Alcohol Drinking Pattern and Risk of Head and Neck Cancer: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Source
2.2. Ethical Approval
2.3. Exposure to Alcohol
2.4. Covariates
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Demographics
3.2. Risk of HNC According to Drinking Status
3.3. Risk of HNC and Drinking Pattern: Drinking Frequency vs. Daily Amount
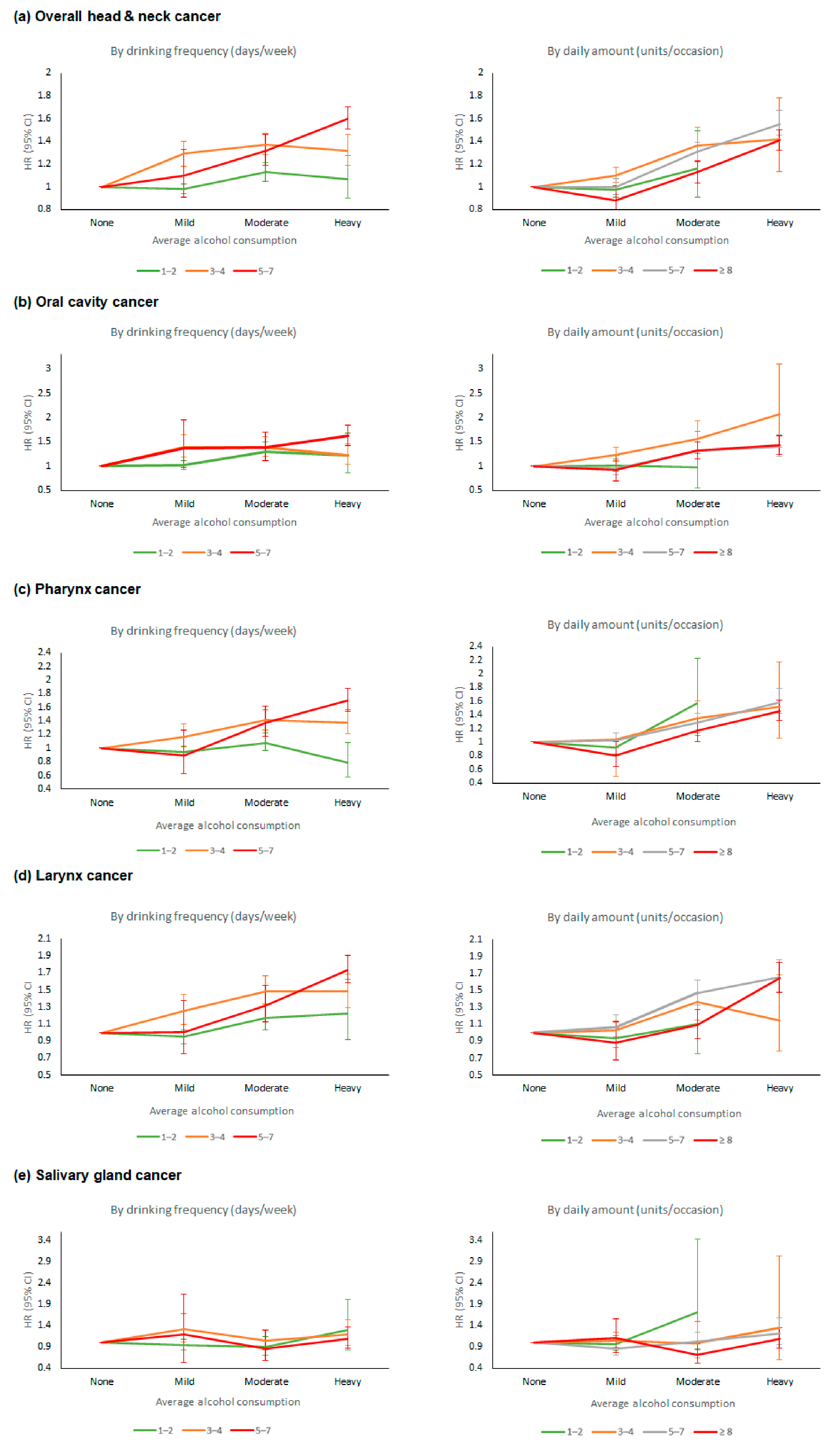
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer 2020. Available online: https://gco.iarc.fr/today (accessed on 17 October 2021).
- Gupta, B.; Johnson, N.W.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef]
- Shaw, R.; Beasley, N. Aetiology and risk factors for head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130 (Suppl. 2), S9–S12. [Google Scholar] [CrossRef]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef]
- Freedman, N.D.; Schatzkin, A.; Leitzmann, M.F.; Hollenbeck, A.R.; Abnet, C. Alcohol and head and neck cancer risk in a prospective study. Br. J. Cancer 2007, 96, 1469–1474. [Google Scholar] [CrossRef]
- Polesel, J.; Maso, L.D.; Bagnardi, V.; Zucchetto, A.; Zambon, A.; Levi, F.; La Vecchia, C.; Franceschi, S. Estimating dose-response relationship between ethanol and risk of cancer using regression spline models. Int. J. Cancer 2005, 114, 836–841. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Miao, L.; Zhu, L.; Jiang, H.; Yuan, H. Different Levels in Alcohol and Tobacco Consumption in Head and Neck Cancer Patients from 1957 to 2013. PLoS ONE 2015, 10, e0124045. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Blangiardo, M.; la Vecchia, C.; Corrao, G. Alcohol consumption and the risk of cancer: A meta-analysis. Alcohol Res. Health 2001, 25, 263–270. [Google Scholar]
- Mørch, L.S.; Johansen, D.; Thygesen, L.; Tjonneland, A.; Løkkegaard, E.; Stahlberg, C.; Grønbaek, M. Alcohol drinking, consumption patterns and breast cancer among Danish nurses: A cohort study. Eur. J. Public Health 2007, 17, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Giovannucci, E. Alcohol Intake, Drinking Patterns, and Risk of Prostate Cancer in a Large Prospective Cohort Study. Am. J. Epidemiol. 2004, 159, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J.; Giovannucci, E.L. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: Results from two prospective US cohort studies. BMJ 2015, 351, h4238. [Google Scholar] [CrossRef] [PubMed]
- Kjærheim, K.; Gaard, M.; Andersen, A. The role of alcohol, tobacco, and dietary factors in upper aerogastric tract cancers: A prospective study of 10,900 Norwegian men. Cancer Causes Control. 1998, 9, 99–108. [Google Scholar] [CrossRef]
- Di Credico, G.; Polesel, J.; Maso, L.D.; Pauli, F.; Torelli, N.; Luce, D.; Radoï, L.; Matsuo, K.; Serraino, D.; Brennan, P.; et al. Alcohol drinking and head and neck cancer risk: The joint effect of intensity and duration. Br. J. Cancer 2020, 123, 1456–1463. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Johnson, N.W. Relationship of Lifetime Exposure to Tobacco, Alcohol and Second Hand Tobacco Smoke with Upper aero-digestive tract cancers in India: A Case-Control Study with a Life-Course Perspective. Asian Pac. J. Cancer Prev. 2017, 18, 347–356. [Google Scholar]
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N.W. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef]
- Du, T.; Chen, K.; Zheng, S.; Bao, M.; Huang, Y.; Wu, K. Association between Alcohol Consumption and Risk of Nasopharyngeal Carcinoma: A Comprehensive Meta-Analysis of Epidemiological Studies. Alcohol Clin. Exp. Res. 2019, 43, 2262–2273. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.-Y.; Park, S.K.; Khang, Y.-H.; Kim, H.C.; Park, J.H.; Kang, H.-J.; Do, C.-H.; Song, J.-S.; Lee, E.-J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Kalinowski, A.; Humphreys, K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction 2016, 111, 1293–1298. [Google Scholar] [CrossRef]
- Furtwaengler, N.A.; de Visser, R.O. Lack of international consensus in low-risk drinking guidelines. Drug Alcohol Rev. 2013, 32, 11–18. [Google Scholar] [CrossRef]
- Kim, Y.G.; Han, K.; Choi, J., II; Boo, K.Y.; Kim, D.Y.; Lee, K.; Shim, J.; Kim, J.S.; Kim, Y.-H. Frequent drinking is a more important risk factor for new-onset atrial fibrillation than binge drinking: A nationwide population-based study. Europace 2020, 22, 216–224. [Google Scholar] [CrossRef]
- Korea Health Promotion Institute. Guideline for Low-Risk Alcohol Consumption. 2013. Available online: https://www.khealth.or.kr/kps/publish/view?menuId=MENU00891&page_no=B2017004&board_idx=7640 (accessed on 5 August 2021).
- Stott-Miller, M.; Chen, C.; Chuang, S.-C.; Lee, Y.-C.A.; Boccia, S.; Brenner, H.; Cadoni, G.; Maso, L.D.; La Vecchia, C.; Lazarus, P.; et al. History of diabetes and risk of head and neck cancer: A pooled analysis from the international head and neck cancer epidemiology consortium. Cancer Epidemiol. Biomark. Prev. 2012, 21, 294–304. [Google Scholar] [CrossRef]
- Tseng, K.S.; Lin, C.; Lin, Y.-S.; Weng, S.-F. Risk of head and neck cancer in patients with diabetes mellitus: A retrospective cohort study in Taiwan. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 746–753. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kim, Y.-D.; Park, C.-S.; Han, K.-D.; Joo, Y.-H. Hypertension is associated with oral, laryngeal, and esophageal cancer: A nationwide population-based study. Sci. Rep. 2020, 10, 10291. [Google Scholar] [CrossRef]
- Kao, L.-T.; Hung, S.-H.; Kao, P.-F.; Liu, J.-C.; Lin, H.-C. Inverse association between statin use and head and neck cancer: Population-based case-control study in Han population. Head Neck 2019, 41, 1193–1198. [Google Scholar] [CrossRef]
- Maasland, D.; van den Brandt, P.A.; Kremer, B.; Goldbohm, R.A.S.; Schouten, L.J. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands Cohort Study. BMC Cancer 2014, 14, 187. [Google Scholar] [CrossRef]
- Grønbaek, M.; Becker, U.; Johansen, D.; Tønnesen, H.; Jensen, G.; Sørensen, T.I. Population based cohort study of the association between alcohol intake and cancer of the upper digestive tract. BMJ 1998, 317, 844–847. [Google Scholar] [CrossRef]
- Goldstein, B.Y.; Chang, S.-C.; Hashibe, M.; La Vecchia, C.; Zhang, Z.-F. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: An update. Eur. J. Cancer Prev. 2010, 19, 431–465. [Google Scholar] [CrossRef]
- Seitz, H.K.; Becker, P. Alcohol metabolism and cancer risk. Alcohol Res. Health 2007, 30, 38–47. [Google Scholar]
- Seitz, H.K.; Stickel, F.; Homann, N. Pathogenetic mechanisms of upper aerodigestive tract cancer in alcoholics. Int. J. Cancer 2004, 108, 483–487. [Google Scholar] [CrossRef]
- Obe, G.; Jonas, R.; Schmidt, S. Metabolism of ethanol in vitro produces a compound which induces sister-chromatid exchanges in human peripheral lymphocytes in vitro: Acetaldehyde not ethanol is mutagenic. Mutat. Res. Lett. 1986, 174, 47–51. [Google Scholar] [CrossRef]
- Yokoyama, A.; Tsutsumi, E.; Imazeki, H.; Suwa, Y.; Nakamura, C.; Mizukami, T.; Yokoyama, T. Salivary acetaldehyde concentration according to alcoholic beverage consumed and aldehyde dehydrogenase-2 genotype. Alcohol Clin. Exp. Res. 2008, 32, 1607–1614. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Monakhova, Y.B. Short-term salivary acetaldehyde increase due to direct exposure to alcoholic beverages as an additional cancer risk factor beyond ethanol metabolism. J. Exp. Clin. Cancer Res. 2011, 30, 3. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, A.; Ohashi, S.; Hirohashi, K.; Amanuma, Y.; Matsuda, T.; Muto, M. Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium. Int. J. Mol. Sci. 2017, 18, 1943. [Google Scholar] [CrossRef] [PubMed]
- Stornetta, A.; Guidolin, V.; Balbo, S. Alcohol-Derived Acetaldehyde Exposure in the Oral Cavity. Cancers 2018, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Filion, E.J.; McClure, L.A.; Huang, D.; Seng, K.; Kaplan, M.J.; Colevas, A.D.; Gomez, S.L.; Chang, E.T.; Le, Q.-T. Higher incidence of head and neck cancers among Vietnamese American men in California. Head Neck 2010, 32, 1336–1344. [Google Scholar] [CrossRef]
- Pan, S.Y.; de Groh, M.; Morrison, H. A Case-Control Study of Risk Factors for Salivary Gland Cancer in Canada. J. Cancer Epidemiol. 2017, 2017, 4909214. [Google Scholar] [CrossRef]
- Zheng, W.; Shu, X.-O.; Ji, B.-T.; Gao, Y.-T. Diet and other risk factors for cancer of the salivary glands:a population-based case-control study. Int. J. Cancer 1996, 67, 194–198. [Google Scholar] [CrossRef]
- Muscat, J.E.; Wynder, E.L. A case/control study of risk factors for major salivary gland cancer. Otolaryngol. Head Neck Surg. 1998, 118, 195–198. [Google Scholar] [CrossRef]
- Hayes, R.; Bravo-Otero, E.; Kleinman, D.V.; Brown, L.M.; Fraumeni, J.F.; Harty, L.C.; Winn, D.M. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control 1999, 10, 27–33. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; Ljung, B.-M.; Morrow, M. Environmental Factors and the Risk of Salivary Gland Cancer. Epidemiology 1997, 8, 414–419. [Google Scholar] [CrossRef]
- Sanderson, R.J.; Ironside, J.A. Squamous cell carcinomas of the head and neck. BMJ 2002, 325, 822–827. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Zhu, Y.-X.; Chen, T.-Z.; Wang, Y.; Sun, G.-H.; Zhang, L.; Huang, C.-P.; Wang, Z.-Y.; Shen, Q.; Li, D.-S.; et al. Clinicopathologic study of 1176 salivary gland tumors in a Chinese population: Experience of one cancer center 1997–2007. Acta Otolaryngol. 2012, 132, 879–886. [Google Scholar] [CrossRef][Green Version]
- Väkeväinen, S.; Tillonen, J.; Agarwal, D.P.; Srivastava, N.; Salaspuro, M. High Salivary Acetaldehyde After a Moderate Dose of Alcohol in ALDH2-Deficient Subjects: Strong Evidence for the Local Carcinogenic Action of Acetaldehyde. Alcohol. Clin. Exp. Res. 2000, 24, 873–877. [Google Scholar] [CrossRef]
- Homann, N.; Jousimies-Somer, H.; Jokelainen, K.; Heine, R.; Salaspuro, M. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis 1997, 18, 1739–1743. [Google Scholar] [CrossRef]
- Talaska, G.; Jaeger, M.; Reilman, R.; Collins, T.; Warshawsky, D. Chronic, topical exposure to benzo[a]pyrene induces relatively high steady-state levels of DNA adducts in target tissues and alters kinetics of adduct loss. Proc. Natl. Acad. Sci. USA 1996, 93, 7789–7793. [Google Scholar] [CrossRef]
- Mennecier, G.; Torres, L.N.; Cogliati, B.; Sanches, D.S.; Mori, C.M.; Latorre, A.O.; Chaible, L.M.; Mackowiak, I.I.; Nagamine, M.K.; Da Silva, T.C.; et al. Chronic exposure of lung alveolar epithelial type II cells to tobacco-specific carcinogen NNK results in malignant transformation: A new in vitro lung carcinogenesis model. Mol. Carcinog. 2014, 53, 392–402. [Google Scholar] [CrossRef]
- Sarkola, T.; Iles, M.R.; Kohlenberg-Mueller, K.; Eriksson, C.J.P. Ethanol, acetaldehyde, acetate, and lactate levels after alcohol intake in white men and women: Effect of 4-methylpyrazole. Alcohol Clin. Exp. Res. 2002, 26, 239–245. [Google Scholar] [CrossRef]
- Belinsky, S.A.; Devereux, T.R.; Foley, J.F.; Maronpot, R.R.; Anderson, M.W. Role of the Alveor Type II Cell in the Development and Progression of Pulmonary Tumors Induced bt 4-(Methylnitrosamino)-(3-pyridyl)-1-butanone in the A/J Mouse. Cancer Res. 1992, 52, 3164–3173. [Google Scholar]
- Oliveira, P.A.; Colaço, A.; Chaves, R.; Guedes-Pinto, H.; De-La-Cruz, P.L.F.; Lopes, C. Chemical carcinogenesis. An. Acad. Bras. Cienc. 2007, 79, 593–616. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Ba, K.S.A.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Willenbring, M.L.; Massey, S.H.; Gardner, M.B. Helping patients who drink too much: An evidence-based guide for primary care clinicians. Am. Fam. Physician 2009, 80, 44–50. [Google Scholar]
- Kannel, W.B.; Ellison, R.C. Alcohol and coronary heart disease: The evidence for a protective effect. Clin. Chim. Acta 1996, 246, 59–76. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Bagnardi, V.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch. Intern. Med. 2006, 166, 2437–2445. [Google Scholar] [CrossRef]
- Stockwell, T.; Zhao, J.; Panwar, S.; Roemer, A.; Naimi, T.; Chikritzhs, T. Do “Moderate” Drinkers Have Reduced Mortality Risk? A Systematic Review and Meta-Analysis of Alcohol Consumption and All-Cause Mortality. J. Stud. Alcohol Drugs 2016, 77, 185–198. [Google Scholar] [CrossRef]
- Goulden, R. Moderate Alcohol Consumption Is Not Associated with Reduced All-cause Mortality. Am. J. Med. 2016, 129, 180–186.e4. [Google Scholar] [CrossRef][Green Version]
- Ko, H.; Chang, Y.; Kim, H.-N.; Kang, J.-H.; Shin, H.; Sung, E.; Ryu, S. Low-level alcohol consumption and cancer mortality. Sci. Rep. 2021, 11, 4585. [Google Scholar] [CrossRef]
- Institute of Medicine and Board National Research Council National Cancer Policy. Fulfilling the Potential of Cancer Prevention and Early Detection; Curry, S.J., Byers, T., Hewitt, M., Eds.; National Academies Press: Washington, DC, USA, 2003; Copyright 2003 by the National Academy of Sciences. [Google Scholar]
- Brennan, P.; Lewis, S.; Hashibe, M.; Bell, D.A.; Boffetta, P.; Bouchardy, C.; Caporaso, N.; Chen, C.; Coutelle, C.; Diehl, S.R.; et al. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: A HuGE review. Am. J. Epidemiol. 2004, 159, 1–16. [Google Scholar] [CrossRef]
- Kawakita, D.; Matsuo, K. Alcohol and head and neck cancer. Cancer Metastasis Rev. 2017, 36, 425–434. [Google Scholar] [CrossRef]
- Chien, H.-T.; Young, C.-K.; Chen, T.-P.; Liao, C.-T.; Wang, H.-M.; Cheng, S.-D.; Huang, S.-F. Alcohol-metabolizing Enzymes’ Gene Polymorphisms and Susceptibility to Multiple Head and Neck Cancers. Cancer Prev. Res. 2019, 12, 247–254. [Google Scholar] [CrossRef]

| Total (n = 11,737,467) | Non-Drinker (0 g) (n = 7,010,332) | Mild Drinker (0 < x < 105 g) (n = 2,781,462) | Moderate Drinker (105 ≤ x < 210 g) (n= 1,113,038) | Heavy Drinker (≥210 g) (n = 832,635) | p-Values | |
|---|---|---|---|---|---|---|
| Mean ± SD, or No. (%) | ||||||
| Age (years) | 54.6 ± 10.4 | 56.4 ± 11.0 | 51.8 ± 9.7 | 51.5 ± 9.1 | 52.3 ± 9.5 | <0.0001 |
| Sex (male) | 5,612,691 (47.8) | 2,027,999 (28.9) | 1,805,061 (64.9) | 992,950 (89.2) | 786,681 (94.5) | <0.0001 |
| Income (lowest quartile) | 2,985,640 (25.4) | 1,883,641 (26.9) | 671,687 (24.2) | 243,933 (21.9) | 186,379 (22.4) | <0.0001 |
| Smoking status | <0.0001 | |||||
| Never smoker | 7,674,834 (65.4) | 5,810,163 (82.9) | 1,413,540 (50.8) | 283,125 (25.4) | 168,006 (20.2) | |
| Ex-smoker, <20 PY | 1,064,858 (9.1) | 330,639 (4.7) | 426,874 (15.4) | 190,356 (17.1) | 116,989 (14.1) | |
| Ex-smoker, ≥20 PY | 672,441 (5.7) | 235,408 (3.4) | 195,990 (7.1) | 122,703 (11.0) | 118,340 (14.2) | |
| Current smoker, <20 PY | 1,042,913 (8.9) | 281,892 (4.0) | 404,652 (14.6) | 227,469 (20.4) | 128,900 (15.5) | |
| Current smoker, ≥20 PY | 1,282,421 (10.9) | 352,230 (5.0) | 340,406 (12.2) | 289,385 (26.0) | 300,400 (36.1) | |
| Physical activity level | <0.0001 | |||||
| Non-regular | 9,408,663 (80.2) | 5,736,859 (81.8) | 2,165,900 (77.9) | 859,791 (77.3) | 646,113 (77.6) | |
| Regular | 2,328,804 (19.8) | 1,273,473 (18.2) | 615,562 (22.1) | 253,247 (22.7) | 186,522 (22.4) | |
| Body mass index (kg/m2) | 24.0 ± 3.0 | 23.9 ± 3.1 | 23.9 ± 2.9 | 24.3 ± 2.9 | 24.4 ± 3.0 | <0.0001 |
| Waist circumference (cm) | 81.1 ± 8.5 | 80.0 ± 8.7 | 81.5 ± 8.4 | 84.1 ± 7.7 | 85.1 ± 7.8 | <0.0001 |
| Systolic blood pressure (mmHg) | 124.1 ± 15.4 | 123.4 ± 15.7 | 123.5 ± 14.9 | 126.8 ± 14.8 | 128.3 ± 15.1 | <0.0001 |
| Diastolic blood pressure (mmHg) | 77.0 ± 10.1 | 76.1 ± 10.1 | 77.2 ± 10.1 | 79.6 ± 10.0 | 80.5 ± 10.1 | <0.0001 |
| Fasting glucose (mg/dL) | 99.9 ± 24.5 | 98.9 ± 24.0 | 99.4 ± 23.4 | 103.0 ± 26.5 | 105.8 ± 29.2 | <0.0001 |
| Total cholesterol (mg/dL) | 199.2 ± 37.0 | 199.8 ± 37.6 | 198.1 ± 35.8 | 199.0 ± 36.1 | 198.4 ± 37.0 | <0.0001 |
| HDL (mg/dL) | 54.8 ± 16.7 | 54.5 ± 17.3 | 55.0 ± 15.4 | 55.1 ± 15.5 | 56.1 ± 16.6 | <0.0001 |
| LDL (mg/dL) | 117.4 ± 34.1 | 119.9 ± 34.3 | 116.1 ± 33.1 | 111.9 ± 34.2 | 107.7 ± 35.3 | <0.0001 |
| eGFR (ml/min/1.73 m2) | 85.8 ± 34.5 | 84.6 ± 31.0 | 86.7 ± 38.1 | 88.3 ± 41.0 | 89.7 ± 40.4 | <0.0001 |
| Hypertension | 3,975,024 (33.9) | 2,406,414 (34.3) | 829,585 (29.8) | 404,736 (36.4) | 334,289 (40.2) | <0.0001 |
| Diabetes mellitus | 1,374,937 (11.7) | 832,222 (11.9) | 274,386 (9.9) | 141,392 (12.7) | 126,937 (15.3) | <0.0001 |
| Dyslipidemia | 2,616,859 (22.3) | 1,815,516 (25.9) | 564,073 (20.3) | 237,270 (21.3) | 181,905 (21.9) | <0.0001 |
| No. (%) | Event Number | Person-Years (PYs) | Incidence Rate (Per 100,000 PYs) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Average alcohol consumption a | |||||||
| Non-drinker | 7,010,332 (59.7) | 7503 | 47,804,729.9 | 15.7 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Mild drinker | 2,781,462 (23.7) | 3746 | 19,061,909.8 | 19.7 | 1.06 (1.02–1.11) | 1.03 (0.99–1.07) | 1.03 (0.98–1.07) |
| Moderate drinker | 1,113,038 (9.5) | 2299 | 7,606,759.9 | 30.2 | 1.42 (1.36–1.50) | 1.27 (1.20–1.33) | 1.26 (1.20–1.32) |
| Heavy drinker | 832,635 (7.1) | 2284 | 5,658,733.2 | 40.4 | 1.74 (1.65–1.83) | 1.47 (1.39–1.55) | 1.46 (1.38–1.53) |
| p-trend | <0.01 | <0.01 | <0.01 | ||||
| Drinking frequency (days per week) | |||||||
| 0 | 7,010,332 (59.7) | 7503 | 47,804,729.9 | 15.7 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 1 | 1,897,327 (16.2) | 2067 | 13,038,365.9 | 15.9 | 0.94 (0.90–0.99) | 0.92 (0.88–0.97) | 0.92 (0.88–0.97) |
| 2 | 1,224,552 (10.4) | 1947 | 8,395,959.4 | 23.2 | 1.18 (1.12–1.25) | 1.11 (1.05–1.17) | 1.10 (1.05–1.16) |
| 3 | 787,753 (6.7) | 1675 | 5,376,661.1 | 31.2 | 1.43 (1.36–1.52) | 1.29 (1.22–1.36) | 1.28 (1.21–1.36) |
| 4 | 272,126 (2.3) | 698 | 1,852,052.9 | 37.7 | 1.63 (1.50–1.76) | 1.42 (1.31–1.54) | 1.41 (1.30–1.53) |
| 5 | 208,885 (1.8) | 575 | 1,415,586 | 40.6 | 1.62 (1.48–1.76) | 1.39 (1.27–1.52) | 1.38 (1.26–1.50) |
| 6 | 117,079 (1.0) | 420 | 787,973.5 | 53.3 | 1.79 (1.62–1.98) | 1.51 (1.37–1.67) | 1.50 (1.36–1.66) |
| 7 | 219,413 (1.9) | 947 | 1,460,804.3 | 64.8 | 1.85 (1.72–1.98) | 1.56 (1.45–1.67) | 1.55 (1.45–1.67) |
| p-trend | <0.01 | <0.01 | <0.01 | ||||
| Daily amount (standard units per occasion) | |||||||
| 0 | 7,010,332 (59.7) | 7503 | 47,804,729.9 | 15.7 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 1–2 | 827,080 (7.0) | 1055 | 5,641,265.8 | 18.7 | 0.99 (0.92–1.05) | 0.98 (0.92–1.05) | 0.98 (0.92–1.05) |
| 3–4 | 1,155,831 (9.8) | 2006 | 7,906,930.4 | 25.4 | 1.22 (1.16–1.29) | 1.15 (1.10–1.21) | 1.15 (1.09–1.21) |
| 5–7 | 1,622,238 (13.8) | 3240 | 11,084,760.3 | 29.2 | 1.41 (1.35–1.48) | 1.26 (1.20–1.32) | 1.25 (1.19–1.31) |
| 8–14 | 923,266 (7.9) | 1668 | 6,330,255.9 | 26.4 | 1.43 (1.35–1.51) | 1.25 (1.18–1.32) | 1.24 (1.17–1.31) |
| >14 | 198,720 (1.7) | 360 | 1,364,190.6 | 26.4 | 1.47 (1.32–1.64) | 1.27 (1.14–1.41) | 1.26 (1.13–1.40) |
| p-trend | <0.01 | <0.01 | <0.01 | ||||
| Cancer Subtypes | ||||
|---|---|---|---|---|
| Oral Cavity HR (95% CI) | Pharynx HR (95% CI) | Larynx HR (95% CI) | Salivary Gland HR (95% CI) | |
| Event N | 4275 | 5598 | 4862 | 1916 |
| Average alcohol consumption a | ||||
| Non-drinker | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Mild drinker | 1.08 (1.00–1.17) | 0.98 (0.92–1.06) | 1.00 (0.93–1.08) | 0.99 (0.88–1.11) |
| Moderate drinker | 1.34 (1.21–1.49) | 1.27 (1.17–1.38) | 1.33 (1.22–1.45) | 0.95 (0.80–1.12) |
| Heavy drinker | 1.44 (1.29–1.60) | 1.51 (1.39–1.64) | 1.62 (1.49–1.76) | 1.14 (0.96–1.36) |
| Drinking frequency (days per week) | ||||
| 0 | 1(ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 1 | 0.98 (0.89–1.08) | 0.91 (0.84–1.00) | 0.86 (0.79–0.95) | 0.91 (0.79–1.05) |
| 2 | 1.19 (1.07–1.33) | 1.03 (0.94–1.12) | 1.13 (1.03–1.24) | 0.99 (0.85–1.17) |
| 3 | 1.28 (1.14–1.44) | 1.26 (1.15–1.38) | 1.40 (1.27–1.54) | 1.10 (0.92–1.32) |
| 4 | 1.47 (1.24–1.73) | 1.48 (1.30–1.68) | 1.37 (1.20–1.57) | 1.30 (1.01–1.68) |
| 5 | 1.51 (1.26–1.80) | 1.32 (1.14–1.53) | 1.40 (1.21–1.61) | 0.88 (0.63–1.23) |
| 6 | 1.56 (1.26–1.92) | 1.57 (1.33–1.84) | 1.71 (1.48–1.99) | 0.79 (0.51–1.24) |
| 7 | 1.53 (1.31–1.78) | 1.71 (1.53–1.91) | 1.61 (1.44–1.79) | 1.29 (1.00–1.67) |
| Daily amount (standard units per occasion) | ||||
| 0 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 1–2 | 1.02 (0.89–1.15) | 0.96 (0.86–1.07) | 0.95 (0.85–1.07) | 1.00 (0.83–1.20) |
| 3–4 | 1.30 (1.18–1.45) | 1.10 (1.01–1.20) | 1.09 (0.99–1.19) | 1.04 (0.89–1.22) |
| 5–7 | 1.19 (1.08–1.31) | 1.26 (1.17–1.35) | 1.39 (1.29–1.50) | 1.00 (0.86–1.15) |
| 8–14 | 1.35 (1.20–1.52) | 1.28 (1.17–1.41) | 1.37 (1.24–1.51) | 0.93 (0.77–1.13) |
| >14 | 1.10 (0.86–1.40) | 1.27 (1.06–1.51) | 1.51 (1.27–1.80) | 1.13 (0.81–1.58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, H.Y.; Han, K.; Shin, D.W.; Yoo, J.E.; Cho, M.H.; Jeon, K.H.; Kim, D.; Hong, S.; Jun, J.K. Alcohol Drinking Pattern and Risk of Head and Neck Cancer: A Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 11204. https://doi.org/10.3390/ijerph182111204
Koo HY, Han K, Shin DW, Yoo JE, Cho MH, Jeon KH, Kim D, Hong S, Jun JK. Alcohol Drinking Pattern and Risk of Head and Neck Cancer: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(21):11204. https://doi.org/10.3390/ijerph182111204
Chicago/Turabian StyleKoo, Hye Yeon, Kyungdo Han, Dong Wook Shin, Jung Eun Yoo, Mi Hee Cho, Keun Hye Jeon, Dahye Kim, Sangduk Hong, and Jae Kwan Jun. 2021. "Alcohol Drinking Pattern and Risk of Head and Neck Cancer: A Nationwide Cohort Study" International Journal of Environmental Research and Public Health 18, no. 21: 11204. https://doi.org/10.3390/ijerph182111204
APA StyleKoo, H. Y., Han, K., Shin, D. W., Yoo, J. E., Cho, M. H., Jeon, K. H., Kim, D., Hong, S., & Jun, J. K. (2021). Alcohol Drinking Pattern and Risk of Head and Neck Cancer: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health, 18(21), 11204. https://doi.org/10.3390/ijerph182111204






