li, Y.X.; Li, Y.W.; Mo, H.; Fu, H.Y.; Li, Z. Phosphate addition diminishes the efficacy of wollastonite in decreasing Cd uptake by rice (Oryza sativa L.) in paddy soil. Sci. Total. Environ. 2019, 687, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Cai, D.J.; Yang, H. Soil Cd pollution and research progress of treatment techniques. Henan Chem. Ind. 2013, 30, 17–22. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total. Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Haider, F.U.; Cai, L.; Coulter, J.A.; Cheema, S.A.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211. [Google Scholar] [CrossRef]
- Zhi, Y.; Deng, Z.; Luo, M. Influence of heavy metals on seed germination and early seedling growth in mill. Am. J. Plant Sci. 2015, 6, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Mayonde, S.; Cron, G.V.; Glennon, K.L.; Byrne, M.J. Effects of cadmium toxicity on the physiology and growth of a halophytic plant, Tamarix usneoides(E. Mey. ex Bunge). Int. J. Phytoremediat. 2020, 23, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sodango, T.H.; Li, X.M.; Sha, J.M.; Bao, Z.C. Review of the Spatial Distribution, Source and Extent of Heavy Metal Pollution of Soil in China: Impacts and Mitigation Approaches. J. Health Pollut. 2018, 8, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef]
- Lee, G.; Suonan, Z.; Kim, S.; Hwang, D.; Lee, K. Heavy metal accumulation and phytoremediation potential by transplants of the seagrass Zostera marina in the polluted bay systems. Mar. Pollut. Bull. 2019, 149, 110509. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.L.; He, H.; Wu, S.; Zhou, Q.; Yang, C.; Zeng, G.; Luo, L.; Lou, W. Responses of microalgae Coelastrella sp. to stress of cupric ions in treatment of anaerobically digested swine wastewater. Bioresour. Technol. 2017, 251, 274. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, C.P.; Zeng, G.M.; Wu, S.H.; Lin, Y.; Zhou, Q.; Lou, W.; Du, C.; Nie, L.J.; Zhong, Y.Y. Nutrient removal from swine wastewater with growing microalgae at various zinc concentrations. Algal Res. 2020, 227, 530791. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.; Ashraf, S.; Asghar, H. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Andonova, T.; Dimitrova-Dyulgerova, I.; Slavov, I.; Muhovski, Y.; Stoyanova, A. A Comparative Study of Koelreuteria paniculata Laxm. Aerial Parts Essential Oil Composition. J. Essent. Oil-Bear. Plants 2021, 23, 1363. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, cd subcellular distribution and chemical forms of koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Fang, S.; Tao, Y.; Zhang, Y.Z.; Kong, F.Y.; Wang, Y.B. Effects of metalaxyl enantiomers stress on root activity and leaf antioxidant enzyme activities in tobacco seedlings. Chirality 2018, 30, 469–474. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Rabiee, R.; Faggio, C. Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish. Immunol. 2020, 106, 852–858. [Google Scholar] [CrossRef]
- Alizadeh-Moghaddam, G.; Rezayatmand, Z.; Esfahani, M.; Khozaei, M. Bio-genetic analysis of resistance in tomato to early blight disease, Alternaria alternata. Phytochemistry 2020, 179, 112486. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.L.; He, Z.Q.; Li, B.; Liu, J.W.; Liu, L.; Liao, W.B.; Xiong, Y.H.; Song, C.; Yang, S.X.; Liu, Y.L. Melatonin inhibits oxalate-induced endoplasmic reticulum stress and apoptosis in HK-2 cells by activating the AMPK pathway. Cell Cycle 2020, 19, 2600–2610. [Google Scholar] [CrossRef]
- Chai, L.J.; Chai, P.; Chen, S.W.; Flaishman, M.A.; Ma, H.Q. Transcriptome analysis unravels spatiotemporal modulation of phytohormone-pathway expression underlying gibberellin-induced parthenocarpic fruit set in San Pedro-type fig (Ficus carica L.). BMC Plant Biol. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, R.J. CTAB-PAGE; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Chai, L.J.; Li, Y.M.; Chen, S.W.; Perl, A.; Zhao, F.X.; Ma, H.Q. RNA sequencing reveals high resolution expression change of major plant hormone pathway genes after young seedless grape berries treated with gibberellin. Plant Sci. 2014, 229, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Poluan, R.H.; Sudigyo, D.; Rahmawati, G.; Setiasari, D.W.; Sesotyosari, S.L.; Wardana, T.; Astuti, I. Transcriptome Related to Avoiding Immune Destruction in Nasopharyngeal Cancer in Indonesian Patients Using Next-Generation Sequencing. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Götz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Najaraj, S.H.; Nueda, M.J.; Robles, M.; Talon, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Sun, B.M.; Zhou, X.; Chen, C.M.; Chen, C.J.; Chen, K.H.; Chen, M.X.; Liu, S.Q.; Chen, G.J.; Cao, B.H.; Cao, F.R.; et al. Coexpression network analysis reveals an MYB transcriptional activator involved in capsaicinoid biosynthesis in hot peppers. Hortic. Res. 2020, 7, 162. [Google Scholar] [CrossRef]
- Gupta, O.P.; Sharma, P.; Gupta, R.K.; Sharma, I. MicroRNA mediated regulation of metal toxicity in plants: Present status and future perspectives. Plant Mol. Biol. 2014, 84, 1–18. [Google Scholar] [CrossRef]
- Zou, C.S.; Wang, Q.L.; Lu, C.R.; Yang, W.C.; Zhang, Y.P.; Cheng, H.L.; Feng, X.X.; Prosper, M.A.; Song, G.L. Transcriptome analysis reveals long noncoding RNAs involved in fiber development in cotton (Gossypium arboreum). Sci. China Life Sci. 2016, 59, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Puente-Sánchez, F.; Díaz, S.; Penacho, V.; Aguilera, A.; Olsson, S. Basis of genetic adaptation to heavy metal stress in the acidophilic green alga Chlamydomonas acidophila. Aquat. Toxicol. 2018, 200, 62–72. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Titov, V.; Hohn, B.; Kovalchuk, O. Transcriptome profiling reveals similarities and differences in plant responses to cadmium and lead. Mutat. Res. 2005, 570, 149–161. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.L.; Feng, X.X.; Li, H.J.; Zhang, G.F. The molecular mechanisms of plant plasma membrane intrinsic proteins trafficking and stress response. Yi Chuan Hered. 2017, 39, 293–301. [Google Scholar] [CrossRef]
- Singh, K.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Mei, J.; Wang, L.; Jiang, X.L.; Wu, B.L.; Li, M. Functions of the C2H2 Transcription Factor Gene thmea1 in Trichoderma harzianum under Copper Stress Based on Transcriptome Analysis. BioMed Res. Int. 2018, 2018, 8149682. [Google Scholar] [CrossRef]
- Kundu, A.; Das, S.; Basu, S.; Kobayashi, Y.; Kobayashi, Y.; Koyama, H.; Ganesan, M. GhSTOP1, a C2H2 type zinc finger transcription factor is essential for aluminum and proton stress tolerance and lateral root initiation in cotton. Plant Biol. 2019, 21, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Li, X.X.; Guo, C.; Ahmad, S.; Wang, Q.; Yu, J.; Liu, C.; Guo, Y. Systematic Analysis of MYB Family Genes in Potato and Their Multiple Roles in Development and Stress Responses. Biomolecules 2019, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Ai, T.N.; Naing, A.H.; Yun, B.W.; Lim, S.H.; Kim, C.K. Overexpression of RsMYB1 Enhances Anthocyanin Accumulation and Heavy Metal Stress Tolerance in Transgenic Petunia. Front. Plant Sci. 2018, 9, 1388. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, R.L.; Ju, Q.; Li, W.Q.; Tran, L.; Xu, J. The R2R3-MYB Transcription Factor MYB49 Regulates Cadmium Accumulation. Plant Physiol. 2019, 180, 529–542. [Google Scholar] [CrossRef]
- Hong, C.Y.; Cheng, D.; Zhang, G.Q.; Zhu, D.D.; Chen, Y.H.; Tan, M.P. The role of ZmWRKY4 in regulating maize antioxidant defense under cadmium stress. Biochem. Biophys. Res. Commun. 2017, 482, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.B.; Yan, X.X.; Huang, Y.; Han, Y.Y.; Zhang, C.; Ren, Y.B.; Fan, T.; Xiao, F.M.; Liu, Y.S.; Cao, S.Q. The WRKY transcription factor, WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Zhou, J.H.; Jie, Y.C.; Xing, H.C.; Zhong, Y.L.; Yu, W.L.; She, W.; Ma, Y.S.; Liu, Z.H. A Ramie bZIP Transcription Factor BnbZIP2 Is Involved in Drought, Salt, and Heavy Metal Stress Response. DNA Cell Biol. 2016, 35, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.G.; Li, J.Y.; Glass, N. Exploring the bZIP transcription factor regulatory network in Neurospora crassa. Microbiol. SGM 2011, 157, 747–759. [Google Scholar] [CrossRef] [Green Version]


| Gene Number | GC Percentage | N50 Number | N50 Length | Max Length | Min Length | Average Length | Total Assembled Bases |
|---|---|---|---|---|---|---|---|
| 65,171 | 44.9824 | 13,438 | 1631 | 16,907 | 201 | 1121 | 73,081,674 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Zhou, T.; Sun, J.; Wang, P.; Yang, C.; Bai, L.; Liu, Z. Transcriptome Profiles of Leaves and Roots of Goldenrain Tree (Koelreuteria paniculata Laxm.) in Response to Cadmium Stress. Int. J. Environ. Res. Public Health 2021, 18, 12046. https://doi.org/10.3390/ijerph182212046
He Q, Zhou T, Sun J, Wang P, Yang C, Bai L, Liu Z. Transcriptome Profiles of Leaves and Roots of Goldenrain Tree (Koelreuteria paniculata Laxm.) in Response to Cadmium Stress. International Journal of Environmental Research and Public Health. 2021; 18(22):12046. https://doi.org/10.3390/ijerph182212046
Chicago/Turabian StyleHe, Qihao, Tao Zhou, Jikang Sun, Ping Wang, Chunping Yang, Lei Bai, and Zhiming Liu. 2021. "Transcriptome Profiles of Leaves and Roots of Goldenrain Tree (Koelreuteria paniculata Laxm.) in Response to Cadmium Stress" International Journal of Environmental Research and Public Health 18, no. 22: 12046. https://doi.org/10.3390/ijerph182212046
APA StyleHe, Q., Zhou, T., Sun, J., Wang, P., Yang, C., Bai, L., & Liu, Z. (2021). Transcriptome Profiles of Leaves and Roots of Goldenrain Tree (Koelreuteria paniculata Laxm.) in Response to Cadmium Stress. International Journal of Environmental Research and Public Health, 18(22), 12046. https://doi.org/10.3390/ijerph182212046







