Impact of Protracted Displacement on Delay in the Diagnosis Associated with Treatment Outcomes: A Cross-Sectional Study in Internally Displaced Tuberculosis Patients of Pakistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
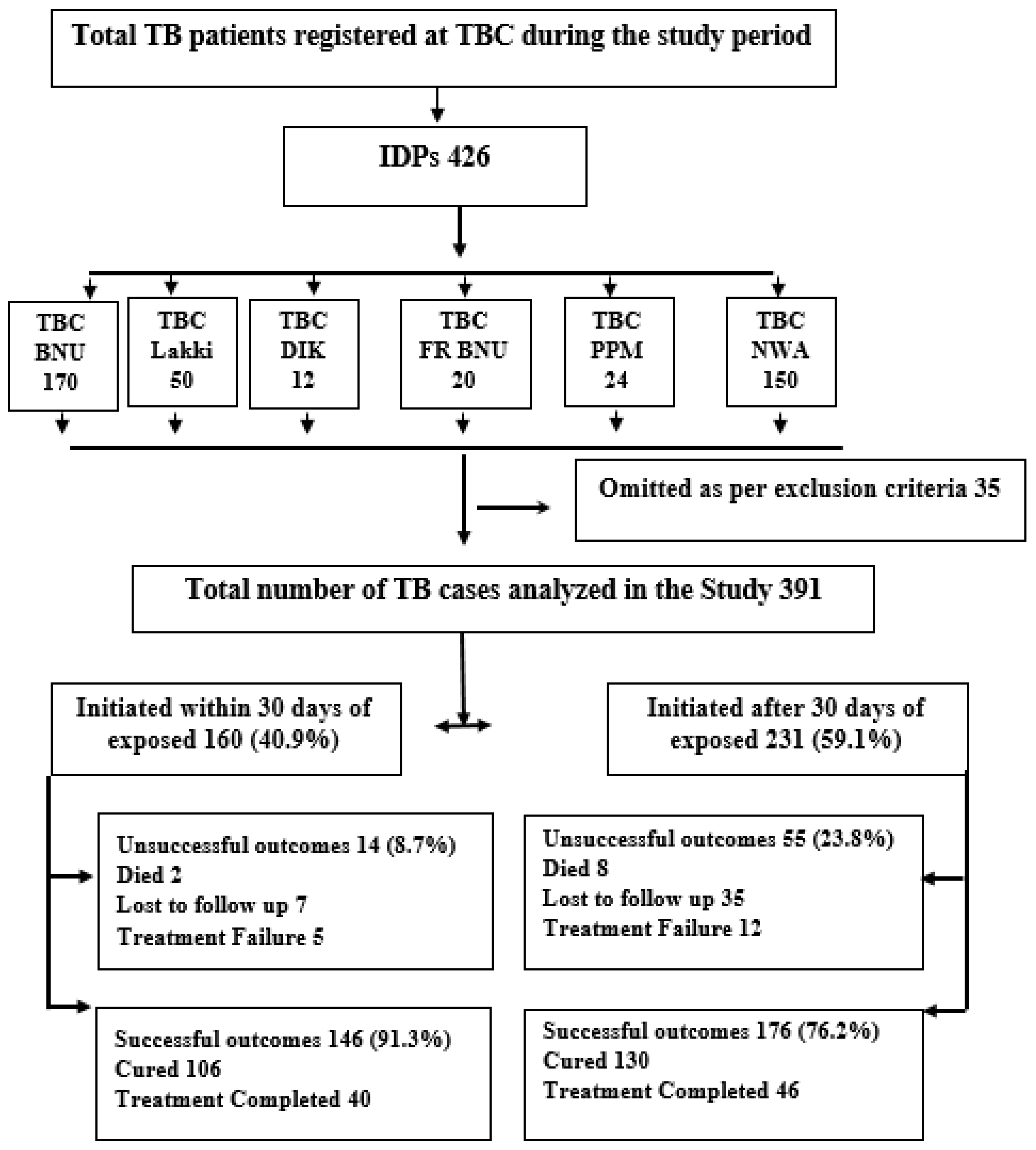
2.2. Study Population and Study Setting
2.3. Study Tool
2.4. Operational Definitions
2.5. Data Analysis
3. Results
3.1. Characteristics of Participants
3.2. Health-Seeking Attitude
3.3. Baseline TB Signs and Symptoms and Transmission amoung Patients
3.4. Predictors of Delay
3.5. Predictors of Tuberculosis Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, S.; Ullah, O.; Khan, M.A.; Gul, T. Optimal control analysis of tuberculosis (TB) with vaccination and treatment. Eur. Phys. J. Plus 2020, 135, 1–27. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 24 January 2021).
- Khan, A.H. Tuberculosis control in Sindh, Pakistan: Critical analysis of its implementation. J. Infect. Public Health 2017, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H. WHO’s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef]
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D. Global Tuberculosis Report 2020–Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Almeida, C.P.B.d.; Skupien, E.C.; Silva, D.R. Health care seeking behavior and patient delay in tuberculosis diagnosis. Cad. Saude Publica 2015, 31, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebreegziabher, S.B.; Bjune, G.A.; Yimer, S.A. Total delay is associated with unfavorable treatment outcome among pulmonary tuberculosis patients in west Gojjam zone, Northwest Ethiopia: A prospective cohort study. PLoS ONE 2016, 11, e0159579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datiko, D.G.; Jerene, D.; Suarez, P. Patient and health system delay among TB patients in Ethiopia: Nationwide mixed method cross-sectional study. BMC Public Health 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Fuge, T.G.; Bawore, S.G.; Solomon, D.W.; Hegana, T.Y. Patient delay in seeking tuberculosis diagnosis and associated factors in Hadiya Zone, Southern Ethiopia. BMC Res. Notes 2018, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shewade, H.D.; Gupta, V.; Satyanarayana, S.; Pandey, P.; Bajpai, U.; Tripathy, J.P.; Kathirvel, S.; Pandurangan, S.; Mohanty, S.; Ghule, V.H. Patient characteristics, health seeking and delays among new sputum smear positive TB patients identified through active case finding when compared to passive case finding in India. PLoS ONE 2019, 14, e0213345. [Google Scholar] [CrossRef]
- Owolabi, O.A.; Jallow, A.O.; Jallow, M.; Sowe, G.; Jallow, R.; Genekah, M.D.; Donkor, S.; Wurrie, A.; Kampmann, B.; Sutherland, J. Delay in the diagnosis of pulmonary tuberculosis in The Gambia, West Africa: A cross-sectional study. Int. J. Infect. Dis. 2020, 101, 102–106. [Google Scholar] [CrossRef]
- Dhavan, P.; Dias, H.; Creswell, J.; Weil, D. An overview of tuberculosis and migration. Int. J. Tuberc. Lung Dis. 2017, 21, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.B.; Rafei, R.; Dabboussi, F.; Hamze, M. Tuberculosis, war, and refugees: Spotlight on the Syrian humanitarian crisis. PLoS Pathog. 2018, 14, e1007014. [Google Scholar] [CrossRef]
- Gilbert, R.L.; Antoine, D.; French, C.; Abubakar, I.; Watson, J.; Jones, J. The impact of immigration on tuberculosis rates in the United Kingdom compared with other European countries. Int. J. Tuberc. Lung Dis. 2009, 13, 645–651. [Google Scholar] [PubMed]
- Pelissari, D.M.; Diaz-Quijano, F.A. Household crowding as a potential mediator of socioeconomic determinants of tuberculosis incidence in Brazil. PLoS ONE 2017, 12, e0176116. [Google Scholar] [CrossRef] [PubMed]
- Internal Displacement Monitoring Centre. Global Report on Internal Displacement, 2021. Noruega Nor. Refug. Counc. 2021. Available online: https://www.internal-displacement.org/publications/2021-global-report-on-internal-displacement (accessed on 24 July 2021).
- Hameed, N. Struggling IDPS of North Waziristan in the Wake of Operation Zarb-e-Azb. NDU J. 2015. Available online: http://www.ndu.edu.Pk/issra/issra_pub/articles/ndujournal/NDU-Journal-2015/05-Struggling-IDPs (accessed on 26 December 2020).
- Javaid, U. Operation Zarb-e-Azb: A Successful Initiative to Curtail Terrorism. South Asian Stud. 2015, 30, 43. [Google Scholar]
- Coninx, R. Tuberculosis in complex emergencies. Bull. World Health Organ. 2007, 85, 637–640. [Google Scholar] [CrossRef]
- Kimbrough, W.; Saliba, V.; Dahab, M.; Haskew, C.; Checchi, F. The burden of tuberculosis in crisis-affected populations: A systematic review. Lancet Infect. Dis. 2012, 12, 950–965. [Google Scholar] [CrossRef]
- Abbara, A.; Almalla, M.; AlMasri, I.; AlKabbani, H.; Karah, N.; El-Amin, W.; Rajan, L.; Rahhal, I.; Alabbas, M.; Sahloul, Z. The challenges of tuberculosis control in protracted conflict: The case of Syria. Int. J. Infect. Dis. 2020, 90, 53–59. [Google Scholar] [CrossRef] [Green Version]
- NTP. Reporting and Recording Tools: National TB Control Program, Pakistan. Available online: http://ntp.gov.pk/wp-content/uploads/2020/02/TB.pdf (accessed on 24 January 2021).
- NTP. PPM DOTS: Training Courses on DOTS for Doctors at Private Hospitals/Clinics; National TB Control Program: Islamabad, Pakistan, 2008; pp. 1–101.
- WHO. Definitions and Reporting Framework for Tuberculosis; World Health Organization: Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/handle/10665/79199 (accessed on 26 December 2020).
- Mesfin, M.M.; Newell, J.N.; Walley, J.D.; Gessessew, A.; Madeley, R.J. Delayed consultation among pulmonary tuberculosis patients: A cross sectional study of 10 DOTS districts of Ethiopia. BMC Public Health 2009, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Asres, A.; Jerene, D.; Deressa, W. Delays to treatment initiation is associated with tuberculosis treatment outcomes among patients on directly observed treatment short course in Southwest Ethiopia: A follow-up study. BMC Pulm. Med. 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Belay, M.; Bjune, G.; Ameni, G.; Abebe, F. Diagnostic and treatment delay among Tuberculosis patients in Afar Region, Ethiopia: A cross-sectional study. BMC Public Health 2012, 12, 369. [Google Scholar] [CrossRef] [Green Version]
- PHOA, L.L.; Teleman, M.D.; WANG, Y.T.; Chee, C.B. Characteristics of patients with delayed diagnosis of infectious pulmonary tuberculosis. Respirology 2005, 10, 196–200. [Google Scholar] [PubMed]
- Li, Y.; Ehiri, J.; Tang, S.; Li, D.; Bian, Y.; Lin, H.; Marshall, C.; Cao, J. Factors associated with patient, and diagnostic delays in Chinese TB patients: A systematic review and meta-analysis. BMC Med. 2013, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Kashif Munir, M.; Iqbal, R.; Ahmed Salam, A.; Saeed, S.; Masud, F.; Aasim, M. Active Case Detection Among Household Contacts of Multi Drug Resistant Tuberculosis Patients in a Tertiary Care Setting. Pak. J. Med Res. 2014, 53. [Google Scholar]
- Steyerberg, E.W. Applications of prediction models. In Clinical Prediction Models; Springer: Berlin/Heidelberg, Germany, 2009; pp. 11–31. [Google Scholar]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS; Routledge: Abingdon-on-Thames, UK, 2020; pp. 1–497. [Google Scholar]
- WHO. Global Tuberculosis Report 2015; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/191102 (accessed on 24 January 2021).
- Hamza, A.; Demissie, M.; Gare, S.; Teshome, G. Delay in Tuberculosis Diagnosis among Tuberculosis Patients at the Three Hospitals: Asella, Robe and Abomsa of Arsi Zone, Oromia Regional State. Open Access Libr. J. 2015, 2, e1947. [Google Scholar]
- Kuznetsov, V.N.; Grjibovski, A.M.; Mariandyshev, A.O.; Johansson, E.; Bjune, G.A. Two vicious circles contributing to a diagnostic delay for tuberculosis patients in Arkhangelsk. Emerg. Health Threat. J. 2014, 7, 24909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gele, A.A.; Bjune, G.A. Armed conflicts have an impact on the spread of tuberculosis: The case of the Somali Regional State of Ethiopia. Confl. Health 2010, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vree, M.; Huong, N.T.; Duong, B.D.; Sy, D.N.; Cobelens, F.G.; Borgdorff, M.W. High mortality during tuberculosis treatment does not indicate long diagnostic delays in Vietnam: A cohort study. BMC Public Health 2007, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Helfinstein, S.; Engl, E.; Thomas, B.E.; Natarajan, G.; Prakash, P.; Jain, M.; Lavanya, J.; Jagadeesan, M.; Chang, R.; Mangono, T. Understanding why at-risk population segments do not seek care for tuberculosis: A precision public health approach in South India. BMJ Glob. Health 2020, 5, e002555. [Google Scholar] [CrossRef]
- Virenfeldt, J.; Rudolf, F.; Camara, C.; Furtado, A.; Gomes, V.; Aaby, P.; Petersen, E.; Wejse, C. Treatment delay affects clinical severity of tuberculosis: A longitudinal cohort study. BMJ Open 2014, 4, e004818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storla, D.G.; Yimer, S.; Bjune, G.A. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008, 8, 15. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Q.; Liu, Q.; Tolhurst, R.; Tang, S. Treatment seeking for symptoms suggestive of TB: Comparison between migrants and permanent urban residents in Chongqing, China. Trop. Med. Int. Health 2008, 13, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.E.; Ahmad, M.M.; Amezcua-Prieto, C.; Virginia, M.-R. Treatment Delay among Pulmonary Tuberculosis Patients within the Pakistan National Tuberculosis Control Program. Am. J. Trop. Med. Hyg. 2018, 99, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Buregyeya, E.; Criel, B.; Nuwaha, F.; Colebunders, R. Delays in diagnosis and treatment of pulmonary tuberculosis in Wakiso and Mukono districts, Uganda. BMC Public Health 2014, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Madebo, T.; Lindtjorn, B. Delay in treatment of pulmonary tuberculosis: An analysis of symptom duration among Ethiopian patients. Medscape Gen. Med. 1999, E6, 11104408. [Google Scholar]
- Demissie, M.; Lindtjorn, B.; Berhane, Y. Patient and health service delay in the diagnosis of pulmonary tuberculosis in Ethiopia. BMC Public Health 2002, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Purty, A.J.; Chauhan, R.C.; Natesan, M.; Cherian, J.; Singh, Z.; Sharma, Y. Patient and health system delays among adult smear-positive tuberculosis patients diagnosed at medical colleges of Puducherry in south India. Indian J. Public Health 2016, 60, 77. [Google Scholar]
- Gebreegziabher, S.B.; Bjune, G.A.; Yimer, S.A. Patients’ and health system’s delays in the diagnosis and treatment of new pulmonary tuberculosis patients in West Gojjam Zone, Northwest Ethiopia: A cross-sectional study. BMC Infect. Dis. 2016, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ayalew, Y.E.; Yehualashet, F.A.; Bogale, W.A.; Gobeza, M.B. Delay for Tuberculosis Treatment and Its Predictors among Adult Tuberculosis Patients at Debremarkos Town Public Health Facilities, North West Ethiopia. Tuberc. Res. Treat. 2020, 2020, 1901890. [Google Scholar] [CrossRef]
- Chen, T.-C.; Lu, P.-L.; Lin, C.-Y.; Lin, W.-R.; Chen, Y.-H. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2011, 15, e211–e216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabin, A.; Kuchukhidze, G.; Sanikidze, E.; Kempker, R.; Blumberg, H. Prescribed and self-medication use increase delays in diagnosis of tuberculosis in the country of Georgia. Int. J. Tuberc. Lung Dis. 2013, 17, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Xingxing, L.; Xun, L.; Tao, L.; Jianjun, Y.; Mengxian, Z.; Yu, Z.; Chengfeng, Y.; Wei, C.; Liping, Z. Factors associated with health care-seeking delay and treatment delay in tuberculosis patients in Shishou and Badong of Hubei. Dis. Surveill. 2021, 36, 1–7. [Google Scholar]
- Santos, E.; Felgueiras, Ó.; Oliveira, O.; Duarte, R. Diagnosis delay of tuberculosis in the Huambo province, Angola. Pulmonology 2018, 24, 294–299. [Google Scholar] [CrossRef]
- Alene, M.; Assemie, M.A.; Yismaw, L.; Gedif, G.; Ketema, D.B.; Gietaneh, W.; Chekol, T.D. Patient delay in the diagnosis of tuberculosis in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Chen, P.; Newell, J.N.; Li, H.; Sun, C.; Mei, J.; Walley, J.D. Barriers to TB care for rural-to-urban migrant TB patients in Shanghai: A qualitative study. Trop. Med. Int. Health 2009, 14, 754–760. [Google Scholar] [CrossRef]
- Huffman, S.A.; Veen, J.; Hennink, M.M.; McFarland, D.A. Exploitation, vulnerability to tuberculosis and access to treatment among Uzbek labor migrants in Kazakhstan. Soc. Sci. Med. 2012, 74, 864–872. [Google Scholar] [CrossRef]
- Peri, A.M.; Bernasconi, D.P.; Galizzi, N.; Matteelli, A.; Codecasa, L.; Giorgio, V.; Di Biagio, A.; Franzetti, F.; Cingolani, A.; Gori, A. Determinants of patient and health care services delays for tuberculosis diagnosis in Italy: A cross-sectional observational study. BMC Infect. Dis. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.; Ma, A.; Wang, Q.; Han, X.; Li, Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120088. [Google Scholar]
- Saqib, M.A.; Awan, I.N.; Rizvi, S.K.; Shahzad, M.I.; Mirza, Z.S.; Tahseen, S.; Khan, I.H.; Khanum, A. Delay in diagnosis of tuberculosis in Rawalpindi, Pakistan. BMC Res. Notes 2011, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- WHO. Regional Office for the Eastern Mediterranean. (2006). Diagnostic and Treatment Delay in Tuberculosis. Available online: https://apps.who.int/iris/handle/10665/116501 (accessed on 24 March 2021).
- Courtwright, A.; Turner, A.N. Tuberculosis and stigmatization: Pathways and interventions. Public Health Rep. 2010, 125, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Getnet, F.; Demissie, M.; Assefa, N.; Mengistie, B.; Worku, A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: Systematic review and meta-analysis. BMC Pulm. Med. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Osei, E.; Akweongo, P.; Binka, F. Factors associated with DELAY in diagnosis among tuberculosis patients in Hohoe Municipality, Ghana. BMC Public Health 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, F.M.; Fiebig, L.; Hauer, B.; Brodhun, B.; Glaser-Paschke, G.; Magdorf, K.; Haas, W. Higher rate of tuberculosis in second generation migrants compared to native residents in a metropolitan setting in Western Europe. PLoS ONE 2015, 10, e0119693. [Google Scholar]
- Belkina, T.V.; Khojiev, D.S.; Tillyashaykhov, M.N.; Tigay, Z.N.; Kudenov, M.U.; Tebbens, J.D.; Vlcek, J. Delay in the diagnosis and treatment of pulmonary tuberculosis in Uzbekistan: A cross-sectional study. BMC Infect. Dis. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stop, T. Partnership: The Potential Impact of the COVID-19 Response on Tuberculosis in High Burden Countries: A Modelling Analysis. 2020. Available online: http://www.stoptb.org/assets/documentsnews/Modeling (accessed on 24 January 2021).
| Variables | IDPs n (%) |
|---|---|
| Residence | |
| Village | 194 (49.6) |
| City | 59 (15.1) |
| Camp | 138 (35.3) |
| Age (years) | |
| 18–25 | 107 (27.4) |
| 26–35 | 93 (23.8) |
| 36–45 | 47 (12) |
| 46–55 | 66 (16.9) |
| 56–65 | 78 (19.9) |
| Gender | |
| Male | 204 (52.2) |
| Female | 187 (47.8) |
| Education | |
| Literate | 57 (14.6) |
| Illiterate | 334 (85.4) |
| Habitation density | |
| Low Density | 66 (16.8) |
| High Density Overcrowded | 159 (40.7) 166 (42.5) |
| Distance From Health Care Center | |
| ≤5 | 32 (8.2) |
| 6–15 km | 92 (23.5) |
| 16–30 km | 133 (34) |
| >30 km | 134 (34.3) |
| Health-seeking behavior | |
| Visited non-formal health provider | 286 (73.1) |
| Visited a formal health provider | 105 (26.9) |
| Self-Medication | |
| Yes | 147 (37.6) |
| No | 244 (62.4) |
| Category of treatment | |
| Category I | 346 (88.5) |
| Category II | 45 (11.5) |
| Have you ever heard of TB before diagnosis? | |
| Yes heard of TB | 49 (12.5) |
| Not heard of TB | 342 (87.5) |
| Number of TB patients at House | |
| Family members have TB | 74 (18.9) |
| Family Member do not have TB | 317 (81.1) |
| Perceived to be stigmatized | |
| Not stigmatized | 112 (28.6) |
| Stigmatized | 279 (71.4) |
| TB Baseline Signs and Symptoms | Total Number n (%) | Delay ≤ 30 Days | Delay > 30 Days | p Value |
|---|---|---|---|---|
| Cough | 381 (97.4) | 158 (41.5) | 203 (58.5) | 0.15 |
| Chest pain | 319 (81.6) | 116 (36.4) | 203 (63.6) | <0.001 |
| Fever | 311 (79.5) | 98 (31.5) | 213 (68.5) | <0.001 |
| Loss of appetite | 345 (88.2) | 154 (39.8) | 219 (60.2) | 0.08 |
| Night sweats | 314 (80.3) | 122 (38.9) | 192 (61.2) | 0.06 |
| Bodyweight loss | 304 (77.7) | 108 (35.5) | 194 (64.5) | <0.001 |
| Factors | Delay ≤ than 30 Days | Delay > than 30 Days | Crude OR (95%-CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Residence | ||||
| Village | 80 (41.2) | 114 (58.8) | Reference | Reference |
| City | 47 (79.7) | 12 (20.3) | 0.34 (0.27–0.93) | 0.42 (0.16–1.18) |
| Camp | 33 (23.9) | 105 (76.1) | 1.01 (0.48–2.11) * | 1.39 (0.55–2.34) |
| Age (years) | ||||
| 15–25 | 65 (60.7) | 42 (39.3) | Reference | Reference |
| 26–35 | 42 (45.2) | 51 (54.8) | 1.87 (1.07–3.30) | 2.17 (0.84–5.34) |
| 36–45 | 17 (36.2) | 30 (63.8) | 2.73 (1.34–5.55) | 1.13 (0.40–3.27) |
| 46–55 | 15 (22.7) | 51 (77.3) | 5.26 (2.62–10.3) * | 3.37 (1.35–9.37) * |
| 56–65 | 21 (26.9) | 57 (73.1) | 4.20 (2.23–7.91) * | 2.66 (1.00–7.07) * |
| Gender | ||||
| Male | 110 (53.9) | 94 (46.1) | Reference | Reference |
| Female | 50 (26.7) | 137 (73.7) | 3.20 (2.09–4.90) * | 2.42 (1.21–4.81) * |
| Education | ||||
| Literate | 24 (42.1) | 33 (57.9) | Reference | Not included |
| Illiterate | 136 (40.7) | 198 (59.3) | 1.05 (0.59–1.87) | |
| Place of living density | ||||
| Low Density | 34 (51.5) | 32 (48.5) | Reference | Not included |
| High Density | 63 (39.6) | 96 (60.4) | 1.61 (0.90–2.88) | |
| Overcrowded | 63 (38) | 103 (62) | 1.73 (0.97–3.08) | |
| Distance From Health Care Center | ||||
| ≤5 | 26 (81.2) | 6 (18.8) | Reference | Reference |
| 6–15 km | 49 (53.3) | 43 (46.7) | 3.80 (1.43–10.1) | 1.77 (0.72–12.8) |
| 16–30 km | 62 (46.6) | 71 (53.4) | 4.96 (1.91–12.8) | 2.13 (0.58–9.25) |
| >30 km | 23 (17.2) | 111 (82.8) | 20.9 (7.73–56.5) * | 4.13 (1.02–16.6) * |
| Health-seeking behavior | ||||
| Visited formal health provider | 87 (82.9) | 18 (17.1) | Reference | Reference |
| Visited non-formal health provider | 73 (25.5) | 213 (74.5) | 14.1 (7.95–25.01) * | 8.81 (1.37–19.46) ** |
| Self-medication | ||||
| Yes | 27 (18.4) | 120 (81.6) | Reference | Reference |
| No | 133 (54.5) | 111 (45.5) | 5.32 (3.27–8.67) * | 2.72 (1.37–5.37) * |
| Category of treatment | ||||
| Category I Category II | 148 (42.8) 12 (26.7) | 198 (57.2) 33 (73.3) | Reference 2.05 (1.02–4.11) * | Reference 1.88 (0.68–5.18) |
| Have you ever heard of TB before diagnosis | ||||
| Yes heard | 44 (89.2) | 5 (10.2) | Reference | Reference |
| Not heard of | 116 (33.9) | 226 (66.1) | 17.1 (6.61–44.0) * | 11.39 (3.31–39.14) ** |
| Number of TB patients at House | ||||
| Family members have TB | 138 (22.5) | 179 (77.5) | Reference | Reference |
| Family members do not have TB | 22 (29.7) | 52 (70.3) | 1.82 (1.05–3.14) * | 1.94 (0.78–4.79) |
| Perceived to be stigmatized | ||||
| Not stigmatized | 95 (84.8) | 17 (15.2) | Reference | Reference |
| Stigmatized | 65 (23.3) | 214 (76.7) | 18.3 (10.2–33) * | 8.81 (3.99–19.4) ** |
| Variables | IDPs n (%) | USO n (%) | Crude OR (95%-CI) | Adjusted OR (95%-CI) |
|---|---|---|---|---|
| Delay diagnosis | ||||
| Less than 30 days | 160 (39.9) | 13 (18.8) | Reference | Reference |
| More than 30 days | 231 (57.6) | 56 (81.2) | 3.61 (1.94–6.8) * | 2.60 (1.06–6.40) * |
| Residency | ||||
| Village | 194 (49.6) | 29 (42.1) | Reference | Reference |
| City | 59 (15.1) | 7 (10.1) | 0.76 (0.31–1.85) | 1.50 (0.55–4.08) |
| Camp | 138 (35.3) | 33 (47.8) | 1.78 (1.02–3.11) * | 1.41 (0.76–2.64) |
| Gender | ||||
| Male | 204 (52.7) | 31 (44.9) | Reference | Not included |
| Female | 187 (47.8) | 38 (55.1) | 1.42 (0.84–2.40) | |
| Age (years) | ||||
| 15–25 | 107 (27.4) | 10 (14.5) | Reference | Reference |
| 26–35 | 93 (23.8) | 10 (14.5) | 1.16 (0.46–0.29) | 0.97 (0.36–2.57) |
| 36–45 | 47 (12) | 7 (10.10) | 1.69 (0.60–4.77) | 1.26 (0.42–3.76) |
| 46–55 | 66 (16.9) | 20 (29.0) | 4.21 (1.82–9.73) * | 3.15 (1.27–7.80) * |
| 56–65 | 78 (19.9) | 22 (31.9) | 3.81 (1.68–8.62) * | 3.00 (1.25–7.19) * |
| Education | ||||
| Literate | 57 (14.6) | 33 (47.8) | Reference | Not Included |
| Illiterate | 334 (85.4) | 36 (52.1) | 1.00 (0.48–2.11) | |
| Number of Room/Tents Per house | ||||
| Low Density | 66 (16.9) | 11 (15.9) | Reference | Not Included |
| High Density | 159 (40.7) | 22 (31.9) | 0.80 (0.36–1.76) | |
| Overcrowded | 166 (42.5) | 36 (52.2) | 1.38 (0.65–2.91) | |
| Distance From Health Care Center | ||||
| ≤5 | 32 (8.2) | 4 (5.8) | Reference | Not Included |
| 6–15 km | 92 (23.5) | 14 (20.3) | 1.25 (0.38–4.13) | |
| 16–30 km | 133 (34) | 17 (24.6) | 1.02 (0.32–3.28) | |
| >30 km | 134 (34.3) | 34 (49.3) | 2.38 (0.77–7.27) | |
| Health-seeking behavior | ||||
| Visited a formal health provider | 105 (26.9) | 10 (14.5) | Reference | Reference |
| Visited non-formal health provider | 286 (73.1) | 59 (85.5) | 1.72 (0.89–3.30) * | 1.43 (0.60–3.35) |
| Self-medication | ||||
| Yes | 147 (37.6) | 37 (53.6) | Reference | Reference |
| No | 244 (62.4) | 32 (46.4) | 1.79 (1.06–3.03) * | 1.42 (0.77–2.62) |
| Have you ever heard of TB before diagnosis? | ||||
| Yes heard | 49 (12.5) | 6 (8.7) | Reference | Reference |
| Not heard of | 342 (87.5) | 63 (91.3) | 1.61 (0.66–3.98) * | 1.40 (0.49–4.008) |
| Number of TB patients at House | ||||
| Family members have TB | 74 (18.9) | 20 (28.9) | Reference | Not Included |
| Family members do not have TB | 317 (81.1) | 49 (71.8) | 0.80 (0.42–1.52) | |
| Category of treatment | ||||
| Category I | 346 (88.5) | 49 (71) | Reference | Reference |
| Category II | 45 (11.5) | 20 (29) | 4.84 (2.50–9.39) * | 4.80 (1.99–8.34) * |
| Perceived to be stigmatized | ||||
| Not stigmatized | 112 (28.6) | 12 (17.4) | Reference | Not Included |
| Stigmatized | 279 (71.4) | 57 (82.6) | 2.14 (1.10–4.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.U.; Khan, F.U.; Hayat, K.; Chang, J.; Kamran, M.; Khan, A.; Malik, U.R.; Khan, A.; Fang, Y. Impact of Protracted Displacement on Delay in the Diagnosis Associated with Treatment Outcomes: A Cross-Sectional Study in Internally Displaced Tuberculosis Patients of Pakistan. Int. J. Environ. Res. Public Health 2021, 18, 11984. https://doi.org/10.3390/ijerph182211984
Khan FU, Khan FU, Hayat K, Chang J, Kamran M, Khan A, Malik UR, Khan A, Fang Y. Impact of Protracted Displacement on Delay in the Diagnosis Associated with Treatment Outcomes: A Cross-Sectional Study in Internally Displaced Tuberculosis Patients of Pakistan. International Journal of Environmental Research and Public Health. 2021; 18(22):11984. https://doi.org/10.3390/ijerph182211984
Chicago/Turabian StyleKhan, Farman Ullah, Faiz Ullah Khan, Khezar Hayat, Jie Chang, Muhammad Kamran, Asad Khan, Usman Rashid Malik, Asif Khan, and Yu Fang. 2021. "Impact of Protracted Displacement on Delay in the Diagnosis Associated with Treatment Outcomes: A Cross-Sectional Study in Internally Displaced Tuberculosis Patients of Pakistan" International Journal of Environmental Research and Public Health 18, no. 22: 11984. https://doi.org/10.3390/ijerph182211984
APA StyleKhan, F. U., Khan, F. U., Hayat, K., Chang, J., Kamran, M., Khan, A., Malik, U. R., Khan, A., & Fang, Y. (2021). Impact of Protracted Displacement on Delay in the Diagnosis Associated with Treatment Outcomes: A Cross-Sectional Study in Internally Displaced Tuberculosis Patients of Pakistan. International Journal of Environmental Research and Public Health, 18(22), 11984. https://doi.org/10.3390/ijerph182211984








