Cardiac Rehabilitation Early after Sternotomy Using New Assistive VR-Enhanced Robotic Exoskeleton—Study Protocol for a Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
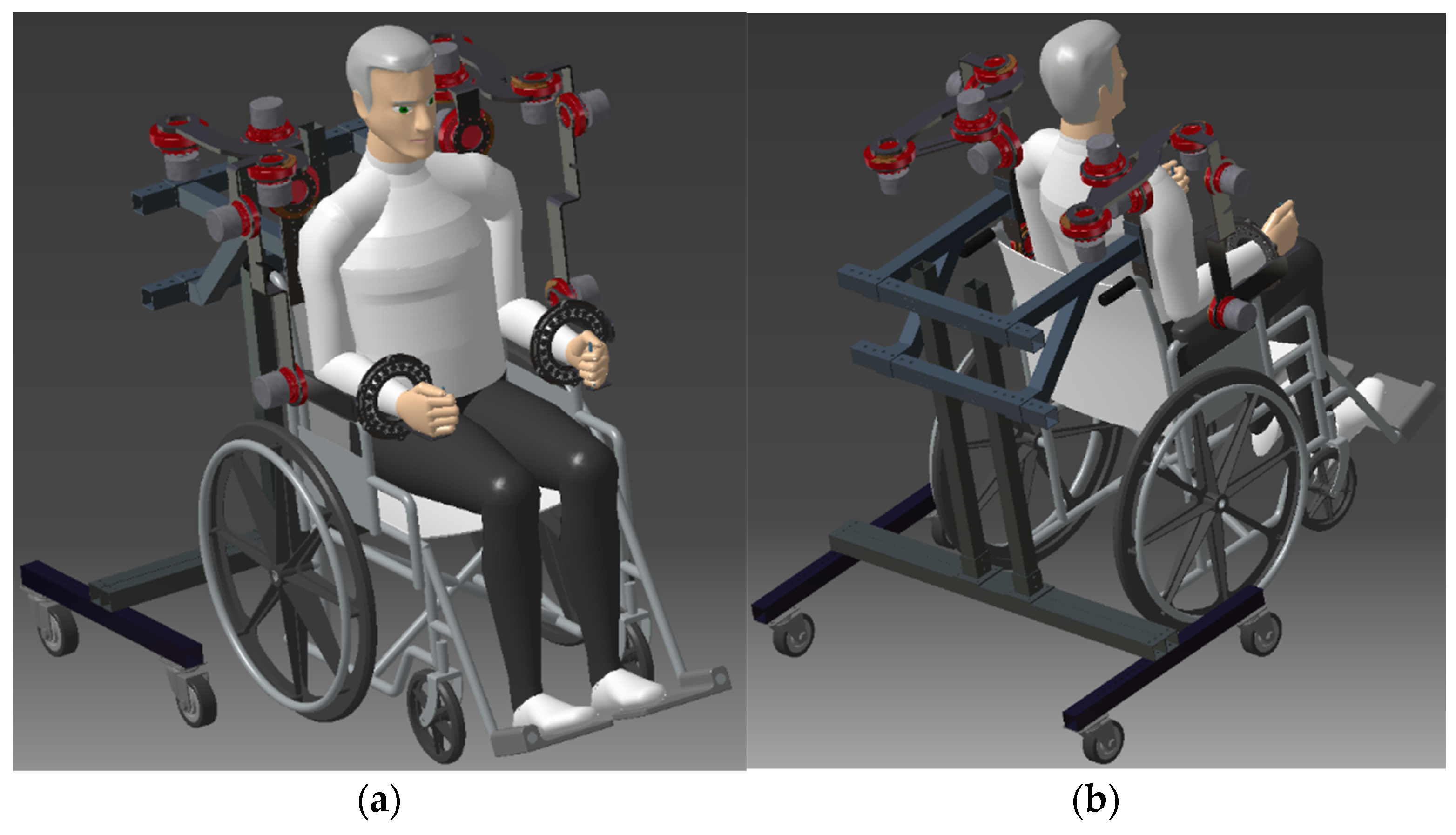
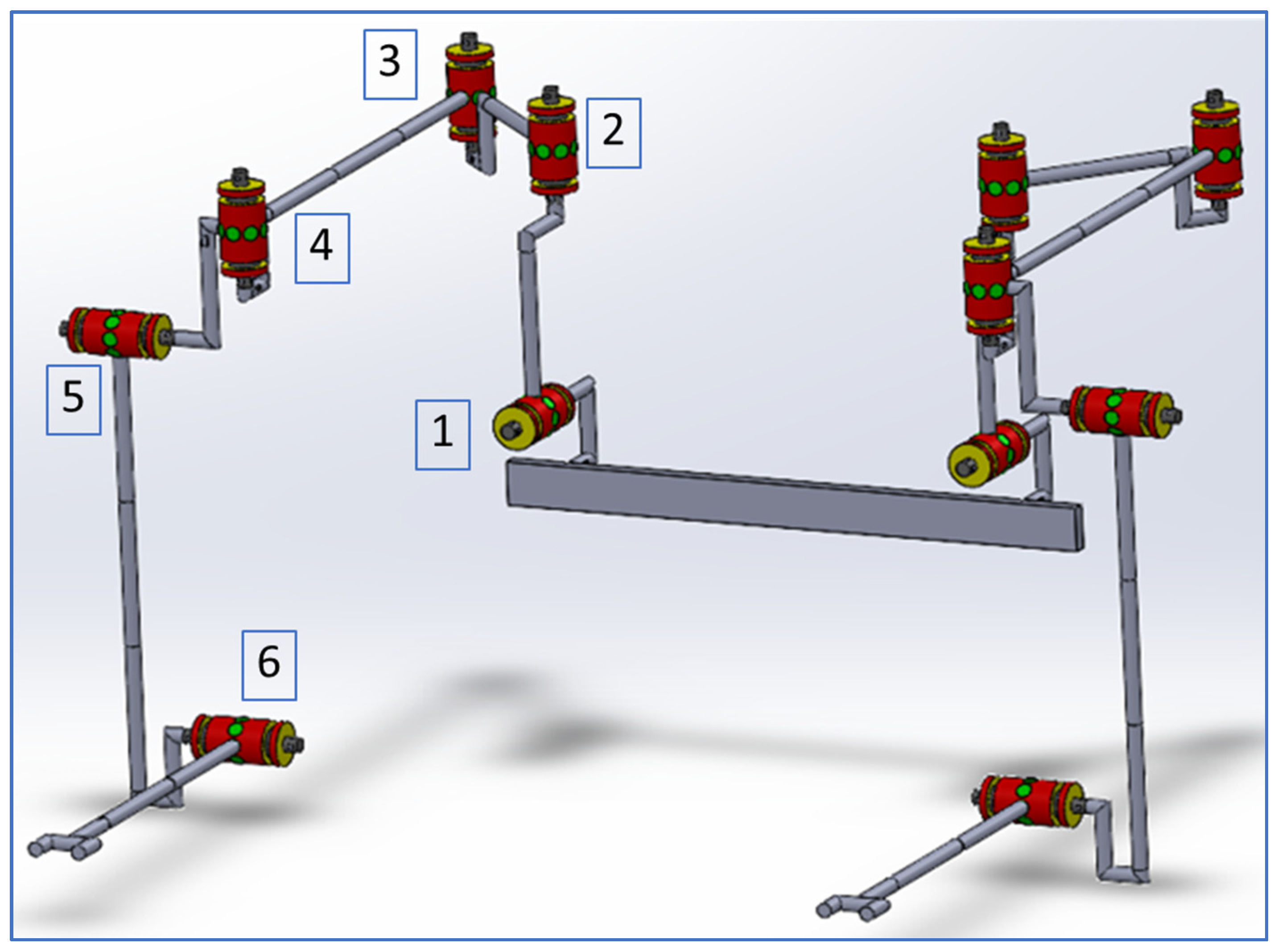
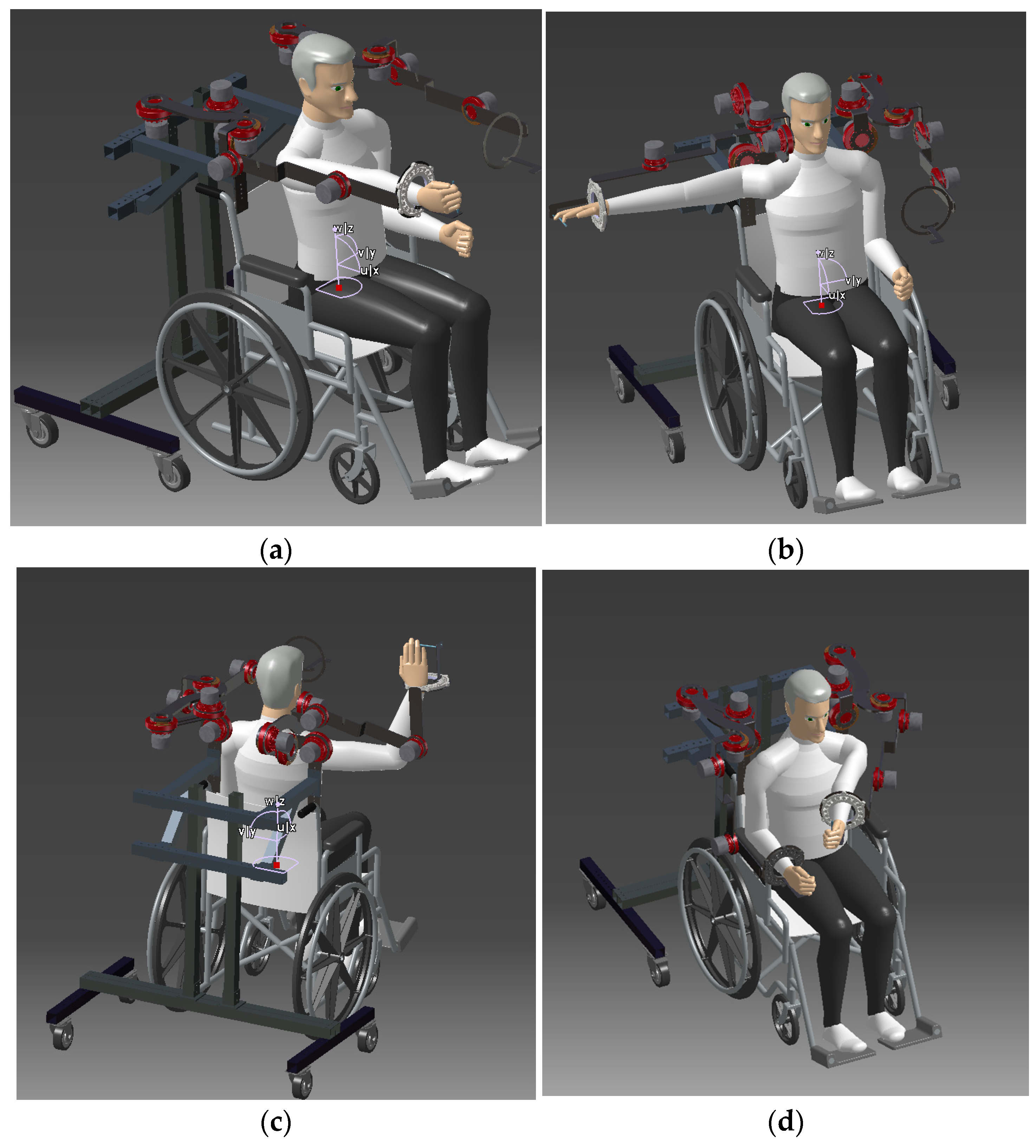
2.1. Description of the CardioVR-ReTone Exoskeleton
2.2. Space Requirements–Specification
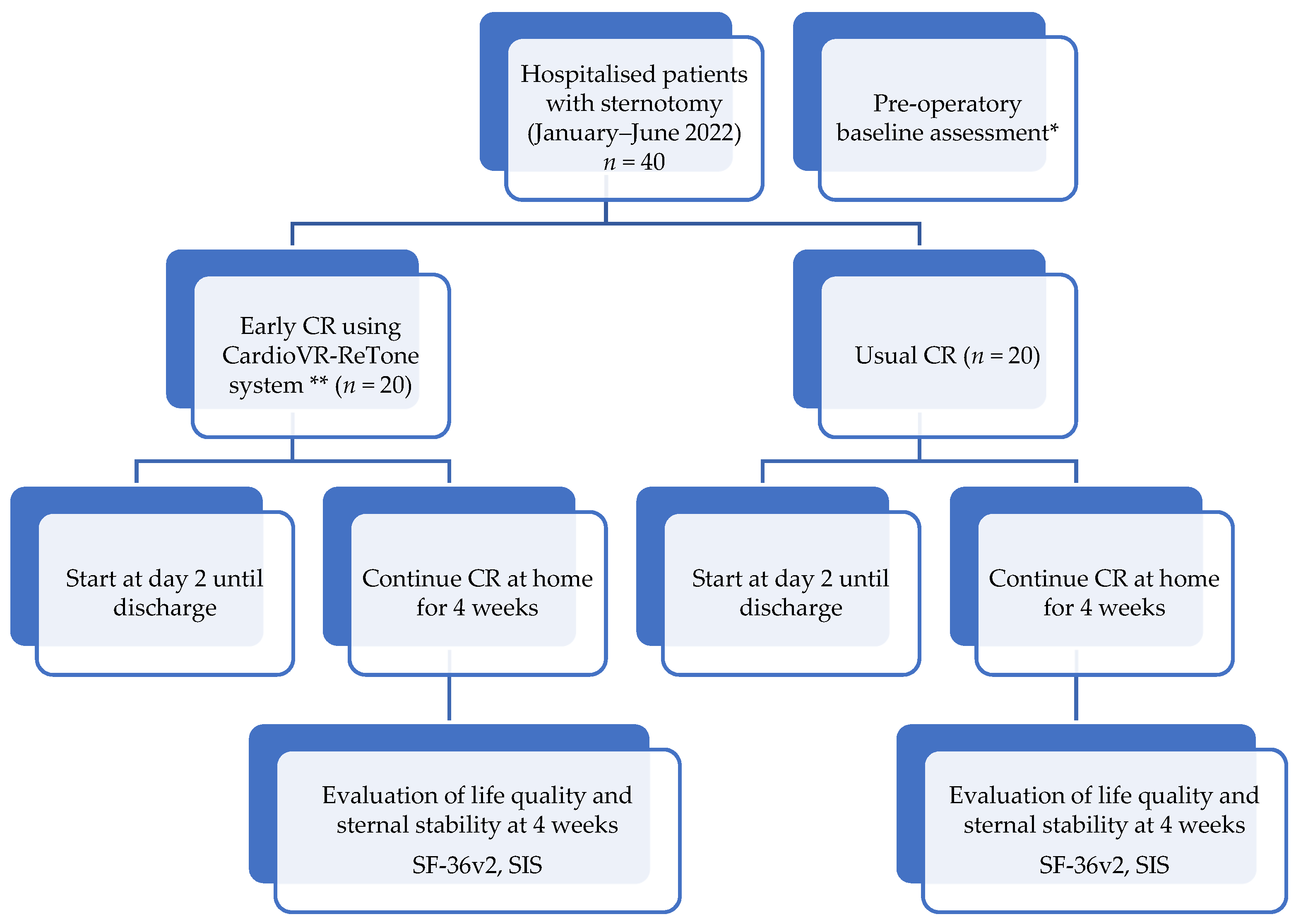
2.3. Setting
2.3.1. Enrolment of the Patients
2.3.2. Eligibility Criteria
2.3.3. Recording of Demographic and Clinical Data
2.3.4. Establishing Testing Protocol
2.3.5. Testing the Exoskeleton and Recording the Results
2.3.6. Appling the Clinical Protocol and Recording the Results to Validate CardioVR-ReTone System
2.3.7. Statistical Analysis
3. Results
3.1. Outcome Measures
3.2. Primary Outcome
3.3. Secondary Outcomes
- skeletal muscle function assessment: the surface electromyographic activities will be recorded for upper limb muscles, using a wireless EMG system.
- cardiac frequency and oxygen saturation will be monitored and recorded for the whole duration of the CR program.
4. Discussion
4.1. Types of Robotic Exoskeleton and Virtual Reality (VR) Used in Cardiac Rehabilitation (CR)
4.2. Dissemination and Impact
4.3. Risk Prediction
- R1: Risk (associated level of likelihood): <medium to low> project scope too narrow; changes in management/financial part. Proposed mitigation measures: (i) the project was designed broad enough to explore different potential approaches; (ii) adapt the budget/consortium structure;
- R2: Risk (associated level of likelihood): <medium> (i) poor mechanical quality of the components; (ii) insufficient details in the design; (iii) improper 3D printed components; (iv) ergonomics issues in energy transfer to human body; (v) faulty assembly the exoskeleton in test laboratory; (vi) incorrect user body size assumptions; (vii) vibrations of actuator, equipment; (viii) harmful levels of acoustic noise for the patient. Proposed mitigation measures: (i) we foresee supplementing the number of experimental investigations; (ii) re-designing the mechanical/electrical component/and/or change the manufacturing procedure; (iii) reprinting the problematic components using different extrude material; (iv) using clickable tools or cables connected for energy supply and communication; (v) reassembling the exoskeleton in the correct way; (vi) the exoskeleton will be designed to permit adjustments/connected (ISO 13482; ISO 13849); (vii) adjusting the control and redesign the linkages; (viii) using noise-absorbing materials;
- R3: Risk (associated level of likelihood): <medium>: (i) incorrect identification of requirements regarding the VR and game module; (ii) the VR game module do not meet the needs; (iii) inconsistency in VR application development. Proposed mitigation measures: (i) applying the iterative “Agile” approach in developing the VR module; (ii) redesigning the application and address the issues regarding VR module;
- R4: Risk (associated level of likelihood): <medium> relevance of data may be limited for the final goal. Proposed mitigation measures: if this is the case, new sets of investigations will be proposed based on a different medical protocol; adjustments in exoskeleton structure may be necessary;
- R5: Risk (associated level of likelihood): <medium> relevance of data may be limited for the final goal: Proposed mitigation measures: if this is the case, new sets of investigations will be proposed based on a different medical protocol; adjustments in exoskeleton structure may be necessary.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Turk-Adawi, K.; Sarrafzadegan, N.; Grace, S.L. Global availability of cardiac rehabilitation. Nat. Rev. Cardiol. 2014, 11, 586–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnason-Wehrens, B.; Nebel, R.; Jensen, K.; Hackbusch, M.; Grilli, M.; Gielen, S.; Schwaab, B.; Rauch, B. Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2020, 27, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Escalona, M.J.; Brosseau, R.; Vermette, M.; Comtois, A.S.; Duclos, C.; Aubertin-Leheudre, M.; Gagnon, D.H. Cardiorespiratory demand and rate of perceived exertion during overground walking with a robotic exoskeleton in long-term manual wheelchair users with chronic spinal cord injury: A cross-sectional study. Ann. Phys. Rehabil. Med. 2018, 61, 215–223. [Google Scholar] [CrossRef]
- Lu, C.K.; Huang, Y.C.; Lee, C.J. Adaptive guidance system design for the assistive robotic walker. Neurocomputing 2015, 170, 152–160. [Google Scholar] [CrossRef]
- Mocan, M.; Mocan, B. Cardiac rehabilitation for older patients with cardiovascular pathology using robotic systems—A survey. Balneo Res. J. 2019, 10, 33–36. [Google Scholar] [CrossRef]
- Mocan, B.; Fulea, M.; Farcas, A.D.; Mocan, M. Exoskeleton robotic systems used as a tool for cardiac rehabilitation. Acta Tech. Napoc.-Ser. Appl. Math. Mech. Eng. 2019, 62, 411–416. [Google Scholar]
- Sitar-Taut, A.-V.; Sitar-Taut, D.-A.; Cramariuc, O.; Negrean, V.; Sampelean, D.; Rusu, L.; Orasan, O.; Fodor, A.; Dogaru, G.; Cozma, A. Smart Homes for Older People Involved in Rehabilitation Activities-Reality or Dream, Acceptance or Rejection? Balneo Res. J. 2018, 9, 291–298. [Google Scholar] [CrossRef]
- Kraal, J.J.; Van Den Akker-Van Marle, M.E.; Abu-Hanna, A.; Stut, W.; Peek, N.; Kemps, H.M.C. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur. J. Prev. Cardiol. 2017, 24, 1260–1273. [Google Scholar] [CrossRef]
- Esquenazi, A.; Talaty, M. Robotics for lower limb rehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 385–397. [Google Scholar] [CrossRef]
- Hamaya, M.; Matsubara, T.; Noda, T.; Teramae, T.; Morimoto, J. Learning assistive strategies for exoskeleton robots from user-robot physical interaction. Pattern Recognit. Lett. 2017, 99, 67–76. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.J.; Lee, S.H.; Chang, W.H.; Jang, J.; Choi, B.O.; Ryu, G.H.; Kim, Y.H. A wearable hip-assist robot reduces the cardiopulmonary metabolic energy expenditure during stair ascent in elderly adults: A pilot cross-sectional study. BMC Geriatr. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Byl, N.N.; Abrams, G.M.; Pitsch, E.; Fedulow, I.; Kim, H.; Simkins, M.; Nagarajan, S.; Rosen, J. Chronic stroke survivors achieve comparable outcomes following virtual task specific repetitive training guided by a wearable robotic orthosis (UL-EXO7) and actual task specific repetitive training guided by a physical therapist. J. Hand Ther. 2013, 26, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Klamroth-Marganska, V.; Blanco, J.; Campen, K.; Curt, A.; Dietz, V.; Ettlin, T.; Felder, M.; Fellinghauer, B.; Guidali, M.; Kollmar, A.; et al. Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial. Lancet Neurol. 2014, 13, 159–166. [Google Scholar] [CrossRef]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P.F.M.J. Effect of specific over nonspecific VR-based rehabilitation on poststroke motor recovery: A systematic meta-analysis. Neurorehabil. Neural Repair 2019, 33, 112–129. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.E.; Schmid, J.P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur. J. Prev. Cardiol. 2020, 460–495. [Google Scholar] [CrossRef] [Green Version]
- Adelmanesh, F.; Jalali, A.; Attarian, H.; Farahani, B.; Mehdi Ketabchi, S.; Arvantaj, A.; Reza Raissi, G. Reliability, validity, and sensitivity measures of expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2) in Iranian patients with neuropathic and non-neuropathic pain. Pain Med. 2012, 13, 1631–1638. [Google Scholar] [CrossRef]
- Lovejoy, T.I.; Turk, D.C.; Morasco, B.J. Evaluation of the psychometric properties of the revised short-form McGill Pain Questionnaire (SF-MPQ-2). J. Pain 2012, 13, 1250–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gull, M.A.; Bai, S.; Bak, T. A review on design of upper limb exoskeletons. Robotics 2020, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Qassim, H.M.; Wan Hasan, W. A Review on upper limb rehabilitation robots. Appl. Sci. 2018, 10, 6976. [Google Scholar] [CrossRef]
- Jakob, I.; Kollreider, A.; Germanotta, M.; Benetti, F.; Cruciani, A.; Padua, L.; Aprile, I. Robotic and sensor technology for upper limb rehabilitation. PM R 2018, 10, S189–S197. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Spiewak, C.; Rahman, M.H.; Fareh, R. A brief review on robotic exoskeletons for upper extremity rehabilitation to find the gap between research porotype and commercial type. Adv. Robot. Autom. 2017, 6, 2. [Google Scholar] [CrossRef]
- Høeg, E.R.; Povlsen, T.M.; Bruun-Pedersen, J.R.; Lange, B.; Nilsson, N.C.; Haugaard, K.B.; Faber, S.M.; Hansen, S.W.; Kimby, C.K.; Serafin, S. System immersion in virtual reality-based rehabilitation of motor function in older adults: A systematic review and meta-analysis. Front. Virtual Real. 2021, 2. [Google Scholar] [CrossRef]
- Elor, A.; Kurniawan, S. The ultimate display for physical rehabilitation: A bridging review on immersive virtual reality. Front. Virtual Real. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Statista Forecast Unit Shipments of Augmented (AR) and Virtual Reality (VR) Headsets from 2019 to 2023 (in Millions). Teh. Rep. 2020. Available online: https://www.statista.com/statistics/653390/worldwide-virtual-and-augmented-reality-headset-shipments/ (accessed on 10 October 2021).
- Tao, G.; Garrett, B.; Taverner, T.; Cordingley, E.; Sun, C. Immersive virtual reality health games: A narrative review of game design. J. Neuroeng. Rehabil. 2021, 18, 1–21. [Google Scholar] [CrossRef]
- García-Bravo, S.; Cuesta-Gómez, A.; Campuzano-Ruiz, R.; López-Navas, M.J.; Domínguez-Paniagua, J.; Araújo-Narváez, A.; Barreñada-Copete, E.; García-Bravo, C.; Flórez-García, M.T.; Botas-Rodríguez, J.; et al. Virtual reality and video games in cardiac rehabilitation programs. A systematic review. Disabil. Rehabil. 2021, 43, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Calborean, A.; Macavei, S.; Mocan, M.; Ciuce, C.; Cordos, A.; Bintintan, A.; Chira, R.; Pestean, C.; Pop, O.; Barbu-Tudoran, L.; et al. Laparoscopic compatible device incorporating inductive proximity sensors for precise detection of gastric and colorectal small tumors. Surg. Oncol. 2020, 35. [Google Scholar] [CrossRef] [PubMed]
- Bințințan, V.; Calborean, A.; Mocan, M.; Macavei, S.; Cordoș, A.; Ciuce, C.; Bințințan, A.; Chira, R.; Nagy, G.; Surlin, V.; et al. New inductive proximity sensor platform for precise localization of small colorectal tumors. Mater. Sci. Eng. C 2020, 106, 110146. [Google Scholar] [CrossRef] [PubMed]
- Mocan, B.; Fulea, M.; Murar, M.; Steopan, M.; Mocan, M. Automatic arterial puncture sensorial device for fast arterial blood gas sampling from radial artery during COVID-19 pandemic. In Joint International Conference of the International Conference on Mechanisms and Mechanical Transmissions and the International Conference on Robotics; Springer International Publishing: Cham, Switzerland, 2020; Volume 88, pp. 533–542. [Google Scholar]

| CardioVR-ReTone Gouge | Dimensions |
|---|---|
| Max. exoskeleton height when in use | 1610 [mm] |
| Max. exoskeleton height when in standby | 1100 [mm] |
| Max. exoskeleton width when in use | 1820 [mm] |
| Max. exoskeleton width when in standby | 1000 [mm] |
| Max. exoskeleton footprint—length/width | 1200 [mm]/800 [mm] |
| Maximum weight of the exoskeleton/and the support | 58 kg |
| Demographic and Clinical Data | Pre-, Intra-, and Post-Surgery Details |
|---|---|
| Schedule of the surgical intervention Medical information (LVEF, NYHA classification, co-morbidities, the type of the graft) Surgical procedure (type of the procedure, elective or emergency procedure,) Intra-operatory details (method of sternal closure, cardiopulmonary bypass time, duration of the intervention, adverse events if any) Time spent in ICU (h) Pain medication (before and after surgical intervention) Other drugs (pre- and post-operative) |
| Measurements | Instruments |
|---|---|
| Quality of life | Short Form Health Survey-36 Questionnaire (SF-36) |
| Sternal stability | Modified Sternal Instability Scale |
| Upper arm skeletal muscle function assessment | Wireless EMG system |
| Pain intensity | Short Form McGill Pain Questionnaire version 2 (SF-MPQ-2) |
| Exercise programme | Total time of the exercise (min) and number of repetitive cycles (No.) |
| Cardiac response to exercise | SaO2, arterial pressure, cardiac frequency |
| Compliance/Adherence | Dropout rates (number of patients that got out of the study/total number of patients) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocan, M.; Vlaicu, S.I.; Farcaș, A.D.; Feier, H.; Dragan, S.; Mocan, B. Cardiac Rehabilitation Early after Sternotomy Using New Assistive VR-Enhanced Robotic Exoskeleton—Study Protocol for a Randomised Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 11922. https://doi.org/10.3390/ijerph182211922
Mocan M, Vlaicu SI, Farcaș AD, Feier H, Dragan S, Mocan B. Cardiac Rehabilitation Early after Sternotomy Using New Assistive VR-Enhanced Robotic Exoskeleton—Study Protocol for a Randomised Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(22):11922. https://doi.org/10.3390/ijerph182211922
Chicago/Turabian StyleMocan, Mihaela, Sonia Irina Vlaicu, Anca Daniela Farcaș, Horea Feier, Simona Dragan, and Bogdan Mocan. 2021. "Cardiac Rehabilitation Early after Sternotomy Using New Assistive VR-Enhanced Robotic Exoskeleton—Study Protocol for a Randomised Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 22: 11922. https://doi.org/10.3390/ijerph182211922
APA StyleMocan, M., Vlaicu, S. I., Farcaș, A. D., Feier, H., Dragan, S., & Mocan, B. (2021). Cardiac Rehabilitation Early after Sternotomy Using New Assistive VR-Enhanced Robotic Exoskeleton—Study Protocol for a Randomised Controlled Trial. International Journal of Environmental Research and Public Health, 18(22), 11922. https://doi.org/10.3390/ijerph182211922









