Evaluation of the Impacts of Abandoned Mining Areas: A Case Study with Benthic Macroinvertebrate Assemblages
Abstract
1. Introduction
2. Materials and Methods
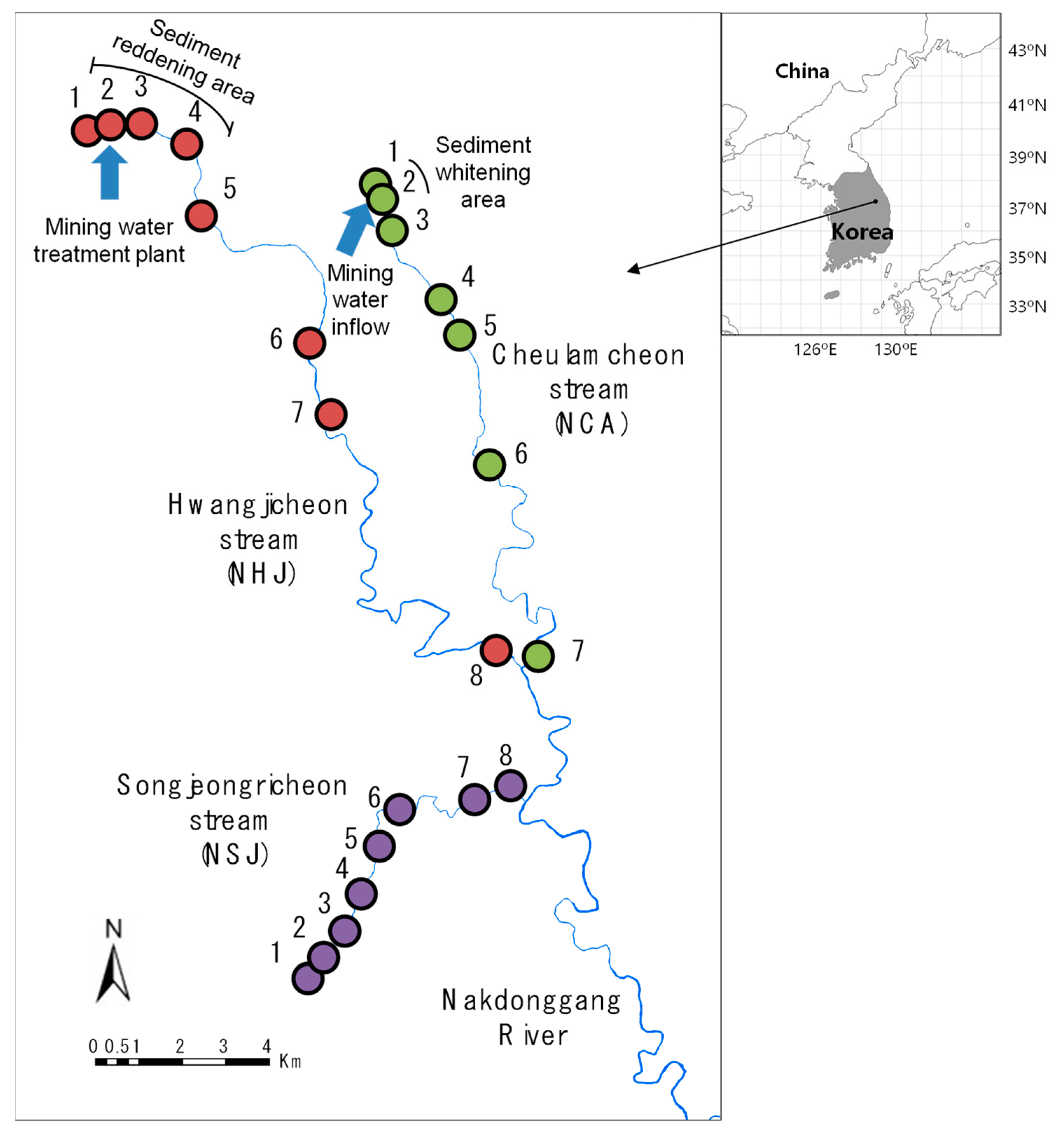
2.1. Study Area
2.2. Ecological Data
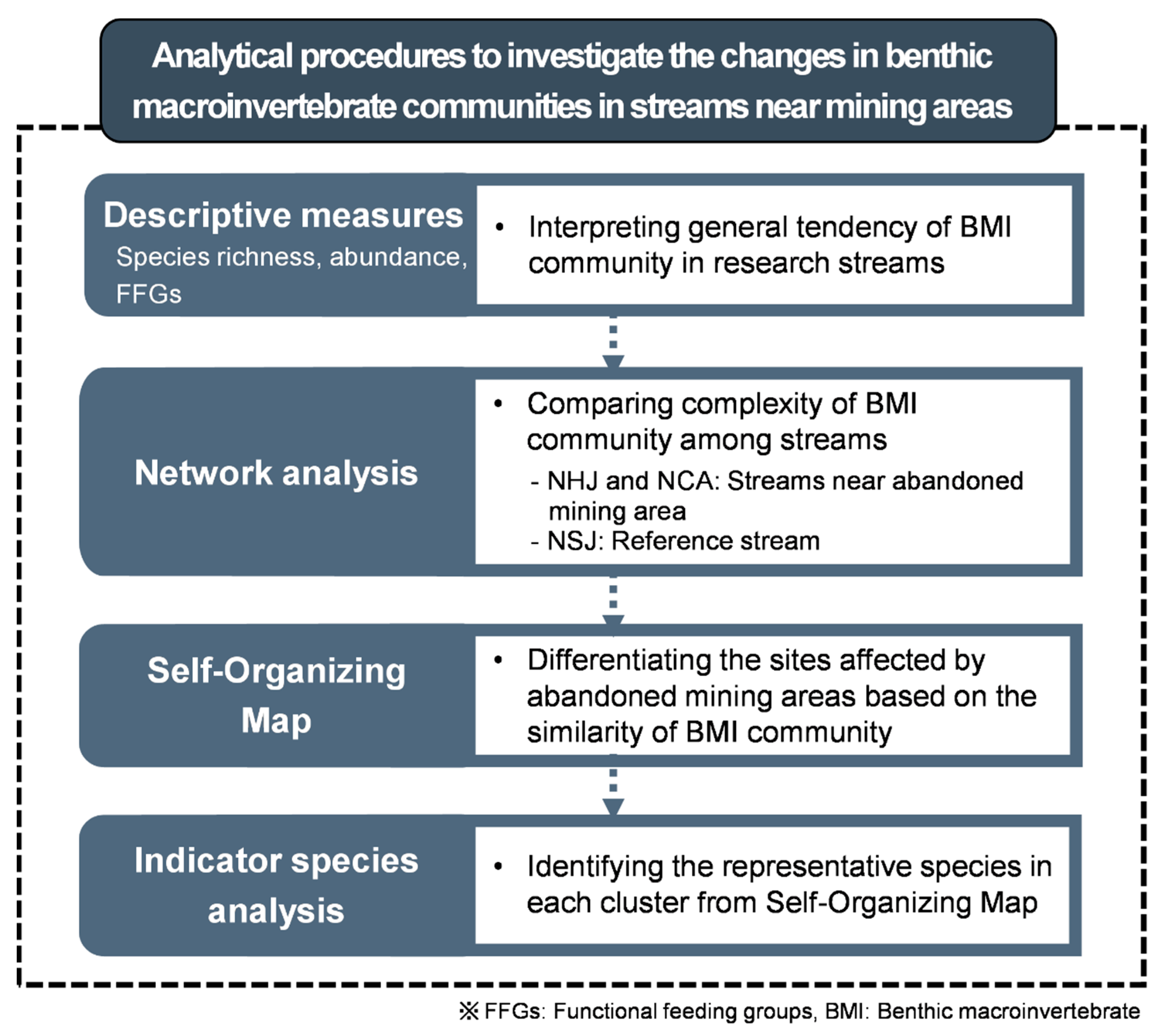
2.3. Data Analysis
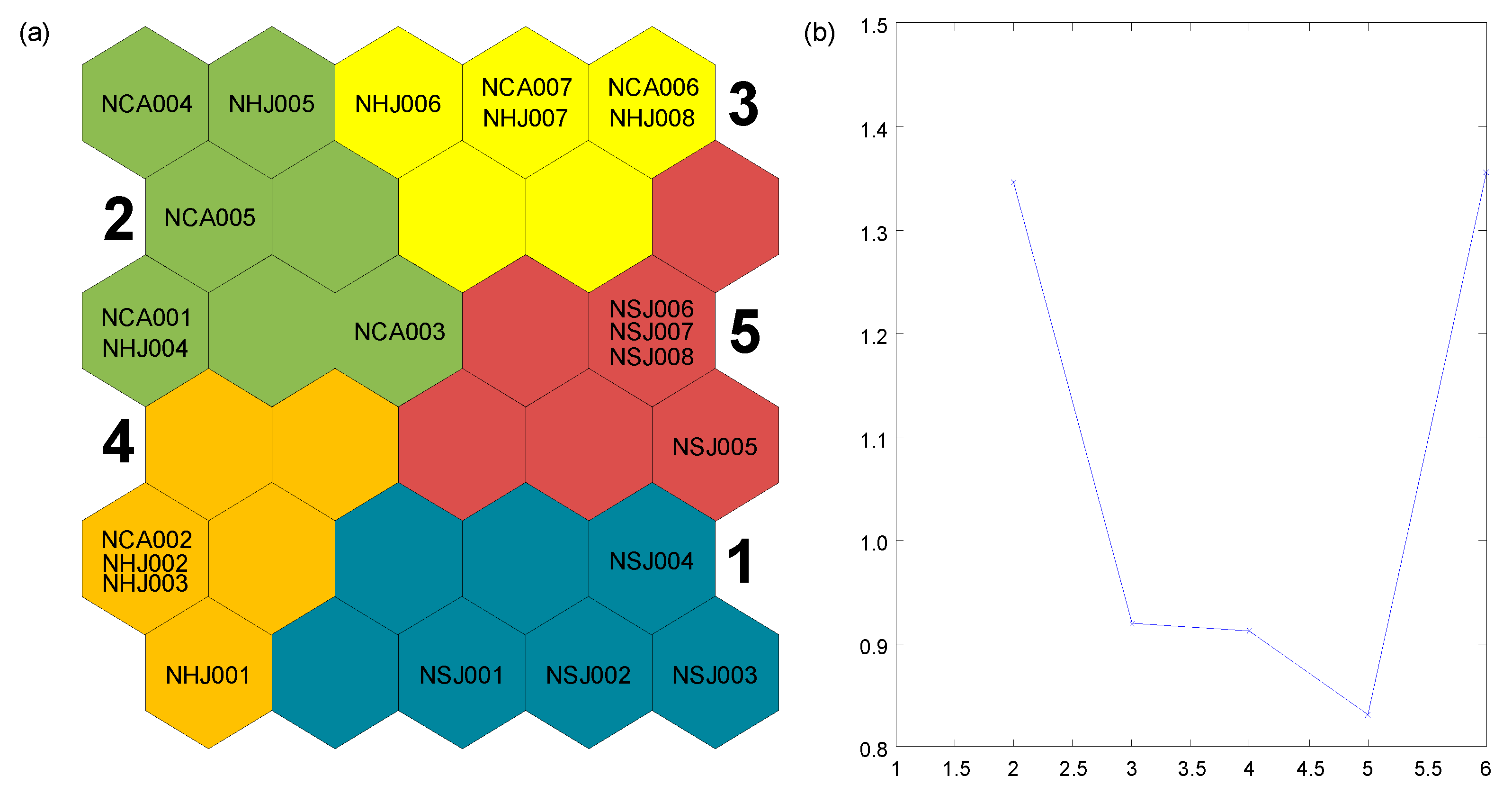
3. Results
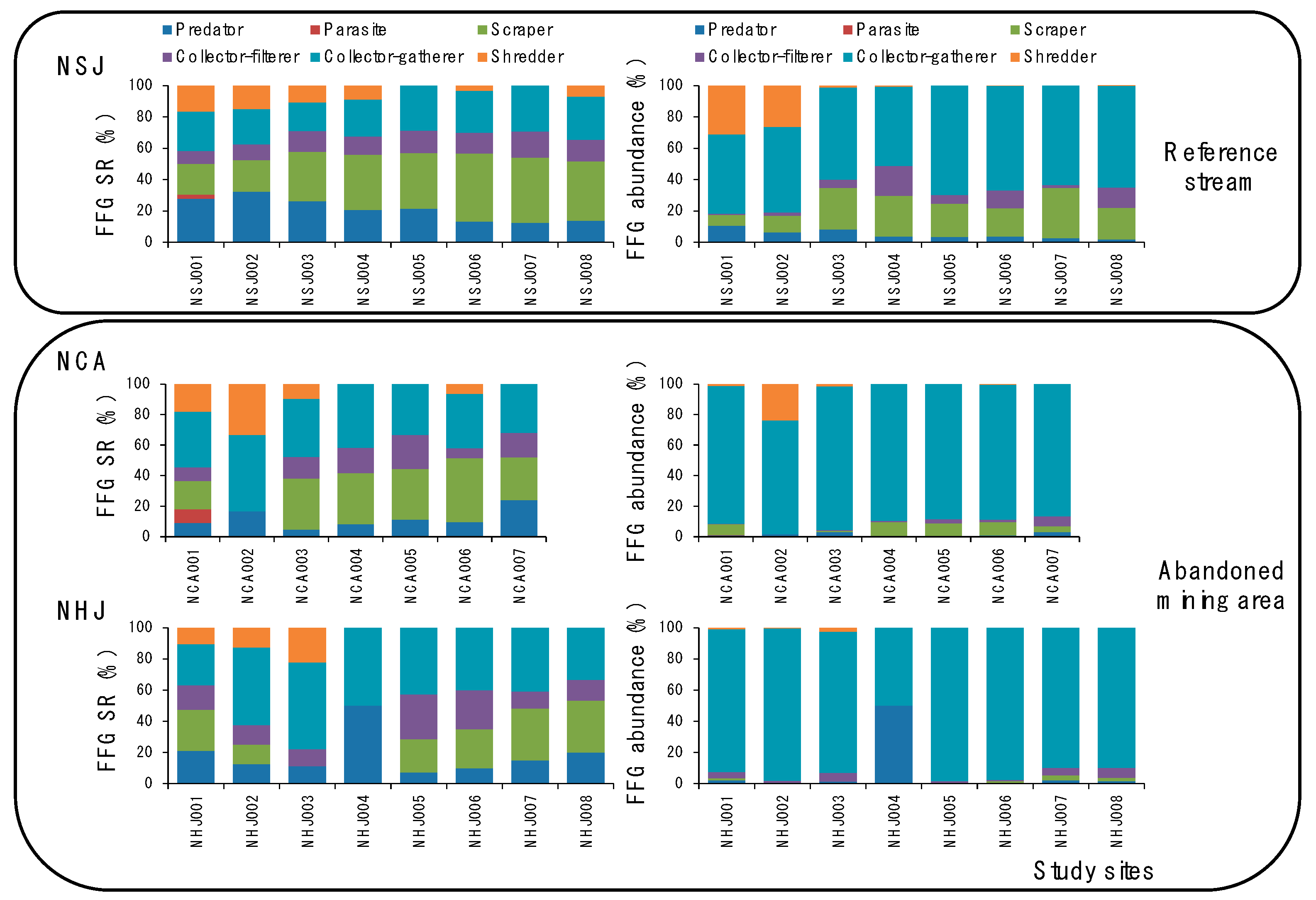
3.1. Differences in Benthic Macroinvertebrate Community among Streams
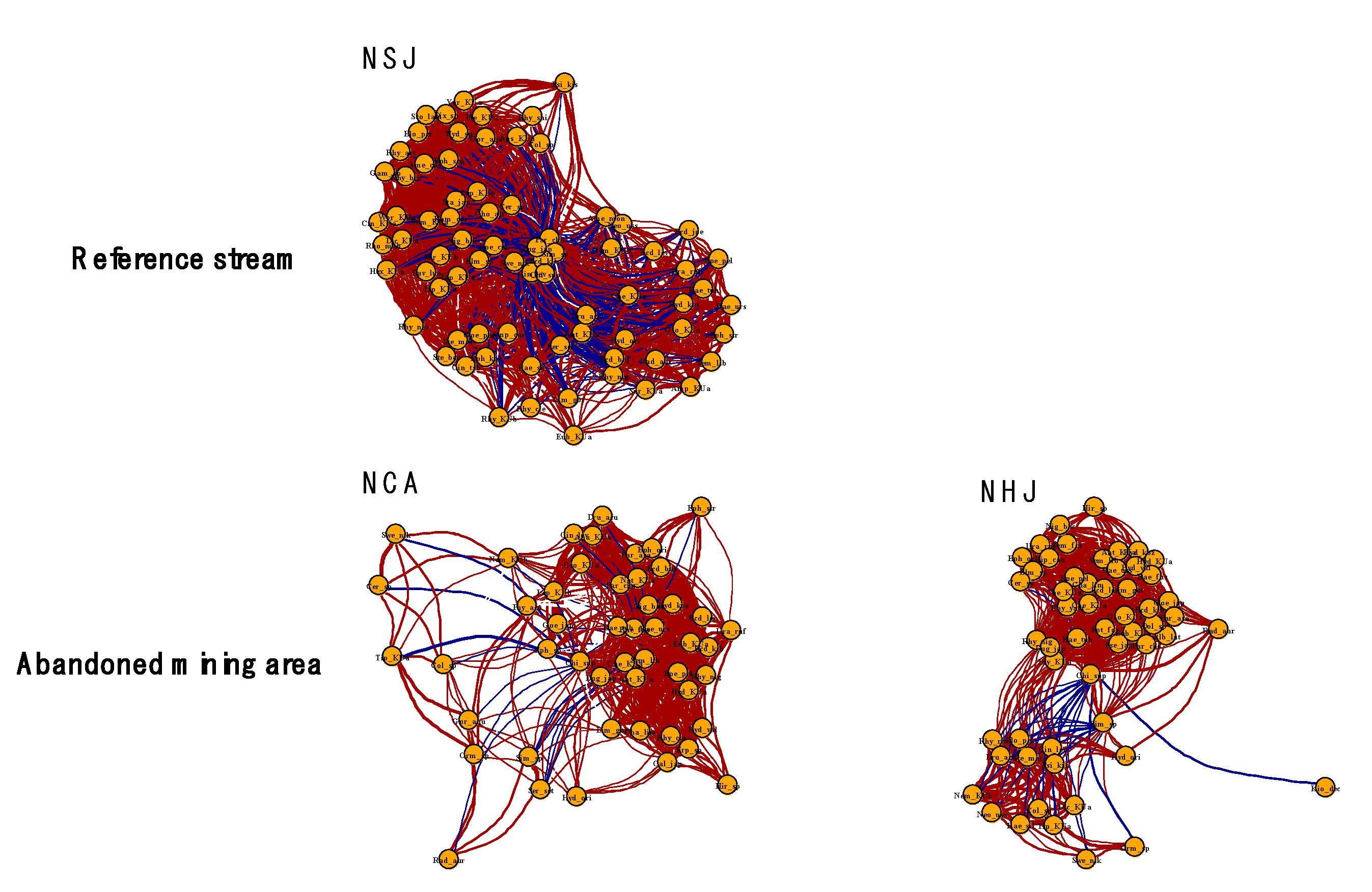
3.2. Species Co-Occurrence Patterns of the Benthic Macroinvertebrate Community
3.3. Patterns of Benthic Macroinvertebrate Community
3.4. Indicator Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, A.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Bae, M.J.; Li, F.; Kwon, Y.S.; Chung, N.; Choi, H.; Hwang, S.J.; Park, Y.S. Concordance of diatom, macroinvertebrate and fish assemblages in streams at nested spatial scales: Implications for ecological integrity. Ecol. Indic. 2014, 47, 89–101. [Google Scholar] [CrossRef]
- Marqués, M.J.; Martínez-Conde, E.; Rovira, J.V.; Ordóñez, S. Heavy metals pollution of aquatic ecosystems in the vicinity of a recently closed underground lead-zinc mine (Basque Country, Spain). Environ. Geol. 2001, 40, 1125–1137. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Harding, J.S.; Simon, K.S. Organic matter breakdown as a measure of stream health in New Zealand streams affected by acid mine drainage. Ecol. Indic. 2013, 24, 510–517. [Google Scholar] [CrossRef]
- Pond, G.J.; Passmore, M.E.; Borsuk, F.A.; Reynolds, L.; Rose, C.J. Downstream effects of mountaintop coal mining: Comparing biological conditions using family-and genus-level macroinvertebrate bioassessment tools. J. North Am. Benthol. Soc. 2008, 27, 717–737. [Google Scholar] [CrossRef]
- Bere, T.; Dalu, T.; Mwedzi, T. Detecting the impact of heavy metal contaminated sediment on benthic macroinvertebrate communities in tropical streams. Sci. Total Environ. 2016, 572, 147–156. [Google Scholar] [CrossRef]
- Jarsjö, J.; Chalov, S.R.; Pietroń, J.; Alekseenko, A.V.; Thorslund, J. Patterns of soil contamination, erosion and river loading of metals in a gold mining region of northern Mongolia. Reg. Environ. Chang. 2017, 17, 1991–2005. [Google Scholar] [CrossRef]
- Mol, J.H.; Ouboter, P.E. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small neotropical rainforest stream. Conserv. Biol. 2004, 18, 201–214. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lock, R.A.C.; Van der Velde, G.; Hoyos, R.M.; Roelofs, J.G.M. Effects of mining activities on heavy metal concentrations in water, sediment, and macroinvertebrates in different reaches of the Pilcomayo River, South America. Arch. Environ. Contam. Toxicol. 2003, 44, 0314–0323. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D. Tropical high-altitude streams. In Tropical Stream Ecology; Dudgeon, D., Ed.; Academic Press: San Diego, CA, USA, 2008; pp. 219–256. [Google Scholar]
- Loayza-Muro, R.A.; Elías-Letts, R.; Marticorena-Ruíz, J.K.; Palomino, E.J.; Duivenvoorden, J.F.; Kraak, M.H.; Admiraal, W. Metal-induced shifts in benthic macroinvertebrate community composition in Andean high altitude streams. Environ. Toxicol. Chem. 2010, 29, 2761–2768. [Google Scholar] [CrossRef]
- Armitage, P.D.; Bowes, M.J.; Vincent, H.M. Long-term changes in macroinvertebrate communities of a heavy metal polluted stream: The river Nent (Cumbria, UK) after 28 years. River Res. Appl. 2007, 23, 997–1015. [Google Scholar] [CrossRef]
- Romero, A.; Flores, S.; Medina, R. Estudio de los metales pesados en el relave abandonado de Ticapampa. Rev. Inst. Inves. Figmmg. 2008, 11, 13–16. [Google Scholar]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Hussain, Q.A.; Pandit, A.K. Macroinvertebrates in streams: A review of some ecological factors. Int. J. Fish. Aquacult. 2012, 4, 114–123. [Google Scholar] [CrossRef]
- Hawkins, C.P.; Murphy, M.L.; Anderson, N.H. Effects of canopy, substrate composition, and gradient on the structure of macroinvertebrate communities in Cascade Range streams of Oregon. Ecology 1982, 63, 1840–1856. [Google Scholar] [CrossRef]
- Minshall, G.W.; Cummins, K.W.; Petersen, R.C.; Gushing, C.E.; Bruns, D.A.; Sedell, J.R.; Vannote, R.L. Developments in stream ecosystem theory. Can. J. Fish. Aquat. Sci. 1985, 42, 1045–1055. [Google Scholar] [CrossRef]
- Grubaugh, J.W.; Wallace, J.B.; Houston, E.S. Longitudinal changes of macroinvertebrate communities along an Appalachian stream continuum. Can. J. Fish. Aquat. Sci. 1996, 53, 896–909. [Google Scholar] [CrossRef]
- Nelson, S.M.; Roline, R.A. Recovery of a stream macroinvertebrate community from mine drainage disturbance. Hydrobiologia 1996, 339, 73–84. [Google Scholar] [CrossRef]
- Solà, C.; Burgos, M.; Plazuelo, Á.; Toja, J.; Plans, M.; Prat, N. Heavy metal bioaccumulation and macroinvertebrate community changes in a Mediterranean stream affected by acid mine drainage and an accidental spill (Guadiamar River, SW Spain). Sci. Total Environ. 2004, 333, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Bataglia, M.; Hose, G.C.; Turak, E.; Warden, B. Depauperate macroinvertebrates in a mine affected stream: Clean water may be key to recovery. Environ. Pollut. 2005, 138, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.A.; Lawrence, E.R.; Jones, T.G. Treated and untreated acid mine drainage effects on stream periphyton biomass, leaf decomposition, and macroinvertebrate diversity. J. Freshw. Ecol. 2005, 20, 413–424. [Google Scholar] [CrossRef][Green Version]
- Tripole, S.; Gonzalez, P.; Vallania, A.; Garbagnati, M.; Mallea, M. Evaluation of the impact of acid mine drainage on the chemistry and the macrobenthos in the Carolina stream (San Luis, Argentina). Environ. Monit. Assess. 2006, 114, 377–389. [Google Scholar] [CrossRef] [PubMed]
- MacCausland, A.; McTammany, M.E. The impact of episodic coal mine drainage pollution on benthic macroinvertebrates in streams in the Anthracite region of Pennsylvania. Environ. Pollut. 2007, 149, 216–226. [Google Scholar] [CrossRef]
- Bott, T.L.; Jackson, J.K.; McTammany, M.E.; Newbold, J.D.; Rier, S.T.; Sweeney, B.W.; Battle, J.M. Abandoned coal mine drainage and its remediation: Impacts on stream ecosystem structure and function. Ecol. Appl. 2012, 22, 2144–2163. [Google Scholar] [CrossRef]
- Malmqvist, B.; Hoffsten, P.O. Influence of drainage from old mine deposits on benthic macroinvertebrate communities in central Swedish streams. Water Res. 1999, 33, 2415–2423. [Google Scholar] [CrossRef]
- Lefcort, H.; Vancura, J.; Lider, E.L. 75 years after mining ends stream insect diversity is still affected by heavy metals. Ecotoxicology 2010, 19, 1416–1425. [Google Scholar] [CrossRef]
- Kiffney, P.M.; Clements, W.H. Structural responses of benthic macroinvertebrate communities from different stream orders to zinc. Environ. Toxicol. Chem. 1994, 13, 389–395. [Google Scholar] [CrossRef]
- Gerhardt, A.; Janssens de Bisthoven, L.; Soares, A.M.V.M. Macroinvertebrate response to acid mine drainage: Community metrics and on-line behavioural toxicity bioassay. Environ. Pollut. 2004, 130, 263–274. [Google Scholar] [CrossRef]
- Clements, W.H. Metal tolerance and predator–prey interactions in benthic macroinvertebrate stream communities. Ecol. Appl. 1999, 9, 1073–1084. [Google Scholar] [CrossRef]
- O’Halloran, K.; Cavanagh, J.-A.; Harding, J.S. Response of a New Zealand mayfly (Deleatidium spp.) to acid mine drainage: Implications for mine remediation. Environ. Toxicol. Chem. 2008, 27, 1135–1140. [Google Scholar] [CrossRef]
- Salinas, G.; Marin, R. Impact of sediment releases on water chemistry and macroinvertebrate communities in clear water Andean streams (Bolivia). Arch. Hydrobiol. 2001, 1, 33–50. [Google Scholar] [CrossRef]
- Herbst, D.B.; Medhurst, R.B.; Black, N.J. Long-term effects and recovery of streams from acid mine drainage and evaluation of toxic metal threshold ranges for macroinvertebrate community reassembly. Environ. Toxicol. 2018, 37, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.H.; Herbst, D.B.; Hornberger, M.I.; Mebane, C.A.; Short, T.M. Long-term monitoring reveals convergent patterns of recovery from mining contamination across 4 western US watersheds. Freshw. Sci. 2021, 40, 407–426. [Google Scholar] [CrossRef]
- Freeman, L.C. Centrality in social networks conceptual clarification. Soc. Netw. 1978, 1, 215–239. [Google Scholar] [CrossRef]
- Bluthgen, N. Why network analysis is often disconnected from community ecology: A critique and an ecologist’s guide. Basic Appl. Ecol. 2010, 11, 185–195. [Google Scholar] [CrossRef]
- Bae, M.J.; Park, Y.S. Evaluation of precipitation impacts on benthic macroinvertebrate communities at three different stream types. Ecol. Indic. 2019, 102, 446–456. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Bae, M.J.; Park, Y.S. Biological early warning system based on the responses of aquatic organisms to disturbances: A review. Sci. Total Environ. 2014, 466, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Peng, W.; Liu, Y.; Zhang, M.; Ren, Z.; Wu, N.; Liu, X. Networks and ordination analyses reveal the stream community structures of fish, macroinvertebrate and benthic algae, and their responses to nutrient enrichment. Ecol. Indic. 2019, 101, 501–511. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Korea Meteorological Administration. Available online: http://www.kma.go.kr/ (accessed on 8 October 2021).
- MIRECO. Yearbook of Mireco Statistics. Mine Reclamation Corporation, 2018. Available online: www.mireco.or.kr (accessed on 5 March 2018).
- Quigley, M. Invertebrates of Streams and Rivers: A Key to Identification; Edward Arnold, Ltd.: London, UK, 1977. [Google Scholar]
- Pennak, R.W. Freshwater Invertebrates of the United States; John Wiley and Sons, Inc.: New York, NY, USA, 1978. [Google Scholar]
- Brigham, A.R.; Brigham, W.U.; Gnilka, A. Aquatic Insects and Oligochaetes of North and South Carolina; Midwest Aquatic Enterprises: Mahomet, IL, USA, 1982; 837p. [Google Scholar]
- Brinkhurst, R.O. Guide to the freshwater aquatic microdrile Oligochaetes of North America. Can. Spec. Publ. Fish. Aquat. Sci. 1986, 84, 259. [Google Scholar]
- Yoon, I.B. Illustrated Encyclopedia of Fauna and Flora of Korea; Ministry of Education: Seoul, Korea, 1988; Volume 30. (In Korean)
- Merritt, R.W.; Cummins, K.W. An Introduction to the Aquatic Insects of North America; Hunt Publishing Company: Dubugue, IA, USA, 2006. [Google Scholar]
- APHA; AWW; WPCF. Standard Methods for the Examination of Water and Wastewater, 21th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Febria, C.M.; Hosen, J.D.; Crump, B.C.; Palmer, M.A.; Williams, D.D. Microbial responses to changes in flow status in temporary headwater streams: A cross-system comparison. Front. Microbiol. 2015, 6, 522. [Google Scholar] [CrossRef] [PubMed]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Inter. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 11 December 2017).
- Vesanto, J.; Himberg, J.; Alhoniemi, E.; Parhankangas, J. SOM Toolbox for Matlab 5; Technical Report A57; Neural Networks Research Centre, Helsinki University of Technology: Helsinki, Finland, 2000. [Google Scholar]
- Céréghino, R.; Park, Y.S. Review of the self-organizing map (SOM) approach in water resources: Commentary. Environ. Model. Softw. 2009, 24, 945–947. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L.F. Numerical Ecology, 3rd ed.; Elsevier Science BV: Amsterdam, The Netherland, 2012. [Google Scholar]
- García, H.L.; González, I.M. Self-organizing map and clustering for wastewater treatment monitoring. Eng. Appl. Artif. Intell. 2004, 17, 215–225. [Google Scholar] [CrossRef]
- The Mathworks, Inc. MATLAB Version 6.1; The Mathworks, Inc.: Natick, MA, USA, 2001. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The Vegan Package: Community Ecology Package. 2007. Available online: http://cran.r-project.org (accessed on 11 December 2017).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Package Version. 2014. Available online: http://cran.r-project.org (accessed on 11 December 2017).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Fortunato, C.S.; Eiler, A.; Herfort, L.; Needoba, J.A.; Peterson, T.D.; Crump, B.C. Determining indicator taxa across spatial and seasonal gradients in the Columbia River coastal margin. ISME J. 2013, 7, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W. Labdsv: Ordination and Multivariate Analysis for Ecology. R Package Version 1.4–1. 2010. Available online: http://CRAN.R-project.org/package¼labdsv (accessed on 11 December 2017).
- Garie, H.L.; McIntosh, A. Distribution of benthic macroinvertebrates in a stream exposed to urban runoff. Water Resour. Bull. 1986, 22, 447–455. [Google Scholar] [CrossRef]
- Freeman, P.L.; Schorr, M.S. Influence of watershed urbanization on fine sediment and macroinvertebrate assemblage characteristics in Tennessee Ridge and Valley Streams. J. Freshw. Ecol. 2004, 19, 353–362. [Google Scholar] [CrossRef]
- Water Environment Information System. Available online: http://water.nier.go.kr/ (accessed on 8 August 2020).
- Pond, G.J.; Passmore, M.E.; Pointon, N.D.; Felbinger, J.K.; Walker, C.A.; Krock, K.J.; Fulton, J.B.; Nash, W.L. Long-term impacts on macroinvertebrates downstream of reclaimed mountaintop mining valley fills in central Appalachia. Environ. Manag. 2014, 54, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, F.; Zhang, Y.; Yang, Z.; Ma, S. Effects of secondary salinisation on macroinvertebrate functional traits in surface mining-contaminated streams, and recovery potential. Sci. Total Environ. 2018, 640, 1088–1097. [Google Scholar] [CrossRef]
- Timpano, A.J.; Schoenholtz, S.H.; Soucek, D.J.; Zipper, C.E. Benthic macroinvertebrate community response to salinization in headwater streams in Appalachia USA over multiple years. Ecol. Indic. 2018, 91, 645–656. [Google Scholar] [CrossRef]
- Timpano, A.J.; Schoenholtz, S.H.; Soucek, D.J.; Zipper, C.E. Salinity as a limiting factor for biological condition in mining-influenced central Appalachian headwater streams. J. Am. Water Resour. Assoc. 2015, 51, 240–250. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Malherbe, W.; Zimmermann, S.; Lorenz, A.W.; Nachev, M.; Wepener, V.; Sures, B.; Smit, N.J. Metal accumulation in riverine macroinvertebrates from a platinum mining region. Sci. Total Environ. 2020, 703, 134738. [Google Scholar] [CrossRef]
- Drover, D.R.; Schoenholtz, S.H.; Soucek, D.J.; Zipper, C.E. Multiple stressors influence benthic macroinvertebrate communities in central Appalachian coalfield streams. Hydrobiologia 2020, 847, 191–205. [Google Scholar] [CrossRef]
- Frick, K.G.; Herrmann, J. Aluminium and pH effects on sodium ion regulation in mayflies. In The Surface Waters Acidification Program; Mason, B.J., Ed.; Cambridge University Press: London, UK, 1990; pp. 409–412. [Google Scholar]
- Frick, K.G.; Herrmann, J. Aluminium accumulation in a lotic mayfly at low pH-A laboratory study. Ecotox. Environ. Saf. 1990, 19, 81–88. [Google Scholar] [CrossRef]
- Clements, W.H.; Kotalik, C. Effects of major ions on natural benthic communities: An experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 2016, 35, 126–138. [Google Scholar] [CrossRef]
- Maret, T.R.; Cain, D.J.; MacCoy, D.E.; Short, T.M. Response of benthic invertebrate assemblages to metal exposure and bioaccumulation associated with hard-rock mining in northwestern streams, USA. J. North Am. Benthol. Soc. 2003, 22, 598–620. [Google Scholar] [CrossRef]
- Conley, J.M.; Funk, D.H.; Cariello, N.J.; Buchwalter, D.B. Food rationing affects dietary selenium bioaccumulation and life cycle performance in the mayfly Centroptilum triangulifer. Ecotoxicology 2011, 20, 1840–1851. [Google Scholar] [CrossRef]
- Arnold, M.C.; Bier, R.L.; Lindberg, T.T.; Bernhardt, E.S.; Di Giulio, R.T. Biofilm mediated uptake of selenium in streams with mountaintop coal mine drainage. Limnologica 2017, 65, 10–13. [Google Scholar] [CrossRef]
- Cianciolo, T.R.; McLaughlin, D.L.; Zipper, C.E.; Timpano, A.J.; Soucek, D.J.; Schoenholtz, S.H. Impacts to water quality and biota persist in mining-influenced Appalachian streams. Sci. Total Environ. 2020, 717, 137216. [Google Scholar] [CrossRef] [PubMed]
- Stagliano, D.M.; Whiles, M.R. Macroinvertebrate production and trophic structure in a tallgrass prairie headwater stream. J. North Am. Benthol. Soc. 2002, 21, 97–113. [Google Scholar] [CrossRef][Green Version]
- Wallace, J.B.; Webster, J.R. The role of macroinvertebrates in stream ecosystem function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef]
- Reinhart, K.O.; VandeVoort, R. Effect of native and exotic leaf litter on macroinvertebrate communities and decomposition in a western Montana stream. Divers. Distrib. 2006, 12, 776–781. [Google Scholar] [CrossRef]
- Qu, X.; Wu, N.; Tang, T.; Cai, Q.; Park, Y.S. Effects of heavy metals on benthic macroinvertebrate communities in high mountain streams. Ann. Limnol. Int. J. Lim. 2010, 46, 291–302. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Reference Stream | Streams Located Near Abandoned Mining Areas | |
|---|---|---|---|
| NSJ | NCA | NHJ | |
| Number of nodes | 76 | 49 | 55 |
| Number of edges | 1635 | 622 | 826 |
| Average node degree | 38.68 | 22.94 | 25.60 |
| Average path length | 1.48 | 1.52 | 1.53 |
| Transitivity | 0.70 | 0.73 | 0.78 |
| Edge density | 0.52 | 0.48 | 0.47 |
| Clusters | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Species richness | 37 (3) a | 12 (6) b | 27 (4) ab | 11 (6) b | 28 (3) a |
| Abundance | 6815 (3391) | 7917 (11,782) | 20,819 (16,946) | 1394 (1324) | 7193 (3431) |
| Ephemeroptera | 1404 (387) a | 99 (157) b | 1181 (513) a | 238 (442) ab | 2921 (1747) a |
| Plecoptera | 1324 (1444) a | 4 (6) b | 2 (5) b | 20 (19) a | 7 (5) ab |
| Trichoptera | 357 (242) | 120 (191) | 957 (933) | 45 (88) | 676 (596) |
| Ephemeroptera | 11 (2) a | 2 (2.3) b | 9.2 (2.9) ab | 1.5 (1.7) b | 12 (0.8) a |
| Plecoptera | 6.3 (0.5) a | 0.5 (0.5) b | 0.2 (0.4) b | 1.3 (0.5) ab | 1.3 (0.5) a |
| Trichoptera | 8.8 (1.0) a | 2.5 (2.6) b | 7.2 (1.1) a | 2 (3.4) b | 6.5 (1.9) ab |
| EPT abundance | 3085 (1258) a | 223 (348) b | 2141 (925) a | 304 (534) ab | 3605 (2267) a |
| ETP richness | 26 (2.2) a | 5 (4.9) b | 16.6 (3.7) a | 4.8 (4.9) ab | 19.8 (1.7) a |
| Non-insecta abundance | 452 (619) a | 185 (165) ab | 456 (280) a | 21 (21) b | 150 (70) a |
| Other-insecta abundance | 3278 (1916) ab | 7509 (11,494) a | 18,222 (16,269) a | 1069 (827) b | 3438 (1207) a |
| Non-insecta species richness | 3 (0.8) ab | 4 (2.4) a | 5.6 (0.9) a | 1.5 (0.6) b | 3 (1.2) a |
| Other-insecta species richness | 8 (2.2) a | 2.5 (0.8) b | 4.4 (1.5) ab | 4.3 (1) a | 5 (0.8) a |
| Dominance index | 0.61 (0.09) b | 0.95 (0.04) a | 0.82 (0.1) ab | 0.89 (0.06) a | 0.64 (0.06) b |
| Shannon diversity | 3.06 (0.34) a | 0.78 (0.28) b | 1.56 (0.71) a | 1.14 (0.63) ab | 2.69 (0.28) a |
| Richness index | 17.71 (1.12) a | 27.93 (24.07) a | 16.12 (1.57) b | 23.03 (4.04) a | 17.58 (1.06) ab |
| Evenness | 0.42 (0.05) a | 0.11 (0.04) b | 0.21 (0.1) a | 0.16 (0.09) ab | 0.37 (0.04) a |
| Category | Cluster | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| FFG abundance | Predator (%) | 7.3 (2.9) | 9.1 (20.1) | 1.6 (1) | 1.4 (0.8) | 3 (0.9) |
| Parasite (%) | 0 (0) | 0 (0.1) | 0 (0) | 0 (0) | 0 (0) | |
| Scraper (%) | 17.4 (10.2) a | 4.4 (4.5) ab | 3.9 (2.9) a | 0.4 (0.6) b | 22.8 (6.2) a | |
| Collector filterer (%) | 6.8 (8.3) | 1 (1) | 4 (2.8) | 2.7 (2.6) | 7.9 (5.1) | |
| Collector gatherer (%) | 53.6 (4) b | 85.1 (17.6) a | 90.5 (4.2) a | 88.6 (9.9) a | 66.2 (2.7) ab | |
| Shredder (%) | 14.8 (16.2) | 0.5 (0.7) | 0.1 (0.1) | 6.9 (11.3) | 0 (0.1) | |
| FFG species richness | Predator (%) | 26.8 (4.9) a | 15.1 (17.2) b | 15.7 (6.3) ab | 15.3 (4.5) a | 15.3 (4.1) a |
| Parasite (%) | 0.7 (1.4) | 1.5 (3.7) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| Scraper (%) | 26.6 (8.1) a | 23.3 (13.2) ab | 32.3 (6.5) a | 9.7 (12.5) b | 39.7 (3.5) a | |
| Collector filterer (%) | 10.8 (2.1) | 15.1 (10) | 14.4 (6.9) | 9.9 (6.9) | 14.5 (1.5) | |
| Collector gatherer (%) | 22.4 (2.8) b | 40.4 (5.9) a | 36.3 (3.9) a | 45.5 (13) a | 28 (1.1) ab | |
| Shredder (%) | 12.8 (3.7) | 4.6 (7.7) | 1.3 (2.9) | 19.6 (10.5) | 2.6 (3.3) |
| Category | Cluster | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Geological factors | Altitude | 738 (85) ab | 741 (66) a | 606 (61) b | 857 (47) a | 556 (42) b |
| DFS (km) | 0.97 (0.36) b | 1.39 (0.51) ab | 2.76 (0.47) a | 0.85 (0.15) b | 2.06 (0.33) a | |
| Slope | 8.27 (2.1) | 9.48 (3.37) | 15.11 (6.17) | 11.03 (7.96) | 12.15 (8.73) | |
| Stream order | 2 (1) b | 3 (1) ab | 5 (1) a | 2 (0) b | 5 (1) a | |
| Hydrology | Depth (cm) | 9.8 (3.2) | 11.4 (2.4) | 16.3 (4.9) | 10.8 (4.1) | 19.5 (10.8) |
| Velocity (cm/s) | 0.53 (0.18) | 0.3 (0.16) | 0.59 (0.15) | 0.49 (0.21) | 0.59 (0.26) | |
| Land use (%) | Urban | 2.9 (3.8) | 6.1 (6.4) | 22.4 (17.1) | 3.2 (2.9) | 4.1 (2.9) |
| Agriculture | 4.8 (5.8) | 10.5 (9.2) | 12.8 (8.7) | 0.6 (0.8) | 16.7 (16.2) | |
| Forest | 92.3 (9.4) a | 77.6 (16.0) a | 55.1 (14.3) b | 93.8 (7.6) a | 76.2 (19.2) ab | |
| Grassland | 0 (0) | 1.2 (2.4) | 0.9 (1) | 1.8 (3.4) | 0.8 (1.1) | |
| Wetland | 0 (0) | 0 (0) | 2.3 (3.1) | 0 (0) | 0.1 (0.1) | |
| Bare land | 0 (0) b | 4.6 (6.8) a | 6.7 (7.1) a | 0.6 (0.9) ab | 2.1 (1.5) a | |
| Substrate composition (%) | <0.063 mm | 0.0 (0.0) ab | 0.2 (0.2) a | 0.4 (0.3) a | 0.5 (0.5) a | 0.0 (0.0) b |
| 0.063–2 mm | 0.9 (0.2) | 0.5 (0.4) | 0.7 (0.2) | 0.9 (0.2) | 1.1 (0.4) | |
| 2–4 mm | 1.1 (0.3) | 0.9 (0.1) | 0.9 (0.2) | 0.9 (0.1) | 1.2 (0.5) | |
| 4–8 mm | 1.4 (0.2) | 1.3 (0.6) | 1 (0.2) | 1.1 (0.4) | 0.9 (0.8) | |
| 8–16 mm | 1.8 (0.7) | 2.3 (1.6) | 1.9 (0.2) | 2 (0.4) | 1.2 (0.8) | |
| 16–32 mm | 4 (0.6) | 4.4 (3.2) | 3.2 (0.8) | 4.2 (1.2) | 2 (1.8) | |
| 32–64 mm | 7.9 (2.6) | 7.9 (4.9) | 6 (2.4) | 10.1 (4.9) | 6.5 (3.2) | |
| 64–128 mm | 22.1 (4.4) | 25.5 (9.2) | 18 (6.2) | 21.6 (8.5) | 23.7 (4.6) | |
| 128–256 mm | 55.4 (7.3) | 56.9 (15) | 65.4 (9.4) | 56.1 (12.5) | 52.8 (12.1) | |
| >256 mm | 5.4 (9.3) | 0 (0) | 2.5 (4.2) | 2.5 (3.3) | 10.7 (21.4) | |
| Water quality | DO (%) | 85.9 (1.8) b | 100.2 (10.2) a | 108.2 (11) a | 88.8 (0.4) a | 88.1 (2.2) ab |
| DO (mg/L) | 9.59 (0.19) | 10.63 (1.43) | 10.73 (1.44) | 9.82 (0.21) | 9.35 (0.1) | |
| pH | 7.57 (0.46) b | 8.35 (0.5) a | 8.73 (0.47) a | 7.73 (0.27) ab | 8.51 (0.23) a | |
| Conductivity (μS/cm) | 116.0 (68.8) ab | 360.8 (11.37) a | 379.4 (112.8) a | 370.3 (188.1) a | 92.3 (23.3) b | |
| Turbidity (NTU) | 1.1 (0.6) | 1 (1.5) | 0.2 (0.3) | 1.1 (1.1) | 1 (0.2) | |
| BOD (mg/L) | 1.13 (0.24) | 0.97 (0.21) | 1.16 (0.29) | 0.7 (0.08) | 1.03 (0.17) | |
| NH3-N (mg/L) | 0.01 (0.003) | 0.016 (0.019) | 0.013 (0.009) | 0.013 (0.004) | 0.008 (0.003) | |
| NO3-N (mg/L) | 0.79 (0.25) b | 1.54 (0.41) a | 1.18 (0.32) ab | 0.71 (0.28) b | 1.22 (0.13) a | |
| T-N (mg/L) | 1.07 (0.36) b | 2.11 (0.47) a | 2.1 (0.11) a | 0.81 (0.28) b | 1.48 (0.13) ab | |
| PO4-P (mg/L) | 0.003 (0) | 0.008 (0.007) | 0.006 (0.004) | 0.003 (0.001) | 0.003 (0) | |
| T-P (mg/L) | 0.008 (0.002) | 0.013 (0.009) | 0.015 (0.008) | 0.006 (0.001) | 0.005 (0) | |
| Chl-a (mg/L) | 0.53 (0.13) | 0.77 (0.53) | 0.5 (0.07) | 0.48 (0.05) | 0.53 (0.05) |
| Cluster | Order | Family | Species (Taxa) | IndVal | p Value |
|---|---|---|---|---|---|
| 1 | Ephemeroptera | Heptageniidae | Cinygmula KUa | 0.75 | 0.009 |
| 1 | Ephemeroptera | Heptageniidae | Epeorus curvatulus | 0.64 | 0.007 |
| 1 | Ephemeroptera | Ephemerellidae | Cincticostella levanidovae | 0.48 | 0.014 |
| 1 | Plecoptera | Nemouridae | Nemoura KUa | 1.00 | 0.002 |
| 1 | Plecoptera | Perlodidae | Stavsolus japonicus | 1.00 | 0.001 |
| 1 | Plecoptera | Perlidae | Kamimuria coreana | 1.00 | 0.001 |
| 1 | Plecoptera | Nemouridae | Amphinemura coreana | 0.75 | 0.006 |
| 1 | Plecoptera | Leuctridae | Rhopalopsole mahunkai | 0.75 | 0.01 |
| 1 | Plecoptera | Chloroperlidae | Sweltsa nikkoensis | 0.67 | 0.002 |
| 1 | Trichoptera | Philopotamidae | Wormaldia KUa | 0.75 | 0.005 |
| 1 | Trichoptera | Hydrobiosidae | Apsilochorema KUa | 0.62 | 0.021 |
| 1 | Trichoptera | Rhyacophilidae | Rhyacophila shikotsuensis | 0.56 | 0.032 |
| 1 | Trichoptera | Lepidostomatidae | Lepidostoma KUb | 0.47 | 0.044 |
| 1 | Coleoptera | Elmidae | Elmidae sp. | 0.80 | 0.004 |
| 1 | Diptera | Tipulidae | Hexatoma KUa | 0.64 | 0.008 |
| 1 | Diptera | Ceratopogonidae | Ceratopogonidae sp. | 0.62 | 0.006 |
| 1 | Diptera | Tipulidae | Dicranota KUa | 0.53 | 0.018 |
| 2 | Basomatophomra | Physidae | Physa acuta | 0.41 | 0.039 |
| 3 | Archioligochaeta | Naididae | Chaetogaster limnaei | 0.72 | 0.003 |
| 3 | Archioligochaeta | Tubificidae | Limnodrilus gotoi | 0.48 | 0.003 |
| 3 | Mesogastropoda | Pleuroceridae | Semisulcospira libertine | 0.41 | 0.046 |
| 3 | Ephemeroptera | Heptageniidae | Ecdyonurus levis | 0.79 | 0.001 |
| 3 | Ephemeroptera | Heptageniidae | Epeorus pellucidus | 0.43 | 0.037 |
| 3 | Ephemeroptera | Baetidae | Baetis fuscatus | 0.80 | 0.001 |
| 3 | Ephemeroptera | Baetidae | Baetis ursinus | 0.61 | 0.001 |
| 3 | Trichoptera | Hydroptilidae | Hydroptila KUa | 0.87 | 0.001 |
| 3 | Trichoptera | Hydropsychidae | Hydropsyche valvata | 0.71 | 0.002 |
| 3 | Trichoptera | Hydropsychidae | Cheumatopsyche KUa | 0.41 | 0.019 |
| 3 | Trichoptera | Hydropsychidae | Hydropsyche kozhantschikovi | 0.40 | 0.04 |
| 3 | Diptera | Tipulidae | Antocha KUa | 0.39 | 0.002 |
| 3 | Diptera | Chironomidae | Chironomidae spp | 0.24 | 0.033 |
| 4 | Plecoptera | Nemouridae | Nemoura KUb | 0.44 | 0.041 |
| 4 | Diptera | Tipulidae | Tipula KUa | 0.65 | 0.007 |
| 5 | Ephemeroptera | Heptageniidae | Ecdyonurus bajkovae | 0.71 | 0.002 |
| 5 | Ephemeroptera | Heptageniidae | Ecdyonurus kibunensis | 0.47 | 0.012 |
| 5 | Ephemeroptera | Ephemerellidae | Uracanthella rufa | 0.57 | 0.004 |
| 5 | Ephemeroptera | Leptophlebiidae | Paraleptophlebia chocorata | 0.51 | 0.012 |
| 5 | Ephemeroptera | Ephemeridae | Ephemera strigata | 0.48 | 0.05 |
| 5 | Trichoptera | Glossosomatidae | Glossosoma KUa | 0.60 | 0.006 |
| 5 | Trichoptera | Hydropsychidae | Hydropsyche orientalis | 0.48 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.-J.; Hong, J.-K.; Kim, E.-J. Evaluation of the Impacts of Abandoned Mining Areas: A Case Study with Benthic Macroinvertebrate Assemblages. Int. J. Environ. Res. Public Health 2021, 18, 11132. https://doi.org/10.3390/ijerph182111132
Bae M-J, Hong J-K, Kim E-J. Evaluation of the Impacts of Abandoned Mining Areas: A Case Study with Benthic Macroinvertebrate Assemblages. International Journal of Environmental Research and Public Health. 2021; 18(21):11132. https://doi.org/10.3390/ijerph182111132
Chicago/Turabian StyleBae, Mi-Jung, Jeong-Ki Hong, and Eui-Jin Kim. 2021. "Evaluation of the Impacts of Abandoned Mining Areas: A Case Study with Benthic Macroinvertebrate Assemblages" International Journal of Environmental Research and Public Health 18, no. 21: 11132. https://doi.org/10.3390/ijerph182111132
APA StyleBae, M.-J., Hong, J.-K., & Kim, E.-J. (2021). Evaluation of the Impacts of Abandoned Mining Areas: A Case Study with Benthic Macroinvertebrate Assemblages. International Journal of Environmental Research and Public Health, 18(21), 11132. https://doi.org/10.3390/ijerph182111132







