Impact of the State of Emergency during the COVID-19 Pandemic in 2020 on Asthma Exacerbations among Children in Kobe City, Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Meteorological Data
2.3. Air Pollutants Data
2.4. Pollen Data
2.5. Statistics
3. Results
3.1. Patient Demographics
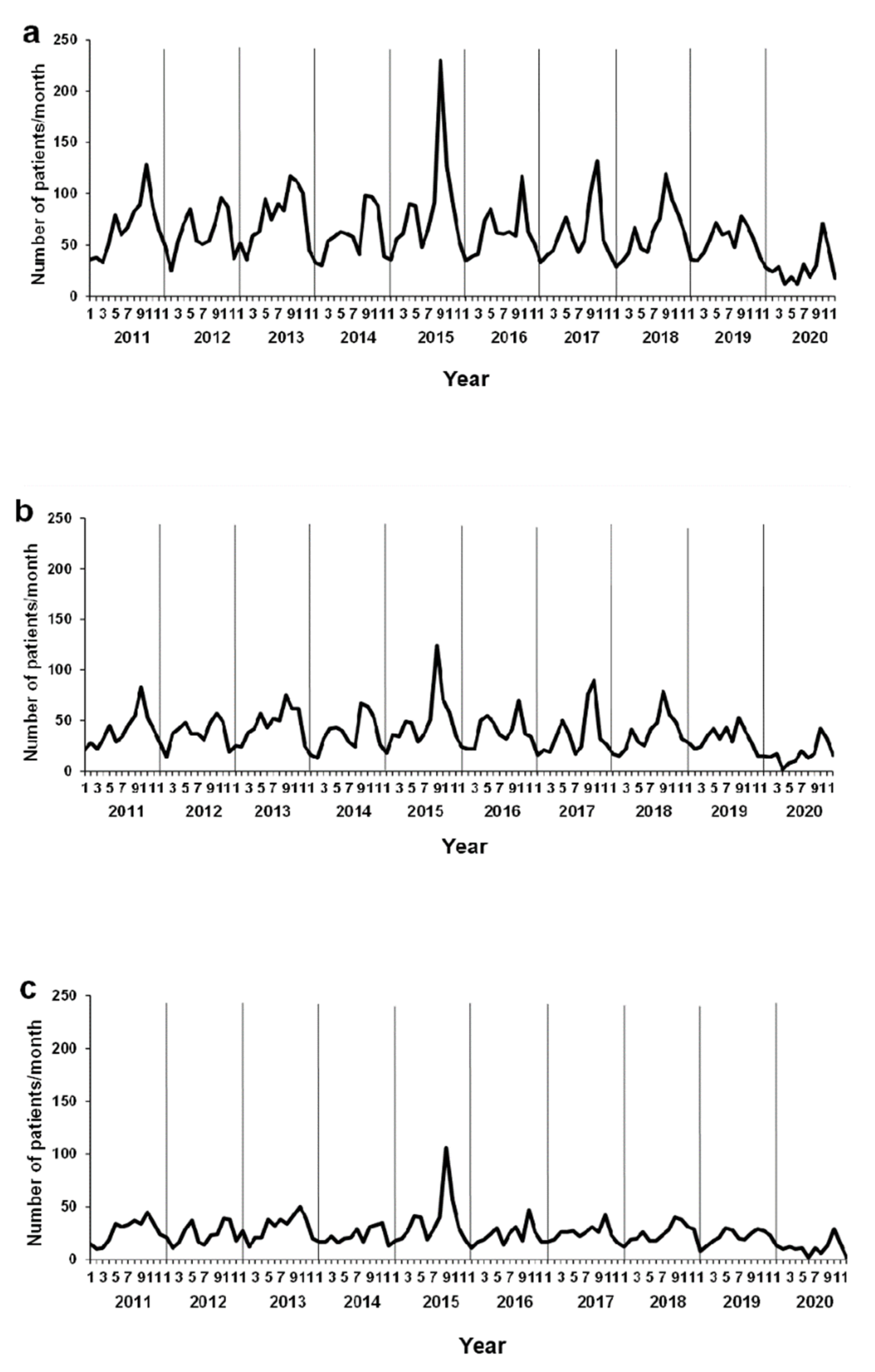
3.2. Monthly Changes in the Number of Patients with AE in Kobe, Japan
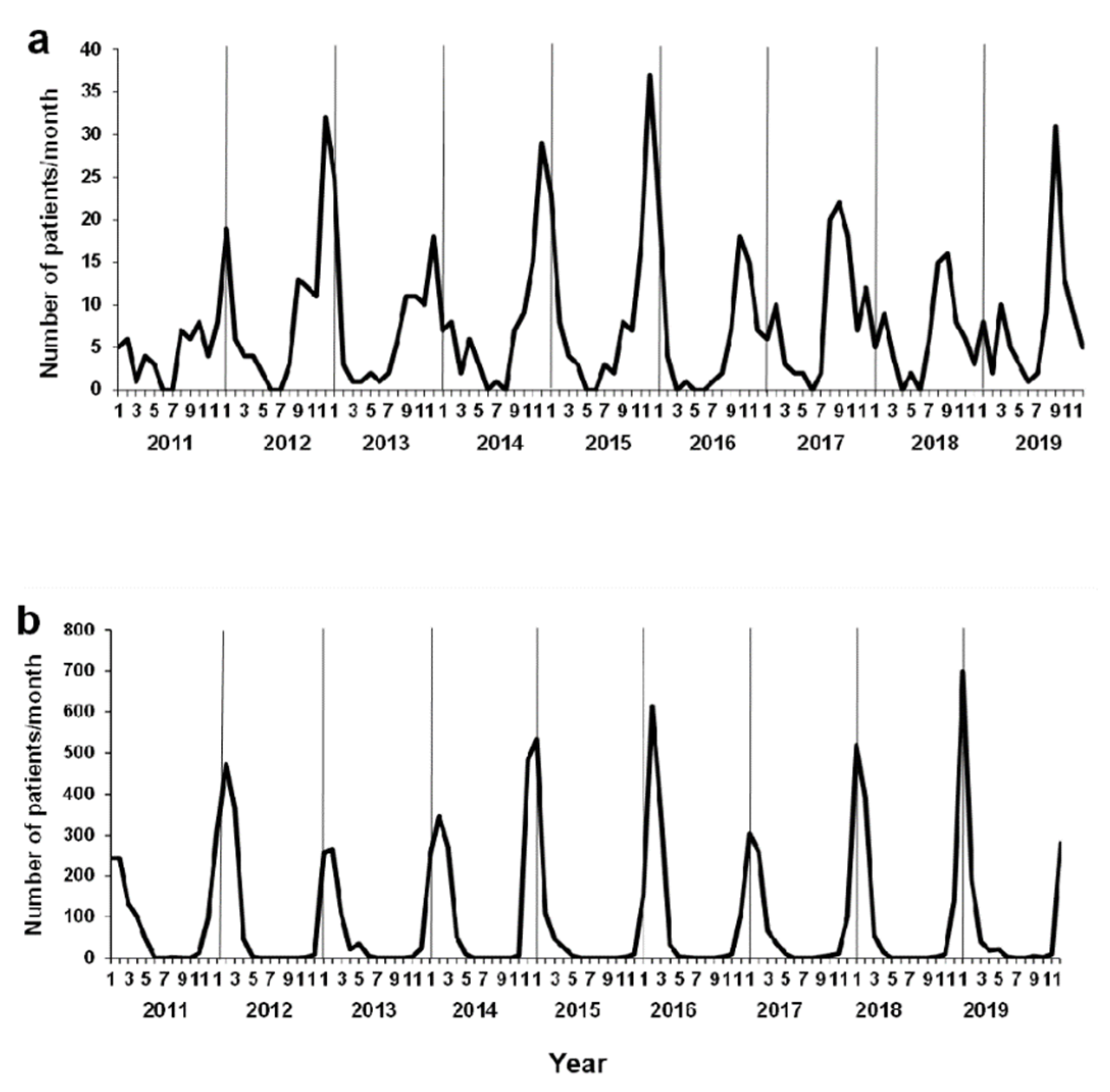
3.3. Background of Environmental Factors and Viral Infections
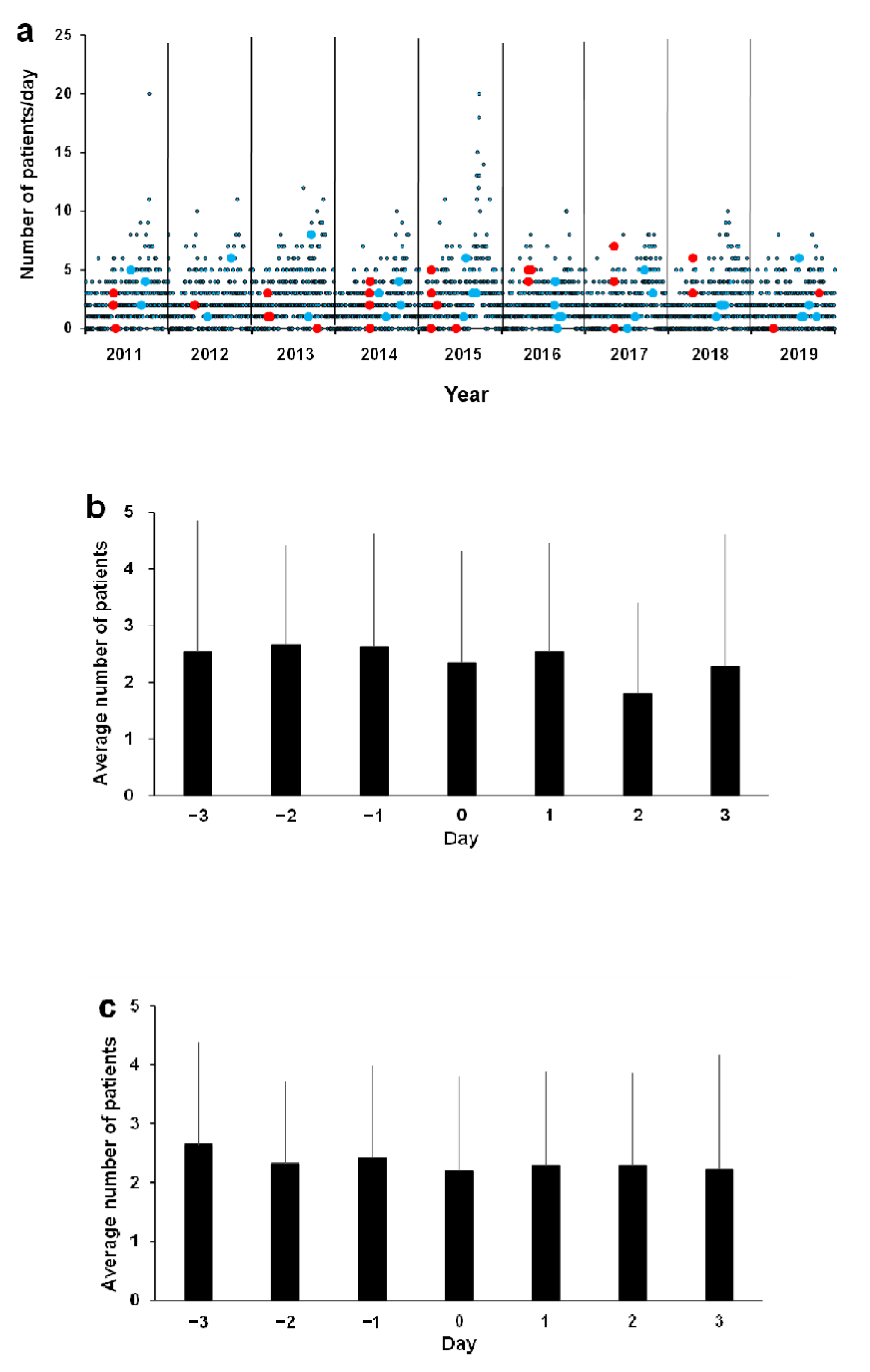
3.4. Association between Asthma ED Visits and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busse, W.W.; Lemanske, R.F., Jr. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef]
- Feddema, J.J.; Claassen, E. Prevalence of viral respiratory infections amongst asthmatics: Results of a meta-regression analysis. Respir. Med. 2020, 173, 106020. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, I.; Grayson, M.H. Asthma and viral infections: An intricate relationship. Ann. Allergy Asthma Immunol. 2019, 123, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques Mejías, M.A.; Tomas Pérez, M.; Hernández, I.; López, I.; Quirce, S. Asthma exacerbations in the pediatric emergency department at a tertiary hospital: Association with environmental factors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 365–370. [Google Scholar] [CrossRef]
- Lovinsky-Desir, S.; Acosta, L.M.; Rundle, A.G.; Miller, R.L.; Goldstein, I.F.; Jacobson, J.S.; Chillrud, S.N.; Perzanowski, M.S. Air pollution, urgent asthma medical visits and the modifying effect of neighborhood asthma prevalence. Pediatr. Res. 2019, 85, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Yon, D.K.; James, C.C.; Lee, S.; Koh, H.Y.; Sheen, Y.H.; Oh, J.W.; Han, M.Y.; Sugihara, G. Short-term effects of multiple outdoor environmental factors on risk of asthma exacerbations: Age-stratified time-series analysis. J. Allergy Clin. Immunol. 2019, 144, 1542–1550.e1. [Google Scholar] [CrossRef] [Green Version]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Seminar Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Kantor, D.B.; Phipatanakul, W.; Hirschhorn, J.N. Gene-environment interactions associated with the severity of acute asthma exacerbation in children. Am. J. Respir. Crit. Care Med. 2018, 197, 545–547. [Google Scholar] [CrossRef]
- Ueda, K.; Nitta, H.; Odajima, H. The effects of weather, air pollutants, and Asian dust on hospitalization for asthma in Fukuoka. Environ. Health Prev. Med. 2010, 15, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Tokuda, Y.; Ohde, S.; Ishimatsu, S.; Nakamura, T.; Birrer, R.B. The relationship of short-term air pollution and weather to ED visits for asthma in Japan. Am. J. Emerg. Med. 2009, 27, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mohr, L.B.; Luo, S.; Mathias, E.; Tobing, R.; Homan, S.; Sterling, D. Influence of season and temperature on the relationship of elemental carbon air pollution to pediatric asthma emergency room visits. J. Asthma 2008, 45, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Deng, Q.; Lu, C.; Qian, H.; Yang, X.; et al. Asthma and allergic rhinitis among young parents in China in relation to outdoor air pollution, climate and home environment. Sci. Total Environ. 2021, 751, 141734. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Schinasi, L.H.; Kenyon, C.C.; Moore, K.; Melly, S.; Hubbard, R.A.; Zhao, Y.; Diez Roux, A.V.; Forrest, C.B.; Maltenfort, M.; et al. Effects of ambient air pollution on childhood asthma exacerbation in the Philadelphia metropolitan Region, 2011–2014. Environ. Res. 2021, 197, 110955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Chen, Z.; Zu, B.; Zhao, Y. Effects of variations in meteorological factors on daily hospital visits for asthma: A time-series study. Environ. Res. 2020, 182, 109115. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, J.; Jiang, F.; Liu, S.; Li, S.; Tan, J.; Yin, Y.; Tong, S. Season-stratified effects of meteorological factors on childhood asthma in Shanghai, China. Environ. Res. 2020, 191, 110115. [Google Scholar] [CrossRef]
- Chang, Q.; Liu, S.; Chen, Z.; Zu, B.; Zhang, H. Association between air pollutants and outpatient and emergency hospital visits for childhood asthma in Shenyang city of China. Int. J. Biometeorol. 2020, 64, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Phelan, A.L.; Katz, R.; Gostin, L.O. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA 2020, 323, 709–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz-Muñoz, I.; Tamames-Gómez, S.; Castrodeza-Sanz, J.; Eiros-Bouza, J.M.; de Lejarazu-Leonardo, R.O. Social distancing, lockdown and the wide use of mask: A magic solution or a double-edged sword for respiratory viruses epidemiology? Vaccines 2021, 9, 595. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, V. Environmental impacts of coronavirus disease 2019 (COVID-19). Bioresour. Technol. Rep. 2021, 15, 100744. [Google Scholar] [CrossRef] [PubMed]
- Gouda, K.C.; Singh, P.; Nikhilasuma, P.; Benke, M.; Kumari, R.; Agnihotri, G.; Hungund, K.M.; Chandrika, M.; Kantha Rao, B.; Ramesh, V.; et al. Assessment of air pollution status during COVID-19 lockdown (March-May 2020) over Bangalore City in India. Environ. Monit. Assess. 2021, 193, 395. [Google Scholar] [CrossRef]
- Simoneau, T.; Greco, K.F.; Hammond, A.; Nelson, K.; Gaffin, J.M. Impact of the COVID-19 pandemic on pediatric emergency department use for asthma. Ann. Am. Thorac. Soc. 2021, 18, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.S.; Baggio, D.M.; Fascina, L.P.; do Prado, C. Impact of social isolation due to COVID-19 on the seasonality of pediatric respiratory diseases. PLoS ONE 2020, 15, e0243694. [Google Scholar] [CrossRef]
- Krivec, U.; Kofol Seliger, A.; Tursic, J. COVID-19 lockdown dropped the rate of paediatric asthma admissions. Arch. Dis. Child. 2020, 105, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.C.; Hill, D.A.; Henrickson, S.E.; Bryant-Stephens, T.C.; Zorc, J.J. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J. Allergy Clin. Immunol. Pract. 2020, 8, 2774–2776.e1. [Google Scholar] [CrossRef]
- Abe, K.; Miyawaki, A.; Nakamura, M.; Ninomiya, H.; Kobayashi, Y. Trends in hospitalizations for asthma during the COVID-19 outbreak in Japan. J. Allergy Clin. Immunol. Pract. 2021, 9, 494–496.e1. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Hamasaki, Y.; Kohno, Y.; Ebisawa, M.; Kondo, N.; Nishima, S.; Nishimuta, T.; Morikawa, A. Japanese guidelines for childhood asthma 2017. Allergol. Int. 2017, 66, 190–204. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nozu, K.; Ishiko, S.; Nagase, H.; Ninchoji, T.; Nagano, C.; Takeda, H.; Unzaki, A.; Ishibashi, K.; Morioka, I.; et al. Multivariate analysis of the impact of weather and air pollution on emergency department visits for nighttime headaches among children: Retrospective, clinical observational study. BMJ Open 2021, 11, e046520. [Google Scholar] [CrossRef]
- Moelling, K.; Broecker, F. Air microbiome and pollution: Composition and potential effects on human health, including SARS coronavirus infection. J. Environ. Public Health 2020, 2020, 1646943. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Korematsu, S.; Nagashima, K.; Sato, Y.; Nagao, M.; Hasegawa, S.; Nakamura, H.; Sugiura, S.; Miura, K.; Okada, K.; Fujisawa, T. “Spike” in acute asthma exacerbations during enterovirus D68 epidemic in Japan: A nation-wide survey. Allergol. Int. 2018, 67, 55–60. [Google Scholar] [CrossRef]
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. Air pollution declines during COVID-19 lockdowns mitigate the global health burden. Environ. Res. 2021, 192, 110403. [Google Scholar] [CrossRef]
- Perera, F.; Berberian, A.; Cooley, D.; Shenaut, E.; Olmstead, H.; Ross, Z.; Matte, T. Potential health benefits of sustained air quality improvements in New York City: A simulation based on air pollution levels during the COVID-19. Environ. Res. 2021, 193, 110555. [Google Scholar] [CrossRef]
- Miyazaki, K.; Bowman, K.; Sekiya, T.; Jiang, Z.; Chen, X.; Eskes, H.; Ru, M.; Zhang, Y.; Shindell, D. Air quality response in China linked to the 2019 novel coronavirus (COVID-19) lockdown. Geophys. Res. Lett. 2020, 47, e2020GL089252. [Google Scholar] [CrossRef] [PubMed]
- Chavasse, R.J. COVID-19: Reduced asthma presentations in children. BMJ 2020, 370, m2806. [Google Scholar] [CrossRef]
- Levene, R.; Fein, D.M.; Silver, E.J.; Joels, J.R.; Khine, H. The ongoing impact of COVID-19 on asthma and pediatric emergency health-seeking behavior in the Bronx, an epicenter. Am. J. Emerg. Med. 2021, 43, 109–114. [Google Scholar] [CrossRef]
- Sheehan, W.J.; Patel, S.J.; Margolis, R.H.F.; Fox, E.R.; Shelef, D.Q.; Kachroo, N.; Pillai, D.; Teach, S.J. Pediatric asthma exacerbations during the COVID-19 pandemic: Absence of the typical fall seasonal spike in Washington, DC. J. Allergy Clin. Immunol. Pract. 2021, 9, 2073–2076. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Hughes, K.M.; Thien, F.; Suphioglu, C. Epidemic thunderstorm asthma: Lessons learned from the storm down-under. J. Allergy Clin. Immunol. Pract. 2021, 9, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ma, P.; Wu, Y.; Ma, Y.; Yang, X.; Li, Y.; Deng, Q. High and low temperatures aggravate airway inflammation of asthma: Evidence in a mouse model. Environ. Pollut. 2020, 256, 113433. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Annesi Maesano, I.; Molino, A.; Vitale, C.; D’Amato, M. Thunderstorm-related asthma attacks. J. Allergy Clin. Immunol. 2017, 139, 1786–1787. [Google Scholar] [CrossRef] [Green Version]
- Grinn-Gofroń, A.; Strzelczak, A. Changes in concentration of Alternaria and Cladosporium spores during summer storms. Int. J. Biometeorol. 2013, 57, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.E.; Flagan, R.C.; Valenta, R.; Glovsky, M.M. Release of allergens as respirable aerosols: A link between grass pollen and asthma. J. Allergy Clin. Immunol. 2002, 109, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Caminati, M.; Senna, G.; Vianello, A.; Ricci, G.; Bellamoli, R.; Longhi, S.; Crivellaro, M.; Marcer, G.; Monai, M.; Passalacqua, G. Thunderstorm asthma: A critical appraisal based on clinical practice. J. Investig. Allergol. Clin. Immunol. 2018, 28, 273–275. [Google Scholar] [CrossRef]
- Bouazza, N.; Foissac, F.; Urien, S.; Guedj, R.; Carbajal, R.; Tréluyer, J.M.; Chappuy, H. Fine particulate pollution and asthma exacerbations. Arch. Dis. Child. 2018, 103, 828–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orellano, P.; Quaranta, N.; Reynoso, J.; Balbi, B.; Vasquez, J. Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS ONE 2017, 12, e0174050. [Google Scholar] [CrossRef]
- O’Connor, G.T.; Neas, L.; Vaughn, B.; Kattan, M.; Mitchell, H.; Crain, E.F.; Evans, R., 3rd; Gruchalla, R.; Morgan, W.; Stout, J.; et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J. Allergy Clin. Immunol. 2008, 121, 1133–1139.e1. [Google Scholar] [CrossRef] [Green Version]
- Spira-Cohen, A.; Chen, L.C.; Kendall, M.; Lall, R.; Thurston, G.D. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx schoolchildren with asthma. Environ. Health Perspect. 2011, 119, 559–565. [Google Scholar] [CrossRef]
- Samoli, E.; Nastos, P.T.; Paliatsos, A.G.; Katsouyanni, K.; Priftis, K.N. Acute effects of air pollution on pediatric asthma exacerbation: Evidence of association and effect modification. Environ. Res. 2011, 111, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.B. Mechanisms of pollution-induced airway disease: In vivo studies. Allergy 1997, 52, 37–44. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Chan, C.K.; Wu, C.Y.; Phan, D.V.; Chan, C.L. The short-term effects of ambient air pollutants on childhood asthma hospitalization in Taiwan: A national study. Int. J. Environ. Res. Public Health 2019, 16, 203. [Google Scholar] [CrossRef] [Green Version]
- Strickland, M.J.; Darrow, L.A.; Klein, M.; Flanders, W.D.; Sarnat, J.A.; Waller, L.A.; Sarnat, S.E.; Mulholland, J.A.; Tolbert, P.E. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am. J. Respir. Crit. Care Med. 2010, 182, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Tétreault, L.F.; Doucet, M.; Gamache, P.; Fournier, M.; Brand, A.; Kosatsky, T.; Smargiassi, A. Severe and moderate asthma exacerbations in asthmatic children and exposure to ambient air pollutants. Int. J. Environ. Res. Public Health 2016, 13, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, M.; Meng, Y.Y.; Rull, R.P.; English, P.; Balmes, J.; Ritz, B. Environmental public health tracking of childhood asthma using California health interview survey, traffic, and outdoor air pollution data. Environ. Health Perspect. 2008, 116, 1254–1260. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, H.; Kweon, J. Hourly differences in air pollution on the risk of asthma exacerbation. Environ. Pollut. 2015, 203, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Shankardass, K.; McConnell, R.; Jerrett, M.; Milam, J.; Richardson, J.; Berhane, K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc. Natl. Acad. Sci. USA 2009, 106, 12406–12411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clougherty, J.E.; Levy, J.I.; Kubzansky, L.D.; Ryan, P.B.; Suglia, S.F.; Canner, M.J.; Wright, R.J. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ. Health Perspect. 2007, 115, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.Y.; Tsai, S.S.; Chang, C.C.; Ho, S.C. Effects of Asian dust storm events on daily admissions for asthma in Taipei, Taiwan. Inhal. Toxicol. 2005, 17, 817–821. [Google Scholar] [CrossRef]
- Nakamura, T.; Nishiwaki, Y.; Hashimoto, K.; Takeuchi, A.; Kitajima, T.; Komori, K.; Tashiro, K.; Hasunuma, H.; Ueda, K.; Shimizu, A.; et al. Association between Asian dust exposure and respiratory function in children with bronchial asthma in Nagasaki Prefecture, Japan. Environ. Health Prev. Med. 2020, 25, 8. [Google Scholar] [CrossRef]
- Merckx, J.; Ducharme, F.M.; Martineau, C.; Zemek, R.; Gravel, J.; Chalut, D.; Poonai, N.; Quach, C. Respiratory viruses and treatment failure in children with asthma exacerbation. Pediatrics 2018, 142, e20174105. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, A.; Sakai, K. Dispersal of allergenic pollen from Cryptomeria japonica and Chamaecyparis obtusa: Characteristic annual fluctuation patterns caused by intermittent phase synchronisations. Sci. Rep. 2019, 9, 11479. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F., Jr.; Gern, J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Bizzintino, J.; Lee, W.M.; Laing, I.A.; Vang, F.; Pappas, T.; Zhang, G.; Martin, A.C.; Khoo, S.K.; Cox, D.W.; Geelhoed, G.C.; et al. Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 2011, 37, 1037–1042. [Google Scholar] [CrossRef]
- Khetsuriani, N.; Lu, X.; Teague, W.G.; Kazerouni, N.; Anderson, L.J.; Erdman, D.D. Novel human rhinoviruses and exacerbation of asthma in children. Emerg. Infect. Dis. 2008, 14, 1793–1796. [Google Scholar] [CrossRef]
- Jartti, T.; Gern, J.E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 2017, 140, 895–906. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.D.C.; Camargos, P.A.M.; Brand, P.L.P.; Fiaccadori, F.S.; de Lima Dias E Souza, M.B.; das Dôres de Paula Cardoso, D.; de Araújo Castro, Í.; Minamisava, R.; Sucasas da Costa, P.S. Asthma exacerbations in a subtropical area and the role of respiratory viruses: A cross-sectional study. BMC Pulm. Med. 2018, 18, 109. [Google Scholar] [CrossRef]
- Romero-Espinoza, J.A.; Moreno-Valencia, Y.; Coronel-Tellez, R.H.; Castillejos-Lopez, M.; Hernandez, A.; Dominguez, A.; Miliar-Garcia, A.; Barbachano-Guerrero, A.; Perez-Padilla, R.; Alejandre-Garcia, A.; et al. Virome and bacteriome characterization of children with pneumonia and asthma in Mexico City during winter seasons 2014 and 2015. PLoS ONE 2018, 13, e0192878. [Google Scholar] [CrossRef] [Green Version]
- Chavasse, R.; Almario, A.; Christopher, A.; Kappos, A.; Shankar, A. The indirect impact of COVID-19 on children with asthma. Arch. Bronconeumol. (Engl. Ed.) 2020, 56, 768–769. [Google Scholar]
- Morgan, W.J.; Crain, E.F.; Gruchalla, R.S.; O’Connor, G.T.; Kattan, M.; Evans, R., 3rd; Stout, J.; Malindzak, G.; Smartt, E.; Plaut, M.; et al. Results of a home-based environmental intervention among urban children with asthma. N. Engl. J. Med. 2004, 351, 1068–1080. [Google Scholar] [CrossRef] [Green Version]
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatr. 2020, 174, 882–889. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, M.; Pawankar, R.; Narazaki, H.; Ueda, T.; Itabashi, T. Coronavirus disease 2019 and asthma, allergic rhinitis: Molecular mechanisms and host-environmental interactions. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Johnston, S.L.; Pattemore, P.K.; Sanderson, G.; Smith, S.; Campbell, M.J.; Josephs, L.K.; Cunningham, A.; Robinson, B.S.; Myint, S.H.; Ward, M.E.; et al. The relationship between upper respiratory infections and hospital admissions for asthma: A time-trend analysis. Am. J. Respir. Crit. Care Med. 1996, 154, 654–660. [Google Scholar] [CrossRef]
- Takashita, E.; Kawakami, C.; Momoki, T.; Saikusa, M.; Shimizu, K.; Ozawa, H.; Kumazaki, M.; Usuku, S.; Tanaka, N.; Okubo, I.; et al. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir. Viruses 2021, 15, 488–494. [Google Scholar] [CrossRef]
- Kloepfer, K.M.; Lee, W.M.; Pappas, T.E.; Kang, T.J.; Vrtis, R.F.; Evans, M.D.; Gangnon, R.E.; Bochkov, Y.A.; Jackson, D.J.; Lemanske, R.F., Jr.; et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J. Allergy Clin. Immunol. 2014, 133, 1301–1307. [Google Scholar] [CrossRef] [Green Version]

| Year | Patient Visits for AE | All Patients | ||
|---|---|---|---|---|
| Total | 0–5 Years Old | 6–15 Years Old | Total | |
| 2011–2020 | 7476 | 4495 (60.1%) | 2981 (39.9%) | 278,465 |
| 2011 | 817 | 489 | 328 | 30,888 |
| 2012 | 734 | 447 | 287 | 30,885 |
| 2013 | 929 | 555 | 374 | 29,340 |
| 2014 | 720 | 449 | 271 | 30,580 |
| 2015 | 1034 | 590 | 444 | 29,426 |
| 2016 | 750 | 471 | 279 | 30,106 |
| 2017 | 744 | 443 | 301 | 26,884 |
| 2018 | 755 | 453 | 302 | 27,751 |
| 2019 | 653 | 392 | 261 | 29,331 |
| 2020 | 340 | 206 | 134 | 13,274 |
| Variable | Overall | Spring (Mar–May) | Summer (Jun–Aug) | Fall (Sep–Nov) | Winter (Dec–Feb) |
|---|---|---|---|---|---|
| Meteorological data | |||||
| Atmospheric pressure (hPa) | 1011.5 | 1011.4 | 1005.2 | 1012.8 | 1016.6 |
| Precipitation (mm) | 3.9 | 3.5 | 5.7 | 4.5 | 1.7 |
| Average temperature (°C) | 17.2 | 15.1 | 26.6 | 19.8 | 7.0 |
| Highest temperature (°C) | 20.7 | 19.0 | 30.0 | 23.2 | 10.3 |
| Lowest temperature (°C) | 14.1 | 11.5 | 24.0 | 16.9 | 4.0 |
| Humidity (%) | 64.9 | 61.3 | 72.2 | 65.1 | 61.1 |
| Average wind speed (m/s) | 3.7 | 3.6 | 3.6 | 3.8 | 3.8 |
| Hours of sunlight (h) | 5.8 | 6.6 | 6.4 | 5.3 | 5.0 |
| Highest temperature − lowest temperature (°C) | 6.5 | 7.5 | 6.0 | 6.3 | 6.3 |
| Average temperature difference (day − previous day, °C) | 6.5 | 7.3 | 5.9 | 6.5 | 6.3 |
| Air pollutants | |||||
| SO2 (ppm) | 0.0028 | 0.0032 | 0.0037 | 0.0021 | 0.0023 |
| NO (ppm) | 0.0039 | 0.0037 | 0.0038 | 0.0031 | 0.0052 |
| NO2 (ppm) | 0.016 | 0.018 | 0.013 | 0.015 | 0.017 |
| NOX (ppm) | 0.020 | 0.022 | 0.017 | 0.018 | 0.022 |
| OX (ppm) | 0.029 | 0.037 | 0.027 | 0.027 | 0.023 |
| CH4 (ppmC) | 1.92 | 1.93 | 1.86 | 1.92 | 1.96 |
| NMHC (ppmC) | 0.11 | 0.12 | 0.10 | 0.11 | 0.12 |
| THC (ppmC) | 2.02 | 2.04 | 1.96 | 2.02 | 2.07 |
| SPM (mg/m3) | 20.3 | 22.5 | 26.5 | 16.5 | 15.6 |
| PM2.5 (µg/m3) | 13.7 | 16.4 | 14.1 | 11.2 | 13.5 |
| Annual Amount (Average) | |
|---|---|
| Year | (/mm3/day) |
| 2011 | 19.6 |
| 2012 | 11.7 |
| 2013 | 12.6 |
| 2014 | 20.3 |
| 2015 | 14.7 |
| 2016 | 20.1 |
| 2017 | 13.4 |
| 2018 | 15.0 |
| 2019 | 21.1 |
| Average Monthly Value (2011–2019) | |
| Month | (/mm3/day) |
| Feb | 6.7 |
| Mar | 20.5 |
| Apr | 23.2 |
| May | 14.8 |
| Meteorological Data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atmospheric Pressure | Precipitation | Average Temperature | Highest Temperature | Lowest Temperature | Humidity | Wind Speed | Hours of Sunlight | Temperature Difference (Day − Previous Day) | ||
| Meteorological data | ||||||||||
| Atmospheric pressure | 1.0 | |||||||||
| Precipitation | −0.3167 **** | 1.0 | ||||||||
| Average temperature | −0.6119 **** | 0.1146 **** | 1.0 | |||||||
| Highest temperature | −0.5896 **** | 0.08268 **** | 0.9919 **** | 1.0 | ||||||
| Lowest temperature | −0.6232 **** | 0.1352 **** | 0.992 **** | 0.9734 **** | 1.0 | |||||
| Humidity | −0.4641 **** | 0.4461 **** | 0.4094 **** | 0.3462 **** | 0.4588 **** | 1.0 | ||||
| Wind speed | −0.2359 **** | 0.2744 **** | −0.0367 * | −0.04834 ** | −0.01835 | 0.02506 | 1.0 | |||
| Hours of sunlight | 0.1584 **** | −0.3538 **** | 0.1375 **** | 0.215 **** | 0.07075 **** | −0.5122 **** | −0.1913 **** | 1.0 | ||
| Temperature difference | 0.1931 **** | −0.2355 **** | −0.07914 **** | 0.03676 * | −0.1933 **** | −0.5182 **** | −0.1269 **** | 0.6116 **** | 1.0 | |
| (day − previous day) | ||||||||||
| Air pollutants | ||||||||||
| SO2 | −0.1529 **** | −0.07922 **** | 0.2868 **** | 0.2991 **** | 0.2656 **** | 0.1642 **** | −0.2524 **** | 0.1547 **** | 0.1227 **** | |
| NO | 0.1284 **** | −0.01743 | −0.07064 **** | −0.07036 **** | −0.08121 **** | 0.1939 **** | −0.2649 **** | −0.04592 * | 0.05235 ** | |
| NO2 | 0.2098 **** | −0.04714 ** | −0.1232 **** | −0.1166 **** | −0.1509 **** | 0.09542 **** | −0.3754 **** | −0.0361 * | 0.1572 **** | |
| NOX | 0.1922 **** | −0.0396 * | −0.1154 **** | −0.1105 **** | −0.1396 **** | 0.1392 **** | −0.3629 **** | −0.03995 * | 0.1340 **** | |
| OX | −0.1190 **** | −0.1471 **** | 0.04158 * | 0.08215 **** | 0.005769 | −0.4305 **** | 0.06489*** | 0.3078 **** | 0.3264 **** | |
| CH4 | 0.4844 **** | −0.1885 **** | −0.6326 **** | −0.6138 **** | −0.6537 **** | −0.3525 **** | −0.1902 **** | −0.03191 | 0.2223 **** | |
| NMHC | 0.1192 **** | −0.02196 | −0.08328 **** | −0.08113 **** | −0.1018 **** | 0.1358 **** | −0.2843 **** | −0.1009 **** | 0.09666 **** | |
| THC | 0.3745 **** | −0.1304 **** | −0.4456 **** | −0.4326 **** | −0.4704 **** | −0.131 **** | −0.2986 **** | −0.08663 **** | 0.1988 **** | |
| SPM | −0.2336 **** | −0.03178 | 0.3668 **** | 0.3747 **** | 0.3503 **** | 0.1566 **** | −0.2255 **** | 0.1480 **** | 0.07641 **** | |
| PM2.5 | 0.03535 | −0.1866 **** | 0.05723 ** | 0.08374 **** | 0.02575 | −0.08667 **** | −0.2925 **** | 0.2284 **** | 0.2442 **** | |
| Air pollutants | ||||||||||
| SO2 | NO | NO2 | NOX | OX | CH4 | NMHC | THC | SPM | PM2.5 | |
| Meteorological data | ||||||||||
| Atmospheric pressure | ||||||||||
| Precipitation | ||||||||||
| Average temperature | ||||||||||
| Highest temperature | ||||||||||
| Lowest temperature | ||||||||||
| Humidity | ||||||||||
| Wind speed | ||||||||||
| Hours of sunlight | ||||||||||
| Temperature difference | ||||||||||
| (day − previous day) | ||||||||||
| Air pollutants | ||||||||||
| SO2 | 1.0 | |||||||||
| NO | 0.4429 **** | 1.0 | ||||||||
| NO2 | 0.5645 **** | 0.6765 **** | 1.0 | |||||||
| NOX | 0.5686 **** | 0.8733 **** | 0.9378 **** | 1.0 | ||||||
| OX | −0.1249 **** | −0.5155 **** | −0.3193 **** | −0.4202 **** | 1.0 | |||||
| CH4 | −0.1791 **** | 0.1570 **** | 0.3835 **** | 0.3244 **** | 0.1549 **** | 1.0 | ||||
| NMHC | 0.4641 **** | 0.7053 **** | 0.7803 **** | 0.8232 **** | −0.3153 **** | 0.2655 **** | 1.0 | |||
| THC | 0.1839 **** | 0.5491 **** | 0.7364 **** | 0.7276 **** | −0.1053 **** | 0.7876 **** | 0.8009 **** | 1.0 | ||
| SPM | 0.7039 **** | 0.2567 **** | 0.3614 **** | 0.3540 **** | 0.0431 * | −0.1848 **** | 0.3421 **** | 0.1027 **** | 1.0 | |
| PM2.5 | 0.6272 **** | 0.3259 **** | 0.5104 **** | 0.4829 **** | 0.1552 **** | 0.2495 **** | 0.4392 **** | 0.4216 **** | 0.8527 **** | 1.0 |
| Total (0–15 Years Old) (n = 7136) | 0–5 Years Old (n = 4289) | 6–15 Years Old (n = 2847) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | SE | p-Value | Parameter | SE | p-Value | Parameter | SE | p-Value | |
| Meteorological data | |||||||||
| Atmospheric pressure (hPa) | 0.0019 | 0.0061 | 0.75 | 0.0027 | 0.0080 | 0.73 | 0.0007 | 0.0096 | 0.94 |
| Precipitation (mm) | 0.0048 | 0.0036 | 0.19 | 0.0063 | 0.0046 | 0.17 | 0.0023 | 0.0058 | 0.69 |
| Average temperature (°C) | 0.0266 | 0.0090 | 0.00324 ** | 0.0315 | 0.0117 | 0.00711 ** | 0.0201 | 0.0143 | 0.16 |
| Humidity (%) | 0.0026 | 0.0039 | 0.51 | 0.0025 | 0.0050 | 0.63 | 0.0025 | 0.0062 | 0.69 |
| Wind speed (m/s) | −0.029 | 0.026 | 0.26 | −0.039 | 0.034 | 0.24 | −0.013 | 0.040 | 0.73 |
| Hours of sunlight (h) | 0.0016 | 0.0094 | 0.87 | −0.0019 | 0.0121 | 0.87 | 0.0064 | 0.0149 | 0.67 |
| Air pollutants | |||||||||
| SO2 (ppm) | 13.7 | 28.7 | 0.63 | 76.0 | 36.4 | 0.03682 * | −85.8 | 46.8 | 0.07 |
| NO (ppm) | 0.29 | 10.1 | 0.98 | 0.82 | 13.2 | 0.95 | −0.8 | 16.0 | 0.96 |
| NO2 (ppm) | −6.5 | 6.3 | 0.31 | −14.9 | 8.1 | 0.065 | 6.8 | 10.3 | 0.51 |
| OX (ppm) | 6.6 | 4.9 | 0.17 | 7.4 | 6.3 | 0.24 | 5.5 | 7.8 | 0.48 |
| CH4 (ppmC) | −0.3 | 1.0 | 0.81 | 0.68 | 1.3 | 0.61 | −1.7 | 1.7 | 0.300 |
| NMHC (ppmC) | −0.71 | 0.83 | 0.39 | −1.20 | 1.08 | 0.27 | −0.01 | 1.3 | 0.99 |
| SPM (mg/m3) | 0.009 | 0.0090 | 0.326 | 0.008 | 0.012 | 0.51 | 0.011 | 0.014 | 0.441 |
| PM2.5 (µg/m3) | −0.010 | 0.012 | 0.401 | −0.013 | 0.016 | 0.41 | −0.007 | 0.019 | 0.73 |
| Infection | |||||||||
| RSV prevalence | 0.029 | 0.076 | 0.70 | 0.057 | 0.10 | 0.56 | −0.009 | 0.12 | 0.94 |
| Influenza prevalence | 0.0045 | 0.0053 | 0.39 | 0.0058 | 0.0069 | 0.40 | 0.0031 | 0.0083 | 0.70 |
| Pollen (/m3) | 0.0008 | 0.0012 | 0.50 | 0.0015 | 0.0015 | 0.34 | −0.0002 | 0.0020 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, H.; Nozu, K.; Ishiko, S.; Kondo, A.; Ninchoji, T.; Nagano, C.; Takeda, H.; Unzaki, A.; Ishibashi, K.; Morioka, I.; et al. Impact of the State of Emergency during the COVID-19 Pandemic in 2020 on Asthma Exacerbations among Children in Kobe City, Japan. Int. J. Environ. Res. Public Health 2021, 18, 11407. https://doi.org/10.3390/ijerph182111407
Yamaguchi H, Nozu K, Ishiko S, Kondo A, Ninchoji T, Nagano C, Takeda H, Unzaki A, Ishibashi K, Morioka I, et al. Impact of the State of Emergency during the COVID-19 Pandemic in 2020 on Asthma Exacerbations among Children in Kobe City, Japan. International Journal of Environmental Research and Public Health. 2021; 18(21):11407. https://doi.org/10.3390/ijerph182111407
Chicago/Turabian StyleYamaguchi, Hiroshi, Kandai Nozu, Shinya Ishiko, Atsushi Kondo, Takeshi Ninchoji, China Nagano, Hiroki Takeda, Ai Unzaki, Kazuto Ishibashi, Ichiro Morioka, and et al. 2021. "Impact of the State of Emergency during the COVID-19 Pandemic in 2020 on Asthma Exacerbations among Children in Kobe City, Japan" International Journal of Environmental Research and Public Health 18, no. 21: 11407. https://doi.org/10.3390/ijerph182111407
APA StyleYamaguchi, H., Nozu, K., Ishiko, S., Kondo, A., Ninchoji, T., Nagano, C., Takeda, H., Unzaki, A., Ishibashi, K., Morioka, I., Nagase, H., Iijima, K., & Ishida, A. (2021). Impact of the State of Emergency during the COVID-19 Pandemic in 2020 on Asthma Exacerbations among Children in Kobe City, Japan. International Journal of Environmental Research and Public Health, 18(21), 11407. https://doi.org/10.3390/ijerph182111407






