Association between Osteoporosis and Previous Statin Use: A Nested Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
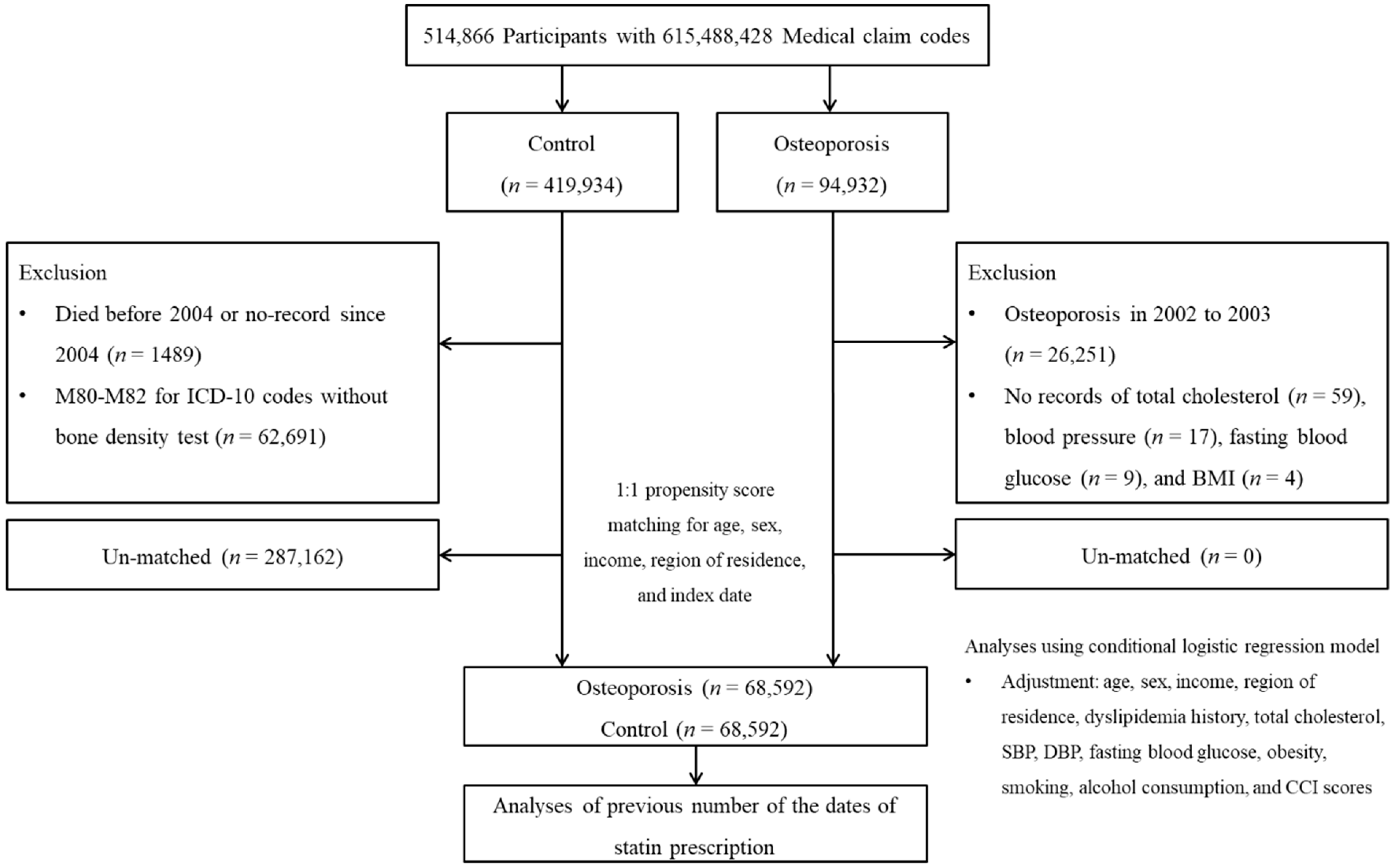
2.2. Study Population and Participant Selection
2.3. Exposure (Dates of Statin Use)
2.4. Outcome (Osteoporosis)
2.5. Covariates
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorentzon, M.; Cummings, S.R. Osteoporosis: The evolution of a diagnosis. J. Intern. Med. 2015, 277, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.B.; Leslie, W.D.; Joseph, L.; Siminoski, K.; Hanley, D.A.; Adachi, J.D.; Brown, J.P.; Morin, S.; Papaioannou, A.; Josse, R.G.; et al. Changes to osteoporosis prevalence according to method of risk assessment. J. Bone Min. Res. 2007, 22, 228–234. [Google Scholar] [CrossRef]
- Armas, L.A.; Recker, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef]
- Foger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef]
- Boling, E.P. Gender and osteoporosis: Similarities and sex-specific differences. J. Gend. Specif. Med. 2001, 4, 36–43. [Google Scholar]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Oesterle, A.; Liao, J.K. The Pleiotropic Effects of Statins-From Coronary Artery Disease and Stroke to Atrial Fibrillation and Ventricular Tachyarrhythmia. Curr. Vasc. Pharmacol. 2019, 17, 222–232. [Google Scholar] [CrossRef]
- Lian, X.L.; Zhang, Y.P.; Li, X.; Jing, L.D.; Cairang, Z.M.; Gou, J.Q. Exploration on the relationship between the elderly osteoporosis and cardiovascular disease risk factors. Eur. Rev. Med. Pharm. Sci. 2017, 21, 4386–4390. [Google Scholar]
- Alagiakrishnan, K.; Juby, A.; Hanley, D.; Tymchak, W.; Sclater, A. Role of vascular factors in osteoporosis. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 362–366. [Google Scholar] [CrossRef]
- Locantore, P.; Del Gatto, V.; Gelli, S.; Paragliola, R.M.; Pontecorvi, A. The Interplay between Immune System and Microbiota in Osteoporosis. Mediat. Inflamm. 2020, 2020, 3686749. [Google Scholar] [CrossRef]
- Mundy, G.R. Osteoporosis and inflammation. Nutr Rev. 2007, 65 Pt 2, 147–151. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhou, F.; Piao, Z.; Hao, J. Effects of Statins on Bone Mineral Density and Fracture Risk: A PRISMA-compliant Systematic Review and Meta-Analysis. Medcine 2016, 95, e3042. [Google Scholar] [CrossRef]
- Toh, S.; Hernandez-Diaz, S. Statins and fracture risk. A systematic review. Pharmacoepidemiol. Drug Saf. 2007, 16, 627–640. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Hao, J.; Sun, S.; Li, R.; Yang, M.; Cheng, G.; Zou, M. Efficacy of statins for osteoporosis: A systematic review and meta-analysis. Osteoporos Int. 2017, 28, 47–57. [Google Scholar] [CrossRef]
- Rizzo, M.; Rini, G.B. Statins, fracture risk, and bone remodeling: What is true? Am. J. Med. Sci. 2006, 332, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Leutner, M.; Matzhold, C.; Bellach, L.; Deischinger, C.; Harreiter, J.; Thurner, S.; Klimek, P.; Kautzky-Willer, A. Diagnosis of osteoporosis in statin-treated patients is dose-dependent. Ann. Rheum. Dis. 2019, 78, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Kapoor, E.; Moyer, A.M.; Hodis, H.N.; Miller, V.M. Statin therapy: Does sex matter? Menopause 2019, 26, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Yoo, D.M.; Chang, J.; Lee, H.J.; Park, B.; Choi, H.G. Hearing Impairment Increases Economic Inequality. Clin. Exp. Otorhinolaryngol. 2021, 14, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef]
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Chou, P.; Lin, C.H.; Hung, Y.J.; Jong, G.P. Long-term effect of statins on the risk of new-onset osteoporosis: A nationwide population-based cohort study. PLoS ONE 2018, 13, e0196713. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Su, P.Y.; Lin, T.K.; Jong, G.P. Association between statin use and osteoporotic fracture in patients with chronic obstructive pulmonary disease: A population-based, matched case-control study. Lipids Health Dis. 2020, 19, 232. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A. Potential mechanisms and applications of statins on osteogenesis: Current modalities, conflicts and future directions. J. Control. Release 2015, 215, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Wei, Z.; Qiu, Z.; Li, Z.; Fu, C.; Ye, Z.; Xu, X. Atorvastatin promotes bone formation in aged apoE−/− mice through the Sirt1-Runx2 axis. J. Orthop. Surg. Res. 2020, 15, 303. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Zhou, X.; Guan, Q.; Zhao, J.; Gao, L.; Yu, C.; Wang, Y.; Zuo, C. Simvastatin Decreases Sex Hormone Levels in Male Rats. Endocr. Pract. 2017, 23, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Guldvang, A.; Hansen, C.H.; Weisser, J.J.; Halling-Sorensen, B.; Styrishave, B. Simvastatin decreases steroid production in the H295R cell line and decreases steroids and FSH in female rats. Reprod. Toxicol. 2015, 58, 174–183. [Google Scholar] [CrossRef]
- Bone, H.G.; Greenspan, S.L.; McKeever, C.; Bell, N.; Davidson, M.; Downs, R.W.; Emkey, R.; Meunier, P.J.; Miller, S.S.; Mulloy, A.L.; et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J. Clin. Endocrinol. Metab. 2000, 85, 720–726. [Google Scholar] [CrossRef][Green Version]
- McKenney, J.M. Pharmacologic characteristics of statins. Clin. Cardiol. 2003, 26 (Suppl. 3), 32–38. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Chen, H.H.; Lin, C.L.; Yeh, S.Y.; Kao, C.H. The Different Cardiovascular Outcomes Between Long-Term Efficacy of Hydrophilic and Lipophilic Statin Therapy in Both Asian Diabetic Sexes. Dose Response 2019, 17, 1559325819876766. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Participants | ||||
|---|---|---|---|---|---|
| Total | Osteoporosis | Control | p-Value | ||
| Age (years old, n, %) | <0.001 * | ||||
| 40–44 | 778 (0.6) | 389 (0.6) | 389 (0.6) | ||
| 45–49 | 6540 (4.8) | 3270 (4.8) | 3270 (4.8) | ||
| 50–54 | 16,350 (11.9) | 8175 (11.9) | 8175 (11.9) | ||
| 55–59 | 23,542 (17.2) | 9582 (40.7) | 13,960 (59.3) | ||
| 60–64 | 31,731 (23.1) | 11,034 (34.8) | 20,697 (65.2) | ||
| 65–69 | 24,109 (17.6) | 14,995 (21.9) | 9114 (13.3) | ||
| 70–74 | 18,960 (13.8) | 11,968 (17.5) | 6992 (10.2) | ||
| 75–79 | 10,661 (7.8) | 6505 (9.5) | 4156 (6.1) | ||
| 80–84 | 3875 (2.8) | 2293 (3.3) | 1582 (2.3) | ||
| 85+ | 638 (0.5) | 381 (0.6) | 257 (0.4) | ||
| Sex (n, %) | 1.000 | ||||
| Male | 17,694 (12.9) | 8847 (12.9) | 8847 (12.9) | ||
| Female | 119,490 (87.1) | 59,745 (87.1) | 59,745 (87.1) | ||
| Income (n, %) | <0.001 * | ||||
| 1 (lowest) | 25,221 (18.4) | 13,218 (19.3) | 12,003 (17.5) | ||
| 2 | 19,679 (14.3) | 9751 (14.2) | 9928 (14.5) | ||
| 3 | 21,440 (15.6) | 10,641 (15.5) | 10,799 (15.7) | ||
| 4 | 28,817 (21.0) | 13,681 (20.0) | 15,136 (22.1) | ||
| 5 (highest) | 42,027 (30.6) | 21,301 (31.1) | 20,726 (30.2) | ||
| Region of residence (n, %) | <0.001 * | ||||
| Urban | 54,018 (39.4) | 26,448 (38.6) | 27,570 (40.2) | ||
| Rural | 83,166 (60.6) | 42,144 (61.4) | 41,022 (59.8) | ||
| Total cholesterol (mg/dL, mean, SD) | 204.7 (39.0) | 203.7 (38.7) | 205.7 (39.3) | <0.001 * | |
| SBP (mmHg, mean, SD) | 127.6 (18.0) | 127.3 (17.8) | 127.9 (18.3) | <0.001 * | |
| DBP (mmHg, mean, SD) | 78.1 (11.1) | 77.8 (10.9) | 78.5 (11.2) | <0.001 * | |
| Fasting blood glucose (mg/dL, mean, SD) | 99.4 (30.0) | 98.1 (27.6) | 100.7 (32.1) | <0.001 * | |
| Obesity (n, %) ‡ | <0.001 * | ||||
| Underweight | 3806 (2.8) | 2281 (3.3) | 1525 (2.2) | ||
| Normal | 48,800 (35.6) | 25,762 (37.6) | 23,038 (33.6) | ||
| Overweight | 36,049 (26.3) | 17,864 (26.0) | 18,185 (26.5) | ||
| Obese I | 43,449 (31.7) | 20,602 (30.0) | 22,847 (33.3) | ||
| Obese II | 5080 (3.7) | 2083 (3.0) | 2997 (4.4) | ||
| Smoking status (n, %) | <0.001 * | ||||
| Nonsmoker | 125,177 (91.3) | 62,797 (91.6) | 62,380 (90.9) | ||
| Past smoker | 4765 (3.5) | 2363 (3.5) | 2402 (3.5) | ||
| Current smoker | 7242 (5.3) | 3432 (5.0) | 3810 (5.6) | ||
| Alcohol consumption (n, %) | <0.001 * | ||||
| <1 time a week | 119,132 (86.8) | 60,336 (88.0) | 58,796 (85.7) | ||
| ≥1 time a week | 18,052 (13.2) | 8256 (12.0) | 9796 (14.3) | ||
| CCI score (score, n, %) | <0.001 * | ||||
| 0 | 89,612 (65.3) | 42,465 (61.9) | 47,147 (68.7) | ||
| 1 | 21,208 (15.5) | 11,807 (17.2) | 9401 (13.7) | ||
| 2 | 11,954 (8.7) | 6622 (9.7) | 5332 (7.8) | ||
| 3 | 6149 (4.5) | 3425 (5.0) | 2724 (4.0) | ||
| ≥4 | 8261 (6.0) | 4273 (6.2) | 3988 (5.8) | ||
| Dyslipidemia (n, %) | 33,899 (24.7) | 18,183 (26.5) | 15,716 (22.9) | <0.001 * | |
| Dates of statin use (days, mean, SD) | 53.7 (158.5) | 56.9 (162.8) | 50.6 (154.1) | <0.001 * | |
| Characteristics | Odds Ratios for Osteoporosis | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 † | p-Value | Model 2 ‡ | p-Value | Model 3 § | p-Value | ||
| Total participants (n = 137,184) | |||||||
| Statin prescription (one year) | 1.04 (1.02–1.07) | 0.001 * | 0.96 (0.93–0.98) | 0.002 * | 0.97 (0.94–1.00) | 0.052 | |
| Age < 60 years old, males (n = 4,178) | |||||||
| Statin prescription (one year) | 1.11 (0.96–1.29) | 0.172 | 0.97 (0.82–1.15) | 0.727 | 0.99 (0.84–1.18) | 0.942 | |
| Age < 60 years old, females (n = 74,763) | |||||||
| Statin prescription (one year) | 0.85 (0.82–0.88) | <0.001 * | 0.80 (0.76–0.83) | <0.001 * | 0.81 (0.77–0.85) | <0.001 * | |
| Age ≥ 60 years old, males (n = 13,516) | |||||||
| Statin prescription (one year) | 0.99 (0.93–1.05) | 0.705 | 0.95 (0.88–1.02) | 0.178 | 0.96 (0.89–1.03) | 0.281 | |
| Age ≥ 60 years old, females (n = 44,727) | |||||||
| Statin prescription (one year) | 1.76 (1.67–1.86) | <0.001 * | 1.36 (1.28–1.44) | <0.001 * | 1.37 (1.30–1.46) | <0.001 * | |
| Low income (n = 66,340) | |||||||
| Statin use (one year) | 1.05 (1.01–1.09) | 0.010 * | 0.95 (0.91–0.99) | 0.022 * | 0.97 (0.93–1.01) | 0.170 | |
| High income (n = 70,844) | |||||||
| Statin use (one year) | 1.04 (1.00–1.07) | 0.043 * | 0.96 (0.92–0.99) | 0.025 | 0.97 (0.94–1.01) | 0.147 | |
| Urban (n = 54,018) | |||||||
| Statin use (one year) | 1.02 (0.98–1.06) | 0.302 | 0.92 (0.88–0.96) | <0.001 * | 0.93 (0.89–0.97) | 0.001 * | |
| Rural (n = 83,166) | |||||||
| Statin use (one year) | 1.08 (1.04–1.11) | <0.001 * | 0.99 (0.96–1.03) | 0.703 | 1.01 (0.97–1.05) | 0.625 | |
| Characteristics | Odds Ratios of Statins Use (One Year) for Osteoporosis | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 † | p-Value | Model 2 ‡ | p-Value | Model 3 § | p-Value | ||
| Obesity | |||||||
| Underweight (n = 3806) | 1.44 (1.12–1.84) | 0.004 * | 1.14 (0.86–1.52) | 0.369 | 1.14 (0.86–1.51) | 0.374 | |
| Normal weight (n = 48,800) | 1.08 (1.03–1.14) | 0.002 * | 0.97 (0.91–1.02) | 0.217 | 0.96 (0.91–1.02) | 0.179 | |
| Overweight (n = 36,049) | 1.10 (1.05–1.15) | <0.001 * | 0.99 (0.94–1.05) | 0.817 | 0.99 (0.94–1.05) | 0.796 | |
| Obese (n = 48,529) | 1.04 (1.01–1.08) | 0.019 * | 0.95 (0.91–0.99) | 0.021 * | 0.95 (0.91–0.99) | 0.025 * | |
| Smoking | |||||||
| Nonsmoker (n = 125,177) | 1.04 (1.01–1.07) | 0.003 * | 0.96 (0.93–0.98) | 0.003 * | 0.97 (0.94–1.00) | 0.066 | |
| Past or current smoker (n = 12,007) | 1.05 (0.97–1.13) | 0.232 | 0.95 (0.87–1.04) | 0.245 | 0.97 (0.89–1.06) | 0.527 | |
| Alcohol consumption | |||||||
| <1 time a week (n = 119,132) | 1.06 (1.03–1.09) | <0.001 * | 0.97 (0.94–1.00) | 0.032 * | 0.98 (0.95–1.01) | 0.269 | |
| ≥1 time a week (n = 18,052) | 0.99 (0.93–1.05) | 0.707 | 0.92 (0.85–0.98) | 0.015 * | 0.93 (0.87–1.00) | 0.045 * | |
| Total cholesterol (mg/dL) | |||||||
| <200 (n = 64,727) | 1.01 (0.98–1.04) | 0.572 | 0.92 (0.88–0.95) | <0.001 * | 0.94 (0.90–0.97) | 0.001 * | |
| ≥200 to <240 (n = 48,835) | 1.08 (1.03–1.14) | 0.002 * | 1.00 (0.94–1.05) | 0.877 | 1.01 (0.95–1.07) | 0.819 | |
| ≥240 (n = 23,622) | 1.13 (1.07–1.21) | <0.001 * | 1.04 (0.97–1.12) | 0.225 | 1.06 (0.99–1.13) | 0.119 | |
| Blood pressure (mmHg) | |||||||
| SBP < 140 and DBP < 90 (n = 97,751) | 0.99 (0.97–1.02) | 0.674 | 0.91 (0.88–0.94) | <0.001 * | 0.93 (0.90–0.96) | <0.001 * | |
| SBP ≥ 140 or DBP ≥ 90 (n = 39,433) | 1.17 (1.12–1.22) | <0.001 * | 1.05 (1.00–1.11) | 0.046 * | 1.07 (1.01–1.12) | 0.013 * | |
| Fasting blood glucose (mg/dL) | |||||||
| <100 (n = 90,834) | 1.07 (1.03–1.11) | <0.001 * | 0.94 (0.90–0.98) | 0.002 * | 0.96 (0.92–0.99) | 0.024 * | |
| ≥100 (n = 46,350) | 1.07 (1.03–1.11) | <0.001 * | 0.98 (0.94–1.02) | 0.386 | 1.00 (0.96–1.04) | 0.845 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Yoo, D.M.; Min, C.; Kim, J.H.; Kwon, M.J.; Kim, J.-H.; Choi, H.G. Association between Osteoporosis and Previous Statin Use: A Nested Case-Control Study. Int. J. Environ. Res. Public Health 2021, 18, 11902. https://doi.org/10.3390/ijerph182211902
Kim SY, Yoo DM, Min C, Kim JH, Kwon MJ, Kim J-H, Choi HG. Association between Osteoporosis and Previous Statin Use: A Nested Case-Control Study. International Journal of Environmental Research and Public Health. 2021; 18(22):11902. https://doi.org/10.3390/ijerph182211902
Chicago/Turabian StyleKim, So Young, Dae Myoung Yoo, Chanyang Min, Ji Hee Kim, Mi Jung Kwon, Joo-Hee Kim, and Hyo Geun Choi. 2021. "Association between Osteoporosis and Previous Statin Use: A Nested Case-Control Study" International Journal of Environmental Research and Public Health 18, no. 22: 11902. https://doi.org/10.3390/ijerph182211902
APA StyleKim, S. Y., Yoo, D. M., Min, C., Kim, J. H., Kwon, M. J., Kim, J.-H., & Choi, H. G. (2021). Association between Osteoporosis and Previous Statin Use: A Nested Case-Control Study. International Journal of Environmental Research and Public Health, 18(22), 11902. https://doi.org/10.3390/ijerph182211902







