The Israeli Experience with the “Green Pass” Policy Highlights Issues to Be Considered by Policymakers in Other Countries
Abstract
:1. Introduction
2. Materials and Methods
3. Results
The Israeli Green Pass Policy—Design and Implementation
4. Discussion
4.1. The Green Pass Policy Can Be an Effective Public Health Tool to Relax COVID-19 Restrictions in a Situation of Great Uncertainty
4.2. What Policymakers Should Be Aware of When Considering a “Green Pass Policy”
5. Conclusions
- Electronic medical records can facilitate the implementation of a GPP, but attention should be paid to data privacy and protection.
- To reduce equity concerns, all age groups should be eligible for the vaccine (contingent on approval for children) and have access to the vaccine. Alternatively, authorities can grant temporary certificates if testing is available.
- Essential activities should be excluded from the GPP requirement. Nevertheless, it is impossible to avoid gaps between GPP regulations and implementation. While some places might demand a GPP even when not legally necessary, others will not implement it despite being legally obligated to do so.
- GPP regulations should have standardised epidemiological criteria, a gradual implementation process, flexibility in their application, and adaptability according to risk.
- GPP limitations should balance epidemiological risks with the economic viability of their implementation; otherwise, businesses may not recover if partial activity does not cover operating costs.
- Consulting with stakeholders can reduce resistance or opposition to a GPP.
- Enforcement is key for the effectiveness of a GPP and should be designed in a way that does not violate civil rights.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Health System Response Monitor. Available online: https://www.covid19healthsystem.org/mainpage.aspx (accessed on 4 May 2021).
- Dubé, E.; Gagnon, D.; MacDonald, N.E. Strategies intended to address vaccine hesitancy: Review of published reviews. Vaccine 2015, 33, 4191–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirico, F. The new Italian mandatory vaccine Law as a health policy instrument against the anti-vaccination movement. Ann. Ig. Med. Prev. E Comunita 2018, 30, 251–256. [Google Scholar]
- WHO. WHO New Yellow Fever Vaccination Requirements for Travelers; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- ALI Ada Lovelace Institute. International Monitor: Vaccine Passports and COVID Status Apps. Available online: https://www.adalovelaceinstitute.org/project/international-monitor-vaccine-passports-covid-status-apps/ (accessed on 14 April 2021).
- BBC. Covid Passports: What are Different Countries Planning; BBC News: London, UK, 2021. [Google Scholar]
- The Institute for Government. Vaccine Passports. Available online: https://www.instituteforgovernment.org.uk/explainers/vaccine-passports (accessed on 3 May 2021).
- Berliner-Zeitung. Berlin Extends Corona Lockdown until May 9th—More Freedom for Vaccinated People [In German]. Available online: https://www.berliner-zeitung.de/news/berlin-verlaengert-erneut-corona-lockdown-li.152352 (accessed on 14 April 2021).
- Spahn: Outdoor Catering Could Soon Open to Vaccinated People [In German]. Available online: https://www.berliner-zeitung.de/news/spahn-aussengastronomie-kann-bald-fuer-geimpfte-oeffnen-li.156694 (accessed on 14 April 2021).
- The Local. Green Pass: Austria’s Coronavirus Immunity Card One Step Closer to Reality—The Local. Available online: https://www.thelocal.at/20210412/step-forward-for-tourism-green-pass-and-entry-tests-in-austria/ (accessed on 14 April 2021).
- EU European Commission. COVID-19: Digital Green Certificates. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-covid-19-vaccines-europeans/covid-19-digital-green-certificates_en#next-steps (accessed on 3 May 2021).
- Waitzberg, R.; Meshulam, A. COVID-19 Health System Response Monitor—Israel Country Page. Available online: https://www.covid19healthsystem.org/countries/israel/countrypage.aspx (accessed on 22 April 2021).
- Ministry of Health. COVID-19 Dashboard. Available online: https://datadashboard.health.gov.il/COVID-19/general (accessed on 28 January 2021).
- Hunter, P.R. Thrombosis after COVID-19 vaccination. BMJ 2021, 373, n958. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. Effectiveness of BNT162b2 mRNA Vaccine Against Infection and COVID-19 Vaccine Coverage in Healthcare Workers in England, Multicentre Prospective Cohort Study (the SIREN Study). SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Benenson, S.; Oster, Y.; Cohen, M.J.; Nir-Paz, R. BNT162b2 mRNA COVID-19 Vaccine Effectiveness among Health Care Workers. N. Engl. J. Med. 2021, 384, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- The Lancet Microbe. Vaccine certificates: Does the end justify the means? Lancet Microbe 2021, 2, e130. [Google Scholar] [CrossRef]
- Lipsitch, M.; Kahn, R. Interpreting vaccine efficacy trial results for infection and transmission. Vaccine 2021, 39, 4082–4088. [Google Scholar] [CrossRef]
- Mahase, E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ 2021, 373, n1513. [Google Scholar] [CrossRef]
- Farinholt, T.; Doddapaneni, H.; Qin, X.; Menon, V.; Meng, Q.; Metcalf, G.; Chao, H.; Gingras, M.C.; Avadhanula, V.; Farinholt, P.; et al. Transmission event of SARS-CoV-2 Delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Kluge, H.; McKee, M. COVID-19 vaccines for the European region: An unprecedented challenge. Lancet 2021, 397, 1689–1691. [Google Scholar] [CrossRef]
- Day, M. COVID-19: Stronger warnings are needed to curb socialising after vaccination, say doctors and behavioural scientists. BMJ 2021, 372, n783. [Google Scholar] [CrossRef]
- Waitzberg, R.; Davidovitch, N. Israel’s Vaccination Rollout: Short Term Success, but Questions for the Long run—The BMJ Blogs. 2021. Available online: https://blogs.bmj.com/bmj/2021/02/05/israels-vaccination-rollout-short-term-success-but-questions-for-the-long-run/ (accessed on 18 October 2021).
- Rosen, B.; Waitzberg, R.; Israeli, A. Israel’s rapid rollout of vaccinations for COVID-19. Isr. J. Health Policy Res. 2021, 10, 6. [Google Scholar] [CrossRef]
- Hall, M.A.; Studdert, D.M. “Vaccine Passport” Certification—Policy and Ethical Considerations. N. Engl. J. Med. 2021, 385, e32. [Google Scholar] [CrossRef] [PubMed]
- Osama, T.; Razai, M.S.; Majeed, A. COVID-19 vaccine passports: Access, equity, and ethics. BMJ 2021, 373, n861. [Google Scholar] [CrossRef]
- Tanner, R.; Flood, C.M. Vaccine Passports Done Equitably. JAMA Health Forum. 2021, 2, e210972. [Google Scholar] [CrossRef]
- Siciliani, L.; Wild, C.; McKee, M.; Kringos, D.; Barry, M.M.; Barros, P.P.; DeMaeseneer, J.; Murauskiene, L.; Ricciardi, W. Strengthening vaccination programmes and health systems in the European Union: A framework for action. Health Policy 2020, 124, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Porat, T.; Burnell, R.; Calvo, R.A.; Ford, E.; Paudyal, P.; Baxter, W.L.; Parush, A. “Vaccine Passports” May Backfire: Findings from a Cross-Sectional Study in the UK and Israel on Willingness to Get Vaccinated against COVID-19. Vaccines 2021, 9, 902. [Google Scholar] [CrossRef] [PubMed]
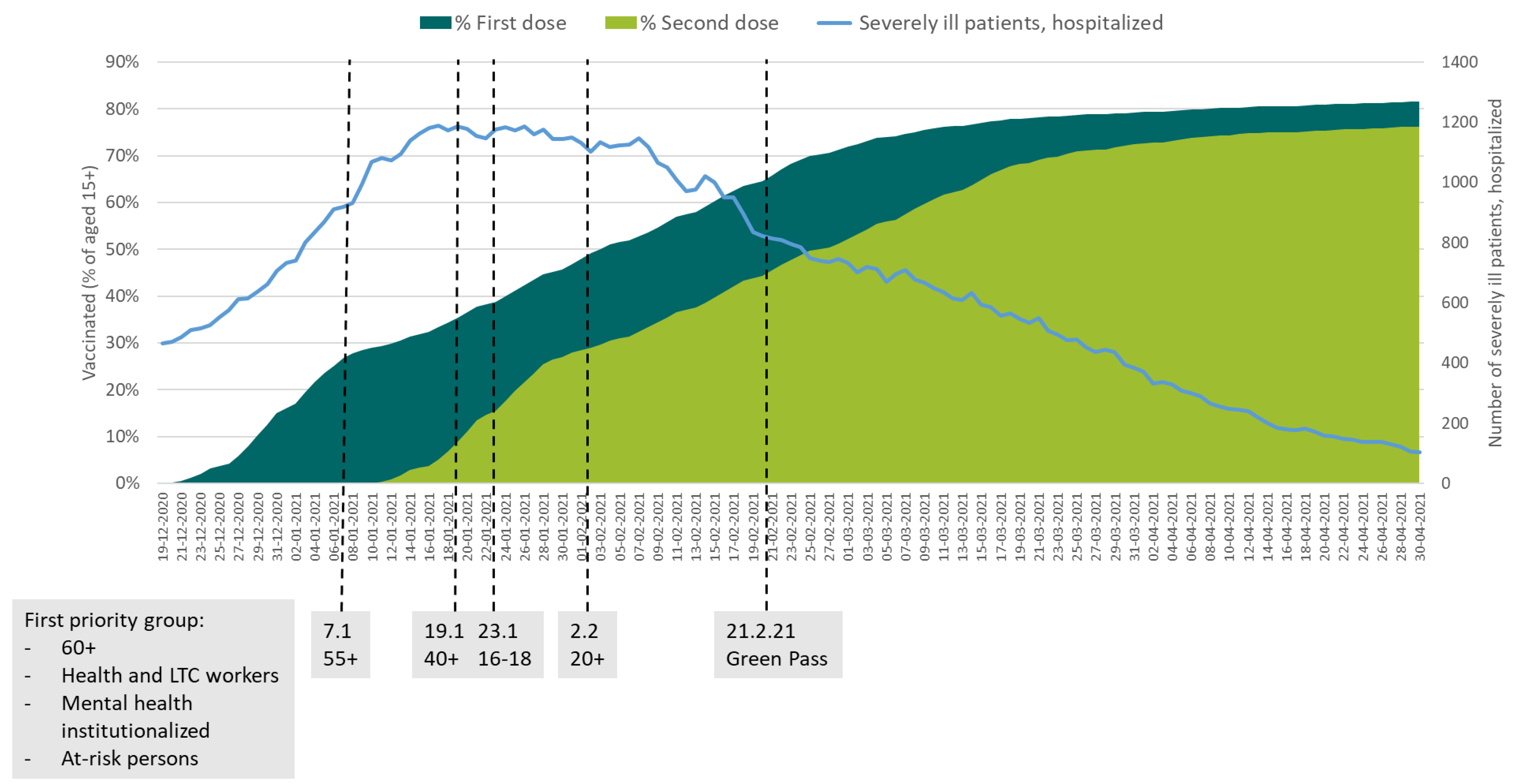
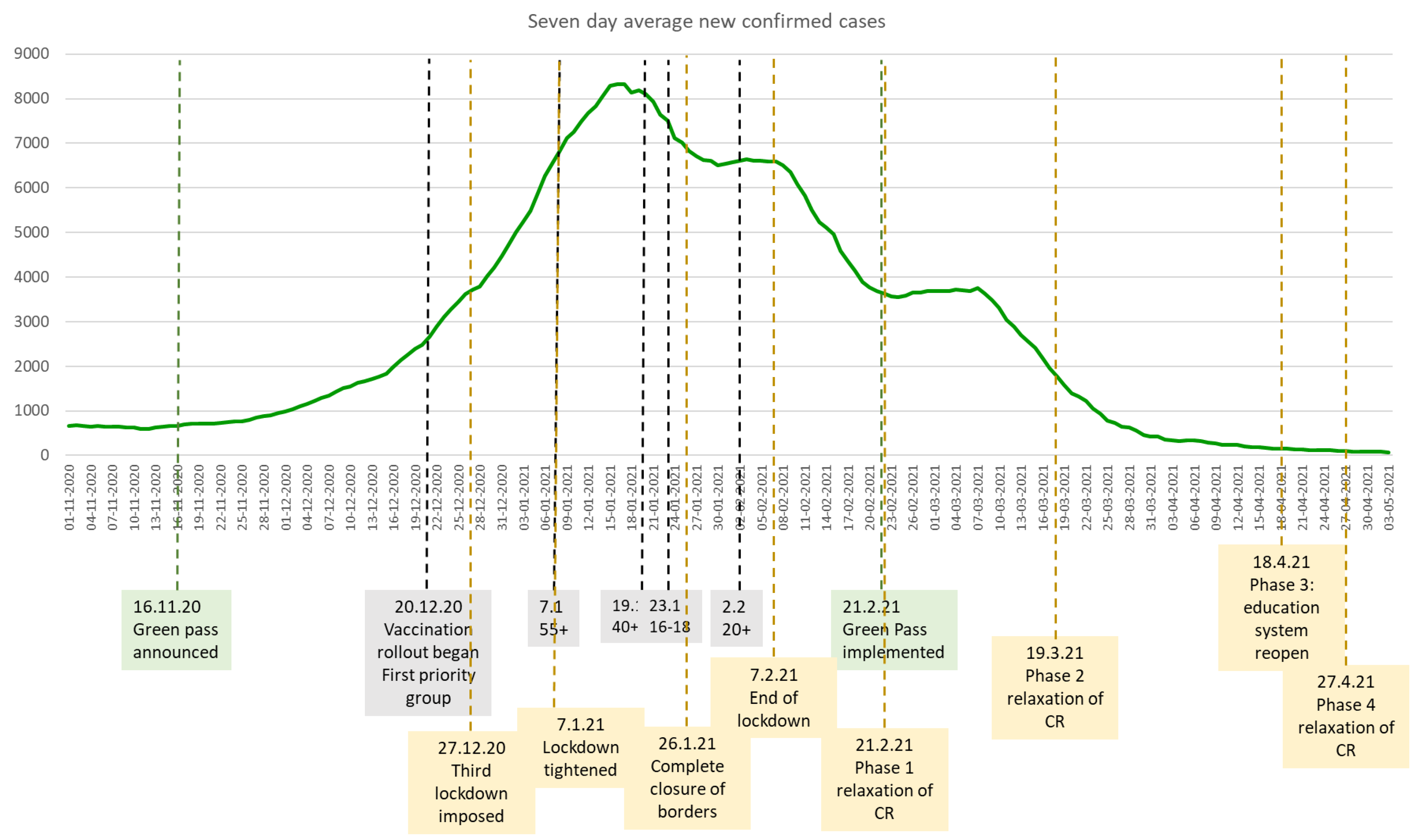
| Phase | Date | Limitations on Gatherings | High-Risk Places | Low-Risk Places | Notes |
|---|---|---|---|---|---|
| First implementation of the GPP | 21.02.21 | Maximum people: 10 indoors 20 outdoors | Remained closed | 75% capacity allowed, 300 indoors, 500 outdoors | Occupancy restrictions of 1 person per 7 sq. metres |
| Opening high-risk events and relaxing restrictions on low-risk events | 07.03.21 | No change | 50% capacity, maximum 300 people. For restaurants capacity 75%, max. 100 people indoors, 100 outdoors | 75% capacity allowed, 500 indoors, 750 outdoors | Babies up to 1 year old exempt from GPP |
| Further easing of restrictions | 19.03.21 | No change | 50% capacity, maximum 300 people indoors, 500 outdoors | Indoors: 75% capacity for places with up to 5000 seats, and 30% capacity otherwise.Outdoors: 75% capacity for places with up to 10,000 places, 30% otherwise | Temporary GPP granted for negatively tested with rapid tests (privately funded). Swimming pools and events in open areas exempt from GPP |
| Further easing of restrictions | 08.04.21 | Maximum people: 20 indoors 100 outdoors | Maximum 300 people indoors, 750 outdoors | Up to 10,000 people at outdoor and 4000 indoor events | Introduction of government-funded PCR tests for children to obtain a temporary GPP |
| Further easing of restrictions | 06.05.21 | Maximum people: 50 indoors 500 outdoors | End of all occupancy restrictions | End of all occupancy restrictions | Exemptions for sporting facilities and events from the GPP |
| Abolition of GPP regulations and all occupancy and gathering restrictions | 01.06.21 | ||||
| Reintroduction of the “Green Pass Policy” for all individuals 1 | 21.07.21 | No limitations | Green Pass required for indoor social events with 100+ people | No limitations | Heavy penalties imposed on owners of businesses that do not require the Green Pass |
| Tighter regulations for the “Green Pass Policy” 2 | 29.07.21 | No limitations | Expansion of the GPP requirements to places with 100+ people such as restaurants, gyms, sporting facilities, hotels | No limitations | Children aged 12+ are exempt from the Green Pass (except social events with 100+ people). Negative tests can be used as “temporary Green Pass”: PCR for 72 h, rapid test for 24 h. |
| Expansion of the requirement of the Green Pass to all public places, except essential places, including children of all ages 3 | 08.08.21 | No limitations | Green Pass required for all public places regardless of the number of people. | Synagogues with more than 50 people must comply with the GPP | Ending of government-funded tests; tests to be covered by the recipient, except for children up to 12 years old |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waitzberg, R.; Triki, N.; Alroy-Preis, S.; Lotan, T.; Shiran, L.; Ash, N. The Israeli Experience with the “Green Pass” Policy Highlights Issues to Be Considered by Policymakers in Other Countries. Int. J. Environ. Res. Public Health 2021, 18, 11212. https://doi.org/10.3390/ijerph182111212
Waitzberg R, Triki N, Alroy-Preis S, Lotan T, Shiran L, Ash N. The Israeli Experience with the “Green Pass” Policy Highlights Issues to Be Considered by Policymakers in Other Countries. International Journal of Environmental Research and Public Health. 2021; 18(21):11212. https://doi.org/10.3390/ijerph182111212
Chicago/Turabian StyleWaitzberg, Ruth, Noa Triki, Sharon Alroy-Preis, Tomer Lotan, Liat Shiran, and Nachman Ash. 2021. "The Israeli Experience with the “Green Pass” Policy Highlights Issues to Be Considered by Policymakers in Other Countries" International Journal of Environmental Research and Public Health 18, no. 21: 11212. https://doi.org/10.3390/ijerph182111212
APA StyleWaitzberg, R., Triki, N., Alroy-Preis, S., Lotan, T., Shiran, L., & Ash, N. (2021). The Israeli Experience with the “Green Pass” Policy Highlights Issues to Be Considered by Policymakers in Other Countries. International Journal of Environmental Research and Public Health, 18(21), 11212. https://doi.org/10.3390/ijerph182111212






