Immunohistochemical Analysis of the Beclin-1 Expression Predicts the Progression of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endpoint
2.2. Inclusion and Exclusion Criteria
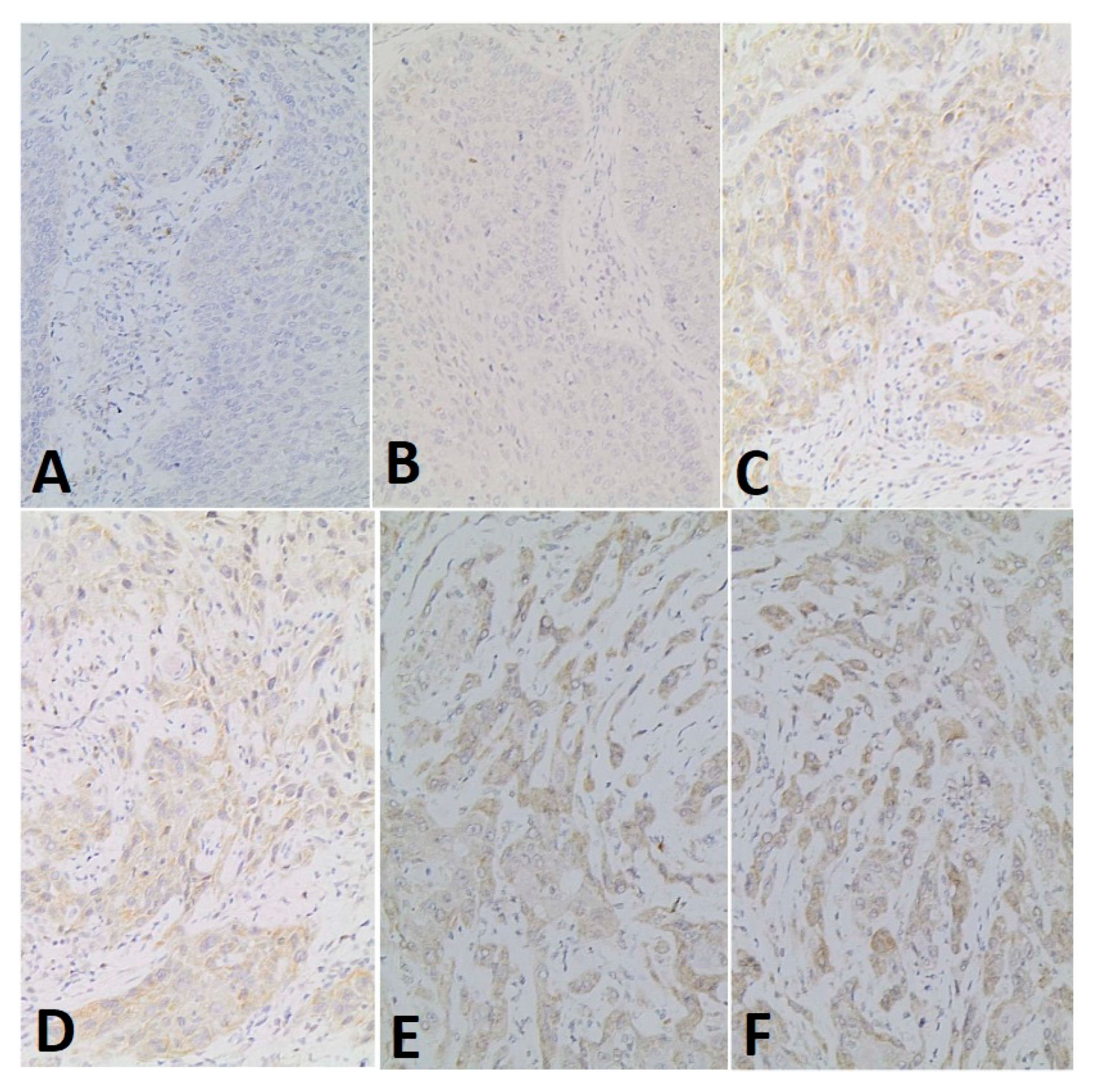
2.3. Immunohistochemical Techniques
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghantous, Y.; Yaffi, V.; Abu-Elnaaj, I. Oral cavity cancer: Epidemiology and early diagnosis. Refuat Hapeh Vehashinayim 2015, 32, 55–63. [Google Scholar]
- Jiang, L.-C.; Xin, Z.-Y.; Deborah, B.; Zhang, J.-S.; Yuan, D.-Y.; Xu, K.; Liu, X.-B.; Jiang, H.-Q.; Fan, Q.-C.; Zhang, B.; et al. Inhibition of autophagy augments apoptosis in human oral squamous cell carcinoma under nutrient depletion. J. Oral Pathol. Med. 2014, 44, 361–366. [Google Scholar] [CrossRef]
- García-Martín, J.M.; Varela-Centelles, P.; González, M.; Seoane-Romero, J.M.; Seoane, J.; García-Pola, M.J. Epidemiology of Oral Cancer. In Oral Cancer Detection; Panta, P., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Novembre, D.; Barca, I.; Cordaro, R.; Kallaverja, E.; Ferragina, F.; Cristofaro, M.G. Malignant transformation of oral lichen planus. A retrospective analysis from 2003–2014: Our experience. Ann. Ital. Chir. 2020, 91, 445–450. [Google Scholar] [PubMed]
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Pastorino, R.; Arzani, D.; Bosetti, C.; Canova, C.; Garavello, W.; La Vecchia, C.; Maule, M.; et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015, 39, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, M.P.; Misra, S.; Rathanaswamy, S.P.; Gupta, S.; Tewari, B.N.; Bhatt, M.L.B. Clinical profile and epidemiological factors of oral cancer patients from North India. Natl. J. Maxillofac. Surg. 2015, 6, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristofaro, M.G.; Scumaci, D.; Fiumara, C.V.; Di Sanzo, M.; Zuccalà, V.; Donato, G.; Caruso, D.; Riccelli, U.; Faniello, M.C.; Cuda, G.; et al. Identification of prognosis-related proteins in gingival squamous cell carcinoma by twodimensional gel electrophoresis and mass spectrometry-based proteomics. Ann. Ital. Chir. 2015, 85, 518–524. [Google Scholar]
- Liang, X.H.; Kleeman, L.K.; Jiang, H.H.; Gordon, G.; Goldman, J.E.; Berry, G.; Herman, B.; Levine, B. Protection against Fatal Sindbis Virus Encephalitis by Beclin, a Novel Bcl-2-Interacting Protein. J. Virol. 1998, 72, 8586–8596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Song, X.; Yang, Y.; Wan, X.; Alvarez, A.A.; Sastry, N.; Feng, H.; Hu, B.; Cheng, S.-Y. Autophagy and Hallmarks of Cancer. Crit. Rev. Oncog. 2018, 23, 247–267. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-J.; Lei, Y.-H.; Yao, N.; Wang, C.-R.; Hu, N.; Ye, W.-C.; Zhang, D.-M.; Chen, Z.-S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, Y.; Sugimoto, M.; Arias, E.; Kasashima, H.; Cordes, T.; Linares, J.F.; Duran, A.; Nakanishi, Y.; Nakanishi, N.; L’Hermitte, A.; et al. PKCλ/ι Loss Induces Autophagy, Oxidative Phosphorylation, and NRF2 to Promote Liver Cancer Progression. Cancer Cell 2020, 38, 247–262.e11. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.M.; Wrobel, L.; Rubinsztein, D.C. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 2019, 26, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation—At the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-L.; Kortylewski, M. Beclin-1 as a neutrophil-specific immune checkpoint. J. Clin. Investig. 2019, 129, 5079–5081. [Google Scholar] [CrossRef]
- Kaur, S.; Changotra, H. The beclin 1 interactome: Modification and roles in the pathology of autophagy-related disorders. Biochimie 2020, 175, 34–49. [Google Scholar] [CrossRef]
- Maejima, Y.; Isobe, M.; Sadoshima, J. Regulation of autophagy by Beclin 1 in the heart. J. Mol. Cell. Cardiol. 2015, 95, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar] [PubMed]
- Mohsin, S.K.; Weiss, H.; Havighurst, T.; Clark, G.M.; Berardo, M.; Roanh le, D.; To, T.V.; Qian, Z.; Love, R.R.; Allred, D.C. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: A validation study. Mod. Pathol. 2004, 17, 1545–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson-Jackson, E.B.; Helm, J.; Strosberg, J.; Nasir, N.A.; Yeatman, T.J.; Kvols, L.K.; Coppola, D.; Nasir, A. Palladin is a marker of liver metastasis in primary pancreatic endocrine carcinomas. Anticancer. Res. 2011, 31, 2957–2962. [Google Scholar]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; Singh, A.K.; Dey, S.; Sharma, S.C.; Das, S.N. Circulating cycloxygenase-2 in patients with tobacco-related intraoral squamous cell carcinoma and evaluation of its peptide inhibitors as potential antitumor agent. J. Cancer Res. Clin. Oncol. 2010, 136, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Kondo, S. Autophagy and Cancer Therapy. Autophagy 2006, 2, 85–90. [Google Scholar] [CrossRef]
- Fu, L.-L.; Cheng, Y.; Liu, B. Beclin-1: Autophagic regulator and therapeutic target in cancer. Int. J. Biochem. Cell Biol. 2013, 45, 921–924. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Georgiou, I.; Kouroupi, M.; Sivridis, E. LC3A, LC3B and Beclin-1 Expression in Gastric Cancer. Anticancer Res. 2018, 38, 6827–6833. [Google Scholar] [CrossRef]
- Shen, H.; Yin, L.; Deng, G.; Guo, C.; Han, Y.; Li, Y.; Cai, C.; Fu, Y.; Liu, S.; Zeng, S. Knockdown of Beclin-1 impairs epithelial-mesenchymal transition of colon cancer cells. J. Cell. Biochem. 2018, 119, 7022–7031. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Q.-H.; Zhou, H.; Wu, J.-L.; Quan, W.-Q.; Ji, P.; Yao, Y.-W.; Li, D.; Sun, Z.-J. Ginkgolide B inhibits lung cancer cells promotion via beclin-1-dependent autophagy. BMC Complement. Med. Ther. 2020, 20, 194. [Google Scholar] [CrossRef]
- Baehrecke, E.H.; Gewirtz, D.A.; Amaravadi, R.K.; Piacentini, M.; Levine, B.; Ryan, K.M.; Penninger, J.; Thorburn, A.M.; Martin, S.J.; Rubinsztein, D.C.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Kapoor, V.; Paliwal, D.; Singh, S.B.; Mohanti, B.K.; Das, S.N. Deregulation of Beclin 1 in patients with tobacco-related oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2012, 422, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Tang, H.; Wang, M.; Weng, J.; Liu, X.; Zhang, R.; Huang, H.; Hou, J. Decrease of autophagy activity promotes malignant progression of tongue squamous cell carcinoma. J. Oral Pathol. Med. 2013, 42, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rubín-De-Celis, S.; Zou, Z.; Fernández, F.; Ci, B.; Kim, M.; Xiao, G.; Xie, Y.; Levine, B. Increased autophagy blocks HER2-mediated breast tumorigenesis. Proc. Natl. Acad. Sci. USA 2018, 115, 4176–4181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignogna, C.; Staropoli, N.; Botta, C.; DE Marco, C.; Rizzuto, A.; Morelli, M.; Di Cello, A.; Franco, R.; Camastra, C.; Presta, I.; et al. Aurora Kinase A expression predicts platinum-resistance and adverse outcome in high-grade serous ovarian carcinoma patients. J. Ovarian Res. 2016, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, I.; Bruno, L.; Maltese, L.; Russo, E.; Donato, A.; Donato, G. Immunohistochemical analysis of the ubiquitin-conjugating enzyme UbcH10 in lung cancer: A useful tool for diagnosis and therapy. J. Histochem. Cytochem. 2012, 60, 359–365. [Google Scholar] [CrossRef]

| N° | Sex | Age | TNM | Neoplastic Infiltration | Beclin-1 Signal Intensity | Percentage of Positive Cells | Total Score |
|---|---|---|---|---|---|---|---|
| 1 | F | 50 | T2 N0 M0 | 10 mm | 1 | 3 | 4 |
| 2 | F | 76 | T1 N2b M0 | 18 mm | 1 | 1 | 2 |
| 3 | F | 71 | T2 N1 M0 | 2 mm | 1 | 1 | 2 |
| 4 | M | 70 | T1 N0 M0 | 7 mm | 1 | 1 | 2 |
| 5 | M | 26 | T1 N0 M0 | 7 mm | 2 | 2 | 4 |
| 6 | F | 75 | T1 N0 M0 | 8 mm | 2 | 4 | 6 |
| 7 | M | 72 | T1 N0 M0 | 2.5 mm | 3 | 5 | 8 |
| 8 | M | 82 | T1 N0 M0 | 1 mm | 3 | 5 | 8 |
| 9 | F | 85 | T1 N0 M0 | 4 mm | 1 | 1 | 2 |
| 10 | F | 60 | T2 N0 M0 | 5 mm | 2 | 4 | 6 |
| 11 | M | 54 | T2 N1 M0 | 10 mm | 1 | 2 | 3 |
| 12 | F | 80 | T1 N0 M0 | 11 mm | 1 | 1 | 2 |
| 13 | F | 75 | T3 N0 M0 | 10 mm | 2 | 4 | 6 |
| 14 | F | 77 | T2 N0 M0 | 5 mm | 1 | 3 | 4 |
| 15 | F | 75 | T2 N0 M0 | 4 mm | 2 | 3 | 5 |
| 16 | M | 45 | T1 N0 M0 | 11 mm | 2 | 3 | 5 |
| 17 | M | 46 | T2 N2b M0 | 12 mm | 2 | 3 | 5 |
| 18 | F | 35 | T1 N0 M0 | 11 mm | 2 | 3 | 5 |
| 19 | M | 69 | T2 N2b M0 | 18 mm | 0 | 0 | 0 |
| 20 | M | 87 | T2 N0 M0 | 7 mm | 1 | 2 | 3 |
| 21 | M | 59 | T2 N0 M0 | 18 mm | 1 | 1 | 2 |
| 22 | F | 50 | T1 N0 M0 | 9 mm | 1 | 1 | 2 |
| 23 | F | 74 | T2 N2b M0 | 5 mm | 1 | 4 | 5 |
| 24 | M | 92 | T2 N0 M0 | 3 mm | 3 | 3 | 6 |
| 25 | F | 93 | T1 N0 M0 | 7 mm | 1 | 3 | 4 |
| 26 | F | 80 | T2 N0 M0 | 3 mm | 2 | 2 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barca, I.; Mignogna, C.; Novembre, D.; Ferragina, F.; Cristofaro, M.G. Immunohistochemical Analysis of the Beclin-1 Expression Predicts the Progression of Oral Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2021, 18, 11125. https://doi.org/10.3390/ijerph182111125
Barca I, Mignogna C, Novembre D, Ferragina F, Cristofaro MG. Immunohistochemical Analysis of the Beclin-1 Expression Predicts the Progression of Oral Squamous Cell Carcinoma. International Journal of Environmental Research and Public Health. 2021; 18(21):11125. https://doi.org/10.3390/ijerph182111125
Chicago/Turabian StyleBarca, Ida, Chiara Mignogna, Daniela Novembre, Francesco Ferragina, and Maria Giulia Cristofaro. 2021. "Immunohistochemical Analysis of the Beclin-1 Expression Predicts the Progression of Oral Squamous Cell Carcinoma" International Journal of Environmental Research and Public Health 18, no. 21: 11125. https://doi.org/10.3390/ijerph182111125
APA StyleBarca, I., Mignogna, C., Novembre, D., Ferragina, F., & Cristofaro, M. G. (2021). Immunohistochemical Analysis of the Beclin-1 Expression Predicts the Progression of Oral Squamous Cell Carcinoma. International Journal of Environmental Research and Public Health, 18(21), 11125. https://doi.org/10.3390/ijerph182111125







