Abstract
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) caused the global pandemic of coronavirus disease 2019 (COVID-19). Rapid identification and isolation of infectious patients are critical methods to block COVID-19 transmission. Antigen tests can contribute to prompt identification of infectious individuals. This meta-analysis aims to evaluate the diagnostic accuracy of antigen tests for SARS-CoV-2. We conducted a literature search in PubMed, Embase, the Cochrane Library, and Biomed Central databases. Studies evaluating the diagnostic accuracy of antigen tests for SARS-CoV-2 in community participants were included. Only English-language articles were reviewed. We included eligible studies that provided available data to construct a 2 × 2 table on a per-patient basis. Overall sensitivity and specificity for antigen tests were generated using a bivariate random-effects model. Eighteen studies with 34,865 participants were retrieved. The meta-analysis for SARS-CoV-2 antigen tests generated a pooled sensitivity of 0.82 and a pooled specificity of 1.00. A subgroup analysis of ten studies that reported outcomes for 5629 symptomatic participants generated a pooled sensitivity of 0.87 and a pooled specificity of 1.00. Antigen tests might have higher sensitivity in detecting SARS-CoV-2 in symptomatic patients in the community and may be an effective tool to identify patients to be quarantined to prevent further SARS-CoV-2 transmission.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) caused the global pandemic of coronavirus disease 2019 (COVID-19). Asymptomatic cases make COVID-19 difficult to monitor and prevent. It is estimated that at least 50% of COVID-19 patients contract the virus from asymptomatic people [1]. To break the transmission chains of SARS-CoV-2, testing infected individuals and tracing and quarantining their contacts have been used as major nonpharmaceutical interventions [2]. Rapid identification and isolation of infectious patients with SARS-CoV-2 are critical methods to block COVID-19 community transmission. Approximately 40% of infected individuals with high viral loads might be asymptomatic [3]. The World Health Organization and Centers for Disease Control and Prevention have implemented reverse-transcription polymerase chain reaction (RT-PCR) technology as the standard diagnostic assay for SARS-CoV-2 detection. RT-PCR has a high sensitivity for SARS-CoV-2. The sensitivity of RT-PCR ranged from 71 to 98%, and the assay was 100% specific [4,5]. However, factors such as the type and quality of the respiratory specimen and the stage of the disease influence testing accuracy. Despite its high sensitivity, RT-PCR has disadvantages, including the necessity of professional lab expertise, costly reagents, and centralized equipment. Therefore, antigen tests that detect viral proteins of SARS-CoV-2 in respiratory samples have been developed [6]. Antigen tests are relatively inexpensive, and most of them can be used at the point of care. Antigen tests can identify individuals with COVID-19 who are highly contagious, namely those whose viral load is likely to be high. Antigen tests have received the U.S. Food and Drug Administration Emergency Use Authorization for use in asymptomatic and symptomatic individuals [7].
The advantages of antigen tests, such as relatively low cost and short turnaround time, can contribute to prompt identification of infectious individuals. RT-PCR testing should be considered after negative antigen test results in symptomatic individuals and after positive antigen test results in asymptomatic individuals [8]. Although antigen tests might not be as accurate as RT-PCR testing, they are more accessible in terms of availability and ease of use and can be used to scale up testing outside of laboratory settings (e.g., frequent repeat testing) [9]. A crucial role for testing in the COVID-19 pandemic response is in identifying people who are not infected with SARS-CoV-2 so that they can travel, return to school or work, and attend mass gatherings. The wide availability of antigen tests and their rapid turnaround time offer the promise of efficiently testing a large number of people in the community [9].
The diagnostic accuracy of using antigen tests for COVID-19 among members of the community at large is still inconclusive. Therefore, the aim of this meta-analysis was to evaluate the accuracy of antigen tests for detecting SARS-CoV-2 among suspected COVID-19 patients in the community.
2. Materials and Methods
2.1. Literature Search Strategy
The study was reported according to “Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement” [10].
We conducted a literature search for relevant studies in PubMed, Embase, the Cochrane Library, and Biomed Central. A literature search was conducted using multiple search terms, including (COVID-19 OR severe acute respiratory syndrome coronavirus 2 OR SARS-CoV-2) AND (antigen test OR SARS-CoV-2 antigens OR Mass Screening OR Community Participation) AND (RT-PCR OR Reverse Transcriptase Polymerase Chain Reaction OR COVID-19 Nucleic Acid Testing) AND (sensitivity OR specificity). A combination of free text and MeSH terms was used to identify relevant studies. We limited our search results to studies performed with human participants. Detailed search strategies are presented in Table S1.
2.2. Inclusion and Exclusion Criteria
Studies evaluating the diagnostic accuracy of antigen tests for SARS-CoV-2 with reference standards in participants with suspected SARS-CoV-2 infection in the community were included, but review articles were excluded. Respiratory specimens were collected from symptomatic or asymptomatic individuals. Studies that defined RT-PCR technology as the reference standard were included. Only English-language articles were reviewed. The literature search was conducted with no time restrictions. Studies that provided sufficient data to construct a 2 × 2 table on a per-patient basis were included. We excluded case reports, case series, proposals, protocols, conference abstracts, in-house tests, and preprint articles. The last literature search was performed on 1 August 2021. One reviewer initially screened titles and abstracts for potentially eligible studies. After eliminating irrelevant studies, two reviewers independently examined full-text articles that met the inclusion criteria. Disagreements between the reviewers were resolved through joint discussions.
2.3. Quality Assessment
The quality of the included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool [11]. Antigen tests for the SARS-CoV-2 virus were the index tests and RT-PCR test results for SARS-CoV-2 were the reference standards. The QUADAS-2 tool consists of the following four domains: patient selection, index test, reference standard, and flow and timing. Each domain includes questions that allow an assessment of the risk of bias. The quality assessment of the diagnostic test comprises the risk of bias and the applicability for individual studies. A study is considered high-quality if each domain in the study exhibits a low risk of bias.
2.4. Statistical Analysis
We extracted data on true positives, true negatives, false positives, and false negatives from each included study to construct 2 × 2 tables for calculating values of the pooled sensitivity, pooled specificity. If 2 × 2 tables could not be extracted from the main text, we searched the supplementary material of the study for additional information. The sensitivity of a test is the proportion of those with the target condition correctly identified as having the condition, whereas the specificity of a test is the proportion of those without the target condition correctly identified as not having the condition [12].
We conducted a meta-analysis using a bivariate random-effects model to generate a summary of sensitivity, specificity on a per-patient basis. We also graphed the summary receiver operating characteristic (SROC) curve to determine the overall diagnostic performance of the index tests. The closer the curve approaches the upper-left corner, the higher the overall performance is [13]. Possible causes of heterogeneity between studies were explored through pre-specified subgroup analysis, which included the following: days after symptom onset, asymptomatic participants, and symptomatic individuals. Summary estimates, including pooled sensitivity, specificity, and DOR, were generated with associated 95% confidence intervals (CIs). All analyses were performed using MetaDiSc version 1.4 (Universidad Complutense, Madrid, Spain) and MetaDTA software (National Institute for Health Research Complex Review Support Unit, Glasgow, UK) [14,15]. A value of p < 0.05 was considered statistically significant.
3. Results
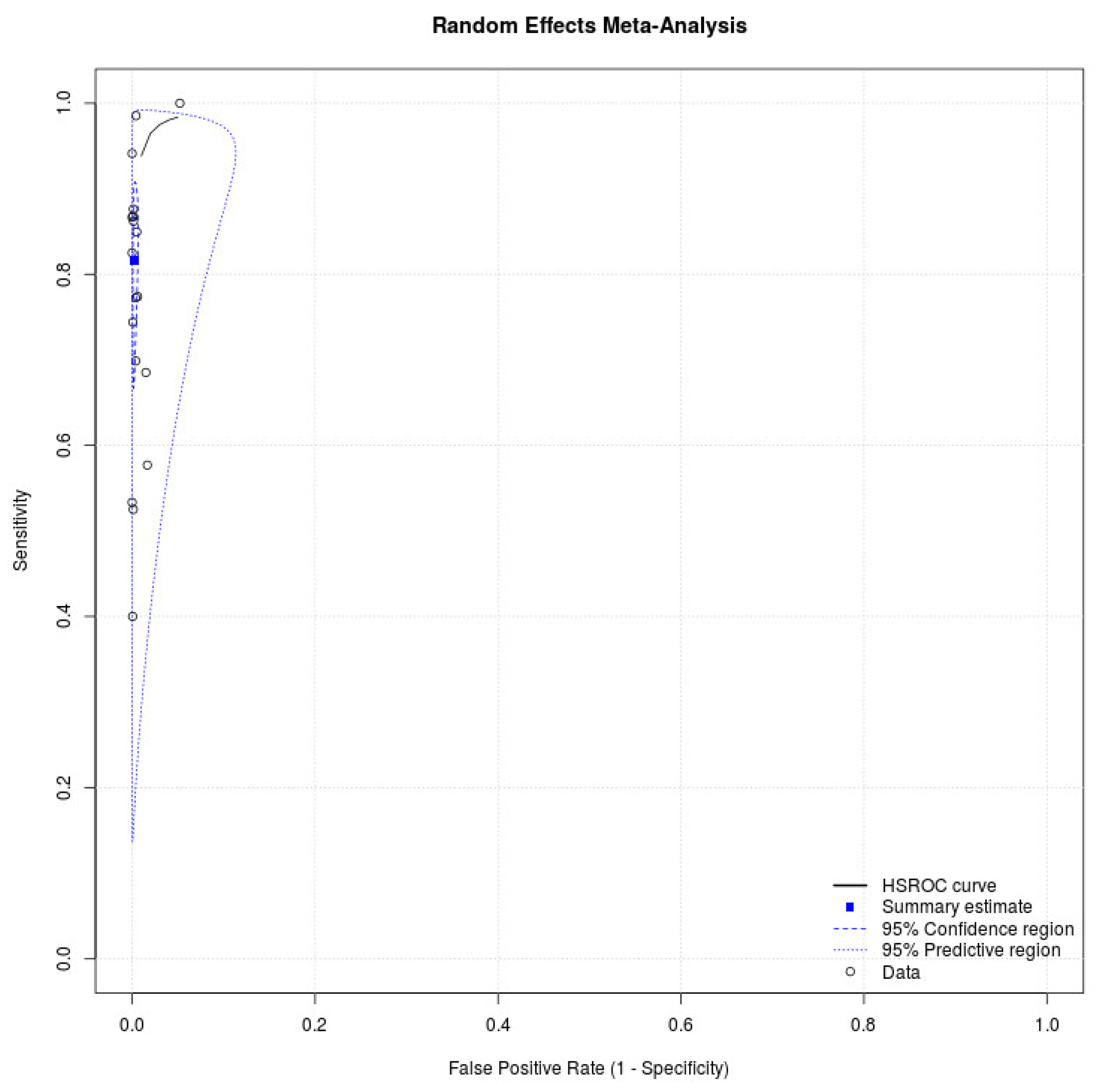
Eighteen studies with 34,865 participants were retrieved [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Figure 1 depicts the process of the literature search, and Table 1 presents detailed characteristics of the studies. All studies in the meta-analysis used a prospective study design, and five studies enrolled participants in the drive-through testing sites [16,19,26,28,31]. Eight studies evaluated the diagnostic performance of antigen tests with nasal swab specimens [16,18,22,23,28,29,30,33], six assessed the accuracy of antigen tests with nasopharyngeal swab specimens [21,24,25,26,27,31], seven provided cycle threshold (Ct) values of positive RT-PCR tests [24,25,28,29,30,31,32], and eight reported cutoff values of Ct [16,21,22,23,25,27,28,33]. Table 2 lists the statistical data. The meta-analysis for antigen tests generated a pooled sensitivity of 0.82 (95% CI: 0.71–0.89) and a pooled specificity of 1.00 (95% CI: 0.99–1.00) (Figure 2). Eight studies with 16,470 patients discussed the accuracy of antigen tests using nasal swab specimens [16,18,22,23,28,29,30,33]. The meta-analysis produced a pooled sensitivity of 0.76 (95% CI: 0.58–0.88) and a pooled specificity of 1.00 (95% CI: 0.99–1.00). Moreover, six studies with 7441 patients reported the accuracy of antigen tests using nasopharyngeal swab specimens [21,24,25,26,27,31]. The meta-analysis produced a pooled sensitivity of 0.90 (95% CI: 0.76–0.96) and a pooled specificity of 1.00 (95% CI: 0.99–1.00). The supplementary information presents the sensitivities and specificities of antigen tests for SARS-CoV-2 from the included studies (see Figure S1).
Figure 1.
Flowchart of literature search.

Table 1.
Characteristics of studies.

Table 2.
Statistical data of included studies.
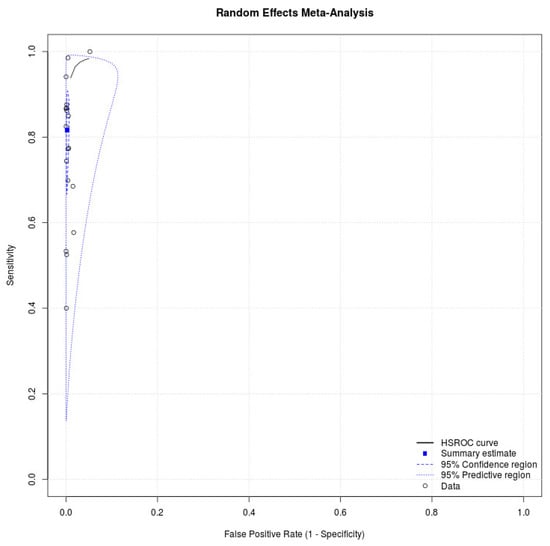
Figure 2.
SROC curve showing the pooled sensitivity and specificity of antigen test for SARS-CoV-2.
3.1. Quality Assessment
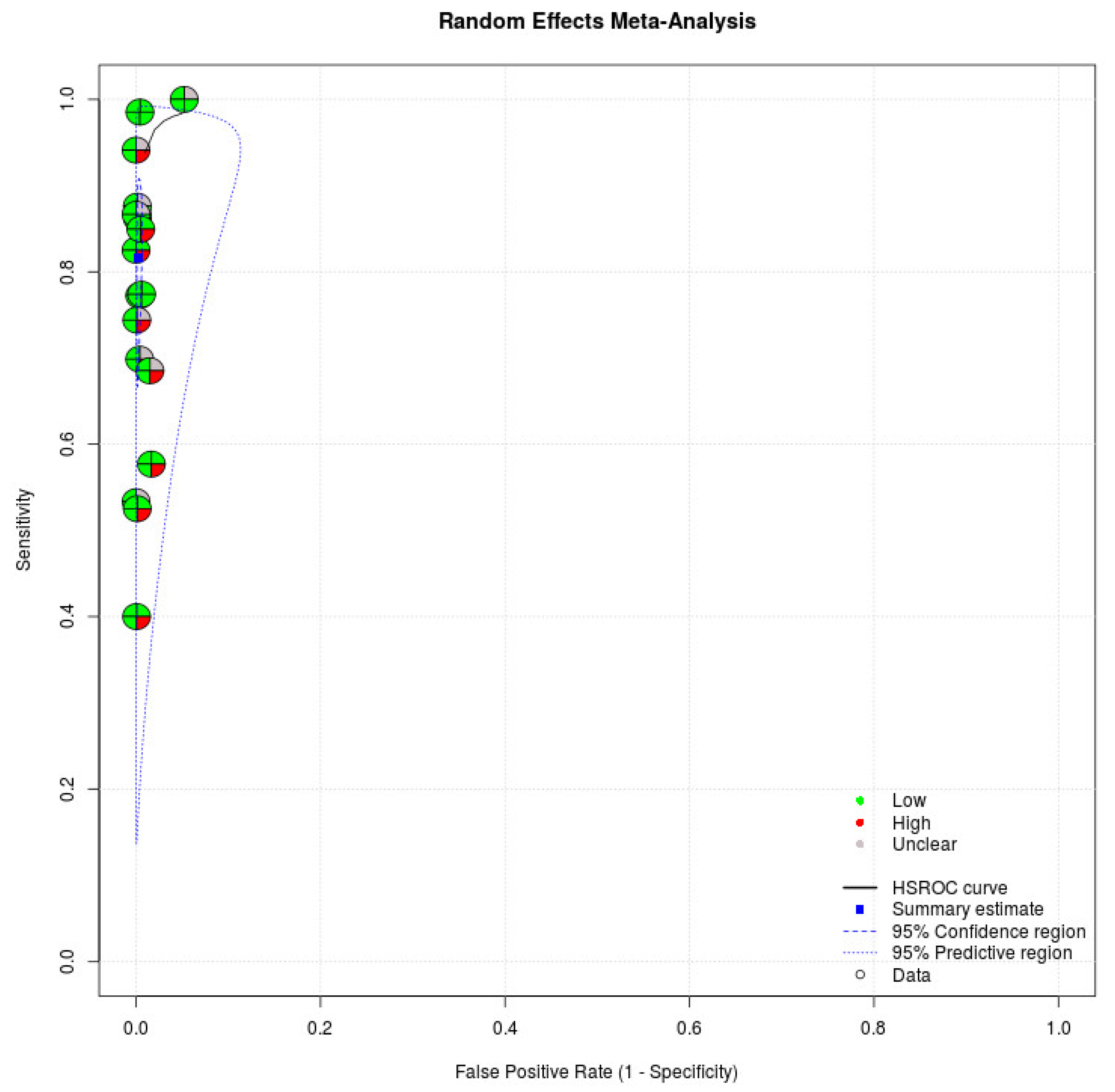
We applied the QUADAS-2, which has four domains to evaluate the quality of studies, in our meta-analysis. Regarding patient selection, eight studies enrolled patients randomly or consecutively. All studies avoided a case–control study design, which might have overestimated the diagnostic accuracy. Based on the rules in this domain, eight studies were judged to have a low risk of bias in the patient selection domain [16,17,18,19,28,30,31,33]. Regarding index tests, all studies recorded that index tests were interpreted without knowledge of the results of the reference standard. All studies in the meta-analysis were judged to have a low risk of bias in the index domain. Regarding the reference standard, all studies indicated that the reference standard likely correctly classified the target condition. Regarding the flow and timing domain, 17 studies demonstrated that all patients received a reference standard [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,32,33]. Seven studies indicated that all patients were included in the analysis [24,25,26,27,28,30,33]. Five articles were judged as having a low risk of bias in the flow and timing domain [24,26,27,28,33]. With regard to applicability, index tests and reference standards of studies in our meta-analysis matched our review title. Table 3 presents the quality of studies. Figure 3 demonstrates the risk of bias of individual studies in the meta-analysis.

Table 3.
Quality of studies.
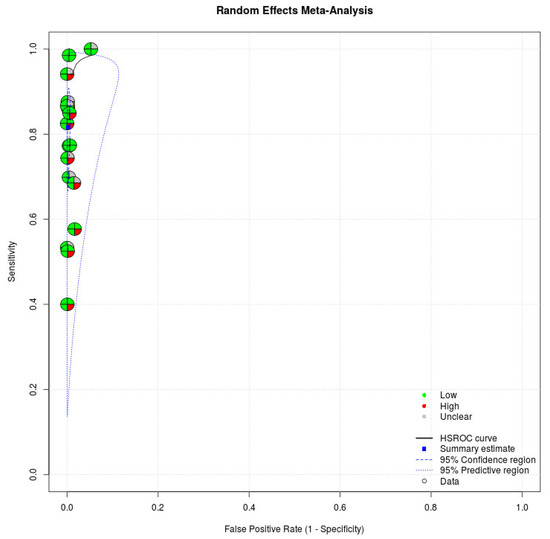
Figure 3.
Risk of bias of individual studies. Circles of the SROC plot in MetaDTA are displayed as pie charts summarizing the risk of bias of individual studies based on the QUADAS-2 tool. The first quadrant of a circle represents patient selection, the second quadrant represents the index test, the third quadrant represents the reference standard, and the fourth quadrant represents flow and timing. Circles on the SROC plot are colored depending on their quality assessment score: green for low, red for high, and gray for unclear risk of bias.
3.2. Investigation of Heterogeneity
Symptoms and the duration from symptom onset to specimen collection could represent sources of heterogeneity in the meta-analysis. We performed subgroup analyses to identify sources of heterogeneity. The I2 index represents heterogeneity across studies, with values of 25%, 50%, and 75% representing low, moderate, and high levels of heterogeneity, respectively [34]. According to the data of antigen tests for symptomatic patients, we performed a subgroup analysis for ten studies that reported outcomes for 5629 symptomatic participants [16,18,19,20,22,23,26,28,30,33]. This analysis generated a pooled sensitivity of 0.87 (95% CI: 0.78–0.93; I2 = 91.0%) and a pooled specificity of 1.00 (95% CI: 0.99–1.00; I2 = 81.7%). This indicates that antigen tests might have high sensitivity in the detection of COVID-19 among symptomatic participants. The subgroup analysis for nine studies that included 16,733 asymptomatic participants generated a pooled sensitivity of 0.57 (95% CI: 0.47–0.66; I2 = 85.0%) and a pooled specificity of 1.00 (95% CI: 0.99–1.00; I2 = 90.4%), respectively [16,17,19,21,22,23,28,29,30]. Based on the data of antigen tests for patients within 7 days after symptom onset, we performed another subgroup analysis. Three studies with 2046 patients in the meta-analysis reported data of antigen tests for participants within 7 days after symptom onset [22,28,33]. The subgroup analysis indicated a pooled sensitivity of 0.97 (95% CI: 0.69–1.00; I2 = 95.2%) and a pooled specificity of 1.00 (95% CI: 0.99–1.00; I2 = 63.3%), indicating that antigen tests might have higher pooled sensitivity in detecting SARS-CoV-2 in symptomatic patients with no more than 7 days of disease evolution. Five studies with 13,236 patients reported data of antigen tests using Ct cutoff value less than or equal to 35 [17,22,25,27,33]. The subgroup analysis indicated a pooled sensitivity of 0.93 (95% CI: 0.60–0.99; I2 = 97.3%) and a pooled specificity of 1.00 (95% CI: 0.98–1.00; I2 = 98.5%), indicating that antigen tests might have higher pooled sensitivity in detecting SARS-CoV-2 using a Ct cutoff value of 35. The supplementary information presents the statistical data of the subgroup analyses (see Table S2).
4. Discussion
Our major findings indicated that antigen tests had high sensitivity and excellent specificity in detecting SARS-CoV-2 in individuals in the community. If a test (in this case, an antigen test) has high specificity and yields a positive result, a clinician can be nearly certain that the disease (in this case, COVID-19) is present [35]. Antibody testing also plays a crucial role in understanding the seroprevalence of COVID-19 in the community and identifying individuals who are immunoreactive against SARS-CoV-2 [34]. RT-PCR is the standard diagnostic tool for SARS-CoV-2 detection. A previous study reported that RT-PCR positivity may persist over 3 weeks after illness onset, although most mild cases will yield a negative result. However, a positive RT-PCR result reveals only SARS-CoV-2 RNA and does not necessarily indicate the presence of a replicating virus [36].
Based on the subgroup analysis of our meta-analysis, antigen tests might have higher sensitivity in detecting SARS-CoV-2 in symptomatic individuals in the community, which indicates that antigen tests might be reliable for SARS-CoV-2 detection among the contagious population. In another subgroup analysis of studies involving asymptomatic participants, antigen tests had insufficient sensitivity in detecting SARS-CoV-2 in the asymptomatic population in the community. Surveillance testing regimens that can sever enough transmission chains to reduce community spread should complement current clinical diagnostic tests. Antigen tests could be used to enable true community-wide surveillance regimens for SARS-CoV-2 [37]. The current meta-analysis provided evidence of high sensitivity of antigen tests in identifying symptomatic individuals in the community. The antigen test is a valuable nonpharmaceutical intervention strategy to contain SARS-CoV-2. Recent research has suggested that when antigen tests are used, a pretest quarantine period of 5 days is not inferior to a quarantine of 10 days for travelers and a postexposure quarantine period of 10 days is not inferior to a quarantine of 14 days [38]. Gradual release of nonpharmaceutical interventions coupled with a high-efficacy vaccine strategy might prevent subsequent waves of SARS-CoV-2 transmission. Although vaccination can allow for some relaxation of nonpharmaceutical control measures, such relaxation should be performed gradually to avoid large-scale public health consequences [39].
Frequent use of antigen tests might help to identify infected individuals and reduce COVID-19 transmission [6] The benefits of administering antigen tests in suspected cases are the rapid diagnosis for clinical treatment and management (including protection of first-line staff) and the ability to quarantine infected individuals. Contact tracing becomes feasible so that positive cases can be isolated to minimize SARS-CoV-2 spread [40]. Diagnostic testing plays a key role in COVID-19 outbreak control. To end the pandemic, the accurate application of high-volume diagnostic testing and the rapid use of the results may help with the timely implementation of appropriate therapies and prevention of further spread [41]. Antigen tests could increase overall COVID-19 testing capacity and have the advantages of shorter turnaround times and reduced costs [42]. Antigen tests are most likely to have high performance in patients with high viral loads (Ct values ≤ 25), which usually appear in the presymptomatic (1–3 days before symptom onset) and early symptomatic (within the first 5–7 days of illness) phases of COVID-19 [43].
A study reported that a requirement to quarantine until an RT-PCR or antigen test on Day 7 after exposure (with early release if negative) might prevent as much transmission as the standard 14-day quarantine period [44]. Testing of asymptomatic health care workers has been suggested to reduce nosocomial transmission of COVID-19 [45]. Therefore, antigen tests can be used for screening and serial testing (every 2–3 days) of residents and staff in health care, home care, and long-term care facilities in areas where there is ongoing community transmission. When a first case is confirmed in a resident or staff member of a closed setting, a comprehensive testing strategy of all residents and staff should be considered [42].
Different testing strategies, including focused symptomatic testing, focused asymptomatic testing, mass testing, and systematic meaningful asymptomatic repeated testing, are adopted to prevent transmission. Many of these strategies use antigen tests [46]. Widespread community transmission has become entrenched in many countries and has required the testing of populations to identify and isolate infected individuals. Although the effects of mass antigen testing are difficult to distinguish from those of concurrent interventions, an obvious reduction in SARS-CoV-2 infections was observed after mass antigen testing in Slovakia. However, this reduction was restricted to regions with high SARS-CoV-2 prevalence, and testing had little effect in areas with lower viral prevalence [47]. Due to an increasing prevalence of new SARS-CoV2 variants with possible clinical implications, monitoring and detecting the spread of different variants in the general population in a timely method is critical [48]. Antigen testing is evolving. Sensitivity of SARS-the CoV-2 antigen test with a self-collected nasal swab is comparable with that of a professional-collected nasopharyngeal swab. Patients suspected of COVID-19 may be able to perform the antigen test and test by themselves [18]. Based on the outcomes of the meta-analysis, antigen tests might have higher sensitivity in symptomatic patients. Hence, we suggested that RT-PCR could be performed after negative antigen test results in symptomatic patients and positive antigen test results in asymptomatic patients in the community transmission screening algorithm for SARS-CoV-2 [8].
Although this meta-analysis demonstrated that antigen tests may be sensitive in detecting SARS-CoV-2 in the community, our study had limitations. The Ct cutoff values of the studies in the meta-analysis were limited. Statistical data of antigen tests stratified by Ct cutoff value are limited. Statistical data of antigen tests for patients under 18 years of age are limited. The majority of studies in this meta-analysis did not report the days after symptom onset of participants. No study in the meta-analysis reported SARS-CoV-2 variants.
5. Conclusions
Our major findings indicated that antigen tests had high sensitivity in detecting the SARS-CoV-2 virus in symptomatic patients in the community. Antigen tests might have a higher sensitivity in detecting SARS-CoV-2 within 7 days after symptom onset. Antigen tests are sensitive in detecting SARS-CoV-2 in patients with a Ct value less than or equal to 35. Therefore, antigen tests might be an effective tool in the effort to block SARS-CoV-2 transmission.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph182111451/s1, Figure S1: Sensitivities and specificities of included studies. Table S1: Search strategy. Table S2: Statistical data of subgroup analyses.
Author Contributions
Conceptualization, C.-C.C.; Data curation, C.-C.C., C.-H.B. and Y.-H.W.; Formal analysis, C.-C.C., C.-H.B. and Y.-H.W.; Investigation, K.-Y.L. and Y.-H.W.; Methodology, C.-H.B., K.-Y.L. and Y.-H.W.; Supervision, Y.-H.W.; Validation, Y.-H.W.; Writing—original draft, S.-C.L. and P.-Y.W.; Writing—review and editing, S.-C.L., P.-Y.W. and K.-Y.L. These authors contributed equally to this work: C.-C.C., S.-C.L. and C.-H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the grants from Shuang Ho Hospital, Taipei Medical University (Grant no.: 109TMU-SHH-22) and the Featured Research Program “Establishment of Tucheng Health Care Cohort” (grant no. 109FRP-01; 110FRP-01) from Shuang Ho Hospital, Taipei Medical University.
Institutional Review Board Statement
The present study is a meta-analysis for examining the effect sizes reported in previously published literature. Therefore, this study was exempt from the Institutional Review Board (IRB) review.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Acknowledgments
We thank the Graduate Institute of Clinical Medicine and Shuang Ho Hospital of Taipei Medical University for technical support in statistical analyses and information collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef]
- Liu, Y.; Morgenstern, C.; Kelly, J.; Lowe, R.; CMMID COVID-19 Working Group; Jit, M. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med. 2021, 19, 40. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef]
- Watson, J.; Whiting, P.F.; Brush, J.E. Interpreting a COVID-19 test result. BMJ 2020, 369, m1808. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Pepper, G.; Naccache, S.N.; Huang, M.L.; Jerome, K.R.; Greninger, A.L. Comparison of Commercially Available and Laboratory-Developed Assays for In Vitro Detection of SARS-CoV-2 in Clinical Laboratories. J. Clin. Microbiol. 2020, 58, e00821-20. [Google Scholar] [CrossRef] [PubMed]
- Toptan, T.; Eckermann, L.; Pfeiffer, A.E.; Hoehl, S.; Ciesek, S.; Drosten, C.; Corman, V.M. Evaluation of a SARS-CoV-2 rapid antigen test: Potential to help reduce community spread? J. Clin. Virol. 2021, 135, 104713. [Google Scholar] [CrossRef] [PubMed]
- Interim Guidance for Antigen Testing for SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed on 20 June 2021).
- Pray, I.W.; Ford, L.; Cole, D.; Lee, C.; Bigouette, J.P.; Abedi, G.R.; Bushman, D.; Delahoy, M.J.; Currie, D.; Cherney, B.; et al. Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses—Wisconsin, September–October 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 69, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling up COVID-19 rapid antigen tests: Promises and challenges. Lancet Infect. Dis. 2021, 21, e290–e295. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Takwoingi, Y.; Riley, R.D.; Deeks, J.J. Meta-analysis of diagnostic accuracy studies in mental health. Evid. Based Ment. Health 2015, 18, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, C.; Leeflang, M.M.; Minion, J.; Brewer, T.; Pai, M. Accuracy of rapid influenza diagnostic tests: A meta-analysis. Ann. Intern. Med. 2012, 156, 500–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef]
- Freeman, S.C.; Kerby, C.R.; Patel, A.; Cooper, N.J.; Quinn, T.; Sutton, A.J. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med. Res. Methodol. 2019, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; Tran, K.; Jacobs, J.R.; Cranston, A.E.; Smith, S.; O’Kane, C.Y.; Roady, T.J.; Moran, A.; Scarry, A.; Carroll, M.; et al. Performance and Operational Evaluation of the Access Bio CareStart Rapid Antigen Test in a High-Throughput Drive-Through Community Testing Site in Massachusetts. Open Forum. Infect. Dis. 2021, 8, ofab243. [Google Scholar] [CrossRef]
- García-Fiñana, M.; Hughes, D.M.; Cheyne, C.P.; Burnside, G.; Stockbridge, M.; Fowler, T.A.; Fowler, V.L.; Wilcox, M.H.; Semple, M.G.; Buchan, I. Performance of the Innova SARS-CoV-2 antigen rapid lateral flow test in the Liverpool asymptomatic testing pilot: Population based cohort study. BMJ 2021, 374, n1637. [Google Scholar] [CrossRef]
- Lindner, A.K.; Nikolai, O.; Rohardt, C.; Kausch, F.; Wintel, M.; Gertler, M.; Burock, S.; Hörig, M.; Bernhard, J.; Tobian, F.; et al. Diagnostic accuracy and feasibility of patient self-testing with a SARS-CoV-2 antigen-detecting rapid test. J. Clin. Virol. 2021, 141, 104874. [Google Scholar] [CrossRef]
- Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F.; Gottschalk, C.; Klein, J.A.F.; Schnitzler, P.; Kräusslich, H.G.; Nikolai, O.; Lindner, A.K.; et al. The Abbott PanBio WHO emergency use listed, rapid, antigen-detecting point-of-care diagnostic test for SARS-CoV-2-Evaluation of the accuracy and ease-of-use. PLoS ONE 2021, 16, e0247918. [Google Scholar] [CrossRef]
- Van der Moeren, N.; Zwart, V.F.; Lodder, E.B.; Van den Bijllaardt, W.; Van Esch, H.R.J.M.; Stohr, J.J.J.M.; Pot, J.; Welschen, I.; Van Mechelen, P.M.F.; Pas, S.D.; et al. Evaluation of the test accuracy of a SARS-CoV-2 rapid antigen test in symptomatic community dwelling individuals in the Netherlands. PLoS ONE 2021, 16, e0250886. [Google Scholar]
- Peña, M.; Ampuero, M.; Garcés, C.; Gaggero, A.; García, P.; Velasquez, M.S.; Luza, R.; Alvarez, P.; Paredes, F.; Acevedo, J.; et al. Performance of SARS-CoV-2 rapid antigen test compared with real-time RT-PCR in asymptomatic individuals. Int. J. Infect. Dis. 2021, 107, 201–204. [Google Scholar] [CrossRef]
- Shah, M.M.; Salvatore, P.P.; Ford, L.; Kamitani, E.; Whaley, M.J.; Mitchell, K.; Currie, D.W.; Morgan, C.N.; Segaloff, H.E.; Lecher, S.; et al. Performance of Repeat BinaxNOW Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Testing in a Community Setting, Wisconsin, November 2020–December 2020. Clin. Infect. Dis. 2021, 73, S54–S57. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Lee, C.; Pray, I.W.; Cole, D.; Bigouette, J.P.; Abedi, G.R.; Bushman, D.; Delahoy, M.J.; Currie, D.W.; Cherney, B.; et al. Epidemiologic characteristics associated with SARS-CoV-2 antigen-based test results, rRT-PCR cycle threshold values, subgenomic RNA, and viral culture results from university testing. Clin. Infect. Dis. 2021, 73, e1348–e1355. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Nsoga, M.T.N.; Perez-Rodriguez, F.J.; Aad, Y.A.; Sattonnet-Roche, P.; Gayet-Ageron, A.; Jaksic, C.; Torriani, G.; Boehm, E.; Kronig, I.; et al. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS ONE 2021, 16, e0248921. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.; Berenger, B.M.; Portnoy, D.; Scott, B.; Szelewicki, J.; Singh, T.; Venner, A.A.; Turnbull, L.; Pabbaraju, K.; Shokoples, S.; et al. Clinical performance of the Abbott Panbio with nasopharyngeal, throat, and saliva swabs among symptomatic individuals with COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Akashi, Y.; Kato, D.; Kuwahara, M.; Muramatsu, S.; Ueda, A.; Notake, S.; Nakamura, K.; Ishikawa, H.; Suzuki, H. The evaluation of a newly developed antigen test (QuickNavi™-COVID19 Ag) for SARS-CoV-2: A prospective observational study in Japan. J. Infect. Chemother. 2021, 27, 890–894. [Google Scholar] [CrossRef]
- Gili, A.; Paggi, R.; Russo, C.; Cenci, E.; Pietrella, D.; Graziani, A.; Stracci, F.; Mencacci, A. Evaluation of Lumipulse® G SARS-CoV-2 antigen assay automated test for detecting SARS-CoV-2 nucleocapsid protein (NP) in nasopharyngeal swabs for community and population screening. Int. J. Infect. Dis. 2021, 105, 391–396. [Google Scholar] [CrossRef]
- Pollock, N.R.; Jacobs, J.R.; Tran, K.; Cranston, A.E.; Smith, S.; O’Kane, C.Y.; Roady, T.J.; Moran, A.; Scarry, A.; Carroll, M.; et al. Performance and Implementation Evaluation of the Abbott BinaxNOW Rapid Antigen Test in a High-Throughput Drive-Through Community Testing Site in Massachusetts. J. Clin. Microbiol. 2021, 59, e00083-21. [Google Scholar] [CrossRef] [PubMed]
- Okoye, N.C.; Barker, A.P.; Curtis, K.; Orlandi, R.R.; Snavely, E.A.; Wright, C.; Hanson, K.E.; Pearson, L.N. Performance Characteristics of BinaxNOW COVID-19 Antigen Card for Screening Asymptomatic Individuals in a University Setting. J. Clin. Microbiol. 2021, 59, e03282-20. [Google Scholar] [CrossRef]
- Prince-Guerra, J.L.; Almendares, O.; Nolen, L.D.; Gunn, J.K.L.; Dale, A.P.; Buono, S.A.; Deutsch-Feldman, M.; Suppiah, S.; Hao, L.; Zeng, Y.; et al. Evaluation of Abbott BinaxNOW Rapid Antigen Test for SARS-CoV-2 Infection at Two Community-Based Testing Sites—Pima County, Arizona, 3–17 November 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Igloi, Z.; Velzing, J.; van Beek, J.; van de Vijver, D.; Aron, G.; Ensing, R.; Benschop, K.; Han, W.; Boelsums, T.; Koopmans, M.; et al. Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands. Emerg. Infect. Dis. 2021, 27, 1323–1329. [Google Scholar] [CrossRef]
- Landaas, E.T.; Storm, M.L.; Tollånes, M.C.; Barlinn, R.; Kran, A.B.; Bragstad, K.; Christensen, A.; Andreassen, T. Diagnostic performance of a SARS-CoV-2 rapid antigen test in a large, Norwegian cohort. J. Clin. Virol. 2021, 137, 104789. [Google Scholar] [CrossRef] [PubMed]
- Pilarowski, G.; Marquez, C.; Rubio, L.; Peng, J.; Martinez, J.; Black, D.; Chamie, G.; Jones, D.; Jacobo, J.; Tulier-Laiwa, V.; et al. Field performance and public health response using the BinaxNOW TM Rapid SARS-CoV-2 antigen detection assay during community-based testing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Baeyens, J.P.; Serrien, B.; Goossens, M.; Clijsen, R. Questioning the “SPIN and SNOUT” rule in clinical testing. Arch. Physiother. 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking COVID-19 Test Sensitivity—A Strategy for Containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef]
- Van der Toorn, W.; Oh, D.Y.; Bourquain, D.; Michel, J.; Krause, E.; Nitsche, A.; von Kleist, M.; Working Group on SARS-CoV-2 Diagnostics at RKI. An intra-host SARS-CoV-2 dynamics model to assess testing and quarantine strategies for incoming travelers, contact management, and de-isolation. Patterns 2021, 2, 100262. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Hill, E.M.; Tildesley, M.J.; Dyson, L.; Keeling, M.J. Vaccination and non-pharmaceutical interventions for COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2021, 21, 793–802. [Google Scholar] [CrossRef]
- Bhopal, R.S. COVID-19 zugzwang: Potential public health moves towards population (herd) immunity. Public Health Pract. 2020, 1, 100031. [Google Scholar] [CrossRef]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef]
- Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. Available online: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-COVID-19-eueea-and-uk (accessed on 20 June 2021).
- WHO. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays: Interim Guidance. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-SARS-CoV-2infection-using-rapid-immunoassays (accessed on 20 June 2021).
- Quilty, B.J.; Clifford, S.; Hellewell, J.; Russell, T.W.; Kucharski, A.J.; Flasche, S.; Edmunds, W.J.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. Quarantine and testing strategies in contact tracing for SARS-CoV-2: A modelling study. Lancet Public Health 2021, 6, e175–e183. [Google Scholar] [CrossRef]
- Chow, A.; Htun, H.L.; Kyaw, W.M.; Lee, L.T.; Ang, B. Asymptomatic health-care worker screening during the COVID-19 pandemic. Lancet 2020, 396, 1393–1394. [Google Scholar] [CrossRef]
- Crozier, A.; Rajan, S.; Buchan, I.; McKee, M. Put to the test: Use of rapid testing technologies for COVID-19. BMJ 2021, 372, n208. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Salit, M. Testing at scale during the COVID-19 pandemic. Nat. Rev. Genet. 2021, 22, 415–426. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Mancini, P.; Bonanno Ferraro, G.; Veneri, C.; Iaconelli, M.; Lucentini, L.; Bonadonna, L.; Brusaferro, S.; Brandtner, D.; Fasanella, A.; et al. Rapid screening for SARS-CoV-2 variants of concern in clinical and environmental samples using nested RT-PCR assays targeting key mutations of the spike protein. Water Res. 2021, 197, 117104. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).