Abstract
Healthcare-associated infections (HAIs) contribute to patient morbidity and mortality with an estimated 1.7 million infections and 99,000 deaths costing USD $28–34 billion annually in the United States alone. There is little understanding as to if current environmental surface disinfection practices reduce pathogen load, and subsequently HAIs, in critical care settings. This evidence map includes a systematic review on the efficacy of disinfecting environmental surfaces in healthcare facilities. We screened 17,064 abstracts, 635 full texts, and included 181 articles for data extraction and study quality assessment. We reviewed ten disinfectant types and compared disinfectants with respect to study design, outcome organism, and fourteen indictors of study quality. We found important areas for improvement and gaps in the research related to study design, implementation, and analysis. Implementation of disinfection, a determinant of disinfection outcomes, was not measured in most studies and few studies assessed fungi or viruses. Assessing and comparing disinfection efficacy was impeded by study heterogeneity; however, we catalogued the outcomes and results for each disinfection type. We concluded that guidelines for disinfectant use are primarily based on laboratory data rather than a systematic review of in situ disinfection efficacy. It is critically important for practitioners and researchers to consider system-level efficacy and not just the efficacy of the disinfectant.
1. Introduction
Healthcare-associated infections (HAIs) contribute to patient morbidity and mortality with an estimated 687,000 infections and 72,000 deaths in the United States in 2015 [1] and an additional 2.6 million annual infections in the European Union [2]. The burden of HAIs is higher in low- and middle-income countries [3,4,5]. HAIs are often correlated with the presence of contaminated environmental surfaces and are exacerbated by multi-drug resistance and compounded by spore-producing or biofilm-associated pathogens that are difficult to disinfect [6]. Healthcare-associated pathogens with high morbidity and mortality, including vancomycin-resistant Enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, and Candida auris, are especially problematic in the intensive care unit (ICU), where patients are often immunocompromised [7,8].
The environmental transmission pathways of pathogens and HAIs are varied. They include medical devices, air ventilation units, environmental surfaces (e.g., floors, bedrails), water, healthcare workers (e.g., hands), and mobile elements (e.g., wheelchairs, shoes, etc.); floors may play a large role [9,10,11,12]. Meta-analyses support the environment as being a transmission pathway through roommates/prior occupants with HAIs in high-income settings [13,14]. Patients hospitalized in rooms previously occupied by people infected with HAIs are at increased odds of HAI acquisition compared to patients whose prior room occupant was negative for HAIs [15,16,17].
Interventions to reduce the environment as a transmission pathway for HAIs are also varied. Improved cleaning procedures [18,19], training environmental service personnel [20,21,22], hand hygiene [10,11,12,23], and bundled disinfection interventions reduce the concentrations of pathogens on environmental surfaces and reduce HAIs in healthcare facilities [19,24]. However, transmission pathways are poorly disaggregated. For bundled interventions, it is challenging to determine each component’s independent effect and the contribution of potential transmission pathways on HAI acquisition. The literature has focused on multimodal strategies in infection prevention and control (IPC) without analyzing the impact of separate components, such as disinfection implementation or disinfection efficacy [25]. Understanding the efficacy of the individual components of multi-modal strategies may help guide bundle development and may aid in decision-making in low-resource settings.
One systematic review found that most studies that included bundled interventions with an environmental cleaning and disinfection component were more effective than bundled interventions without the component at reducing HAIs [26]. Nevertheless, the extent to which surface disinfection contributes to HAI reductions is unclear.
The hierarchy of studies for assessing the impact of infection control is outcomes from (1) in vitro reduction of reference pathogens → (2) in situ reduction of environmental pathogens → (3) colonization and pathogen transmission to patients → (4) patient HAIs [27,28]. In vitro studies, such as quantitative carrier tests, are appropriate for determining the disinfectant concentration and contact time necessary to provide a log reduction target of pathogens on surfaces [29,30]. Large bodies of in vitro surface disinfection research exist for agriculture, food production and preparation, and biodefense but are not always applicable to pathogens that are regularly associated with HAIs. In vitro studies on surface disinfection provide the necessary disinfection kinetics to justify in situ studies yet lack the variance in surfaces, environmentally derived pathogens, and inadequate terminal cleaning methods. There are reported reductions in disinfection efficacy in the healthcare facility setting in situ when compared to reported in vitro efficacy (see, e.g., [31]). Additionally, pathogens remain viable on porous and non-porous surfaces for extended times in ambient conditions [32,33,34,35].
There is still little understanding as to if current disinfection practices on environmental surfaces reduce pathogen load and subsequently HAIs in critical care settings. There has not been a rigorous systematic review of the efficacy of disinfection interventions in situ. While a prior systematic review [28] and related technical brief [36] identified the disinfection methods used in healthcare facilities on environmental surfaces, the work was restricted to publications in English and to efficacy on specific Gram-positive pathogens (MRSA, VRE, C. difficile). The literature primarily concerns multimodal strategies in infection prevention and control (IPC) without analyzing the impact of separate components [25]. This is exemplified in a systematic review assessing the effect of multi-modal interventions on HAIs, which reported that 35%–55% of HAIs are preventable but did not differentiate the multi-faceted components of the interventions [37]. In situ evidence for the efficacy of disinfection interventions are based on non-systematic methods such as narrative review [38], literature reviews [19], commentary [39], and clinical guidance [40]. Furthermore, clinical practice guidance for environmental surface cleaning is disparate between evidence-based or consensus-driven and narrative-based (i.e., logically justified) recommendations. Guidelines vary based on country of origin with government, independent associations, and professional societies issuing 69 separate guidance documents [28].
We conducted a systematic review to develop an evidence map that (1) catalogues in situ disinfection interventions on environmental surfaces (excepting UV); (2) identifies gaps in the research and areas for improvement; (3) catalogues the in situ efficacy of environmental surface disinfection interventions in healthcare facilities on all HAI and organism outcomes; and (4) summarizes important components of IPC strategies for the disinfection of environmental surfaces in a proposed framework for ideal disinfection.
2. Materials and Methods
Search Strategy and Machine Learning: We searched PubMed, Embase, Scopus, and Web of Science in January 2020 for studies related to healthcare facilities and disinfectants (as described in Supplementary Material 1). Healthcare facility terms included inpatient and outpatient environments and spanned global healthcare facilities in a variety of critical care environments. Disinfection terms included specific chemical disinfectants identified by the Centers for Disease Control and Prevention (CDC) [41] and the World Health Organization (WHO) [42] for use in health care disinfection, such as alcohols, chlorine and demand-release chlorine compounds, formaldehyde, glutaraldehyde, hydrogen peroxide, iodophors, ortho-phthalaldehyde, peracetic acid, phenolics, and quaternary ammonium compounds as well as non-touch interventions such as vapors and antimicrobial surfaces. Disinfection terms also included generic terms such as “decontaminant” and “disinfectant” to identify studies for which we did not specify the disinfectant in the search terms. We excluded reviews and other article types such as commentaries, as specified in Supporting Information Supplementary Material 1. After the duplicates were removed, we used machine learning to prioritize studies to be screened manually for relevance using Document Classification and Topic Extraction Resource (DoCTER) software (ICF, Fairfax, VA, USA). All of the studies that were predicted to be relevant by DoCTER were imported to Covidence reference management software (Veritas Health Innovation, Melbourne, Australia) for title and abstract screening.
We used supervised clustering with an ensemble approach to prioritize studies for manual screening using the text of titles and abstracts (similar to the approach described in [43]). Supervised clustering is a form of semi-supervised learning that uses known relevant studies (i.e., seeds) to identify unclassified studies that are likely to be relevant. Seed studies are a form of training data but require fewer positive studies than typically necessary for machine learning algorithms.
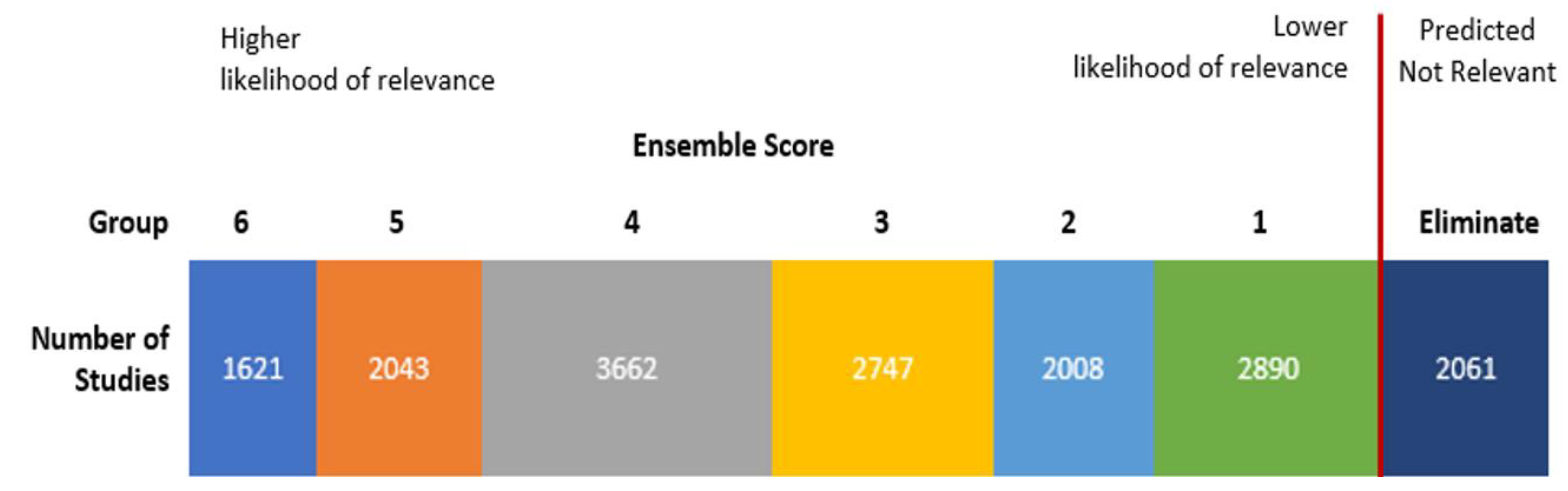
To identify seeds, we screened 750 randomly selected studies from which 32 qualifying studies served as seeds for supervised clustering. One person reviewed studies for use as seeds, and these studies were confirmed by a subject matter expert. The ensemble approach uses two algorithms: k-means and non-negative matrix factorization, and three cluster sizes: 10, 20, and 30. Using each algorithm with the three different cluster numbers yields six different clustering models (e.g., KM-10 model is the k-means algorithm with 10 clusters, and KM-20 is the k-means algorithm with 20 clusters). The six models were applied to the title and abstract text. The output of supervised clustering with a six-model ensemble approach had an ensemble score ranging from 0 to 6 for each study based on the number of models where the study was found in a relevant cluster (i.e., a cluster with a high proportion of seed studies). We ran supervised clustering with the 32 seed studies, and all non-seed studies were given an ensemble score (Figure 1). We specified at least 90 percent recall of relevant studies from the unclassified corpus in DoCTER but a recall closer to 100 percent was anticipated because all 32 seeds were captured by one or more clusters. Overall, we expected approximately 95 percent recall by reviewing all of the studies with an ensemble score of 1 or higher.
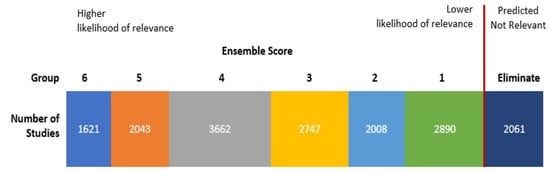
Figure 1.
Results of ensemble supervised clustering using 32 seed studies. Studies with an ensemble score of 1 or more were screened manually for relevance.
Inclusion Criteria: Titles and abstracts of all of the studies with an ensemble score of 1 or higher for relevance, which included the 32 seed studies, were screened. After the titles and abstracts were screened, the full text was read to determine if the study would be included. Two reviewers independently screened all of the titles and abstracts, and disputes were resolved through discussion. One reviewer independently screened the full texts for inclusion. The 2061 studies not found to be in a relevant cluster by any model (score of 0) were removed from analysis without manual screening (Figure 1).
Inclusion criteria for title and abstract and full text screening were (1) disinfection interventions that did not include UV or other light-based interventions to reduce the scope of the systematic review and excluded any study that had a disinfection component that was part of a bundled or multi-modal intervention package (e.g., a training intervention was implemented simultaneously to disinfection intervention). Studies were excluded if the disinfectant was not specified and if the study was cross-sectional in nature (e.g., no comparator). (2) We excluded articles that did not sample environmental surfaces, which were defined as non-porous surfaces that are either part of the built environment (e.g., walls, toilet) of a healthcare facility or remain in the critical care environment during the patient’s stay (e.g., bedside table), and did not include studies that focused solely on mobile elements such as doctors’ hands, wheelchairs, or medical instruments (e.g., stethoscopes, endoscopes). We excluded equipment surfaces, including studies that focused solely on central-line and dialysis. We excluded studies that focused on sink traps, the inside of showerheads, and porous surfaces (e.g., curtains, linens). If studies included surfaces in addition to environmental surfaces in the sampling protocol, we included the study. (3) The critical care environment included all healthcare facilities except veterinary, long-term residential care, and dental facilities. We excluded areas in healthcare facilities that patients would not visit, such as laboratory, laundry, and preparatory areas. We excluded long-term care facilities because IPC management and implementation may be different than other healthcare facilities. (4) Only original, peer-reviewed research was included. Systematic reviews, meta-analyses, poster abstracts, and any conference proceedings were not included. (5) Outcome measurements had to target organisms from surfaces, rather than from, e.g., air. We included HAI outcomes.
Data Extraction and Risk of Bias: Multiple reviewers independently extracted data from studies meeting the inclusion criteria. All data were reviewed for quality control by one reviewer. Interventions were categorized as being manually applied, antimicrobial surfaces applications, or vapors. Disinfectants with multiple active ingredients were categorized based on the active ingredient with the highest percentage by volume. Antimicrobial surfaces were comprised of inherently antibacterial surfaces (e.g., copper) or were coated with a product that bonded with the surface to inhibit growth. Coatings that were re-applied more than once a week were considered manually applied products rather than surface interventions (e.g., [44]). Outcome organisms were grouped into Gram-positive cocci, Gram-positive bacilli, Gram-negative bacteria, fungi, viruses, and “all viable organisms” (non-specific culture media or outcomes that combined multiple organism types, e.g., multi-drug resistant organisms that combine Gram-negative and Gram-positive organisms). Outcome measurement quality was ranked in descending order from organism concentration followed by percent surfaces positive, followed by adenosine triphosphate (ATP) measurements or qualitative observations; they were then classified according to highest quality outcome. HAI and antibiotic resistance outcomes were also identified. Study design was categorized for studies with outcome organisms (i.e., excluding studies with only HAI outcomes) as crossover design, controlled design (controlled before-after or controlled cohort study design), or uncontrolled study design (studies without a contemporary control). All studies were classified according to the World Bank country income group [45] for study location.
Risk of bias was assessed for each study by two reviewers using a fourteen-point study quality assessment instrument adapted from the National Institutes of Health (NIH) Study Quality Assessment Tool [46]. The study quality instrument included fourteen indicators to assess bias across setting, methods, outcomes, and conclusions of the included studies with heterogeneous study design; for contemporary controls, baseline equivalence, bias due to deviation from protocol, blind evaluation, bias due to missing data, bias in selective reporting, conflicts of interest, and others were considered Supplementary Material 2, Table S5). Each indicator received a score of 0, 0.5, or 1, such that the maximum total score for each study was 14. Twenty-three percent of studies were randomly selected for secondary independent review. Cohen’s kappa statistics and raw percent agreement were calculated to compare inter-rater reliability for each of the indicators [47].
This review was not registered nor was the review protocol registered. This systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Checklist [48] (see Supplementary Material 3).
3. Results
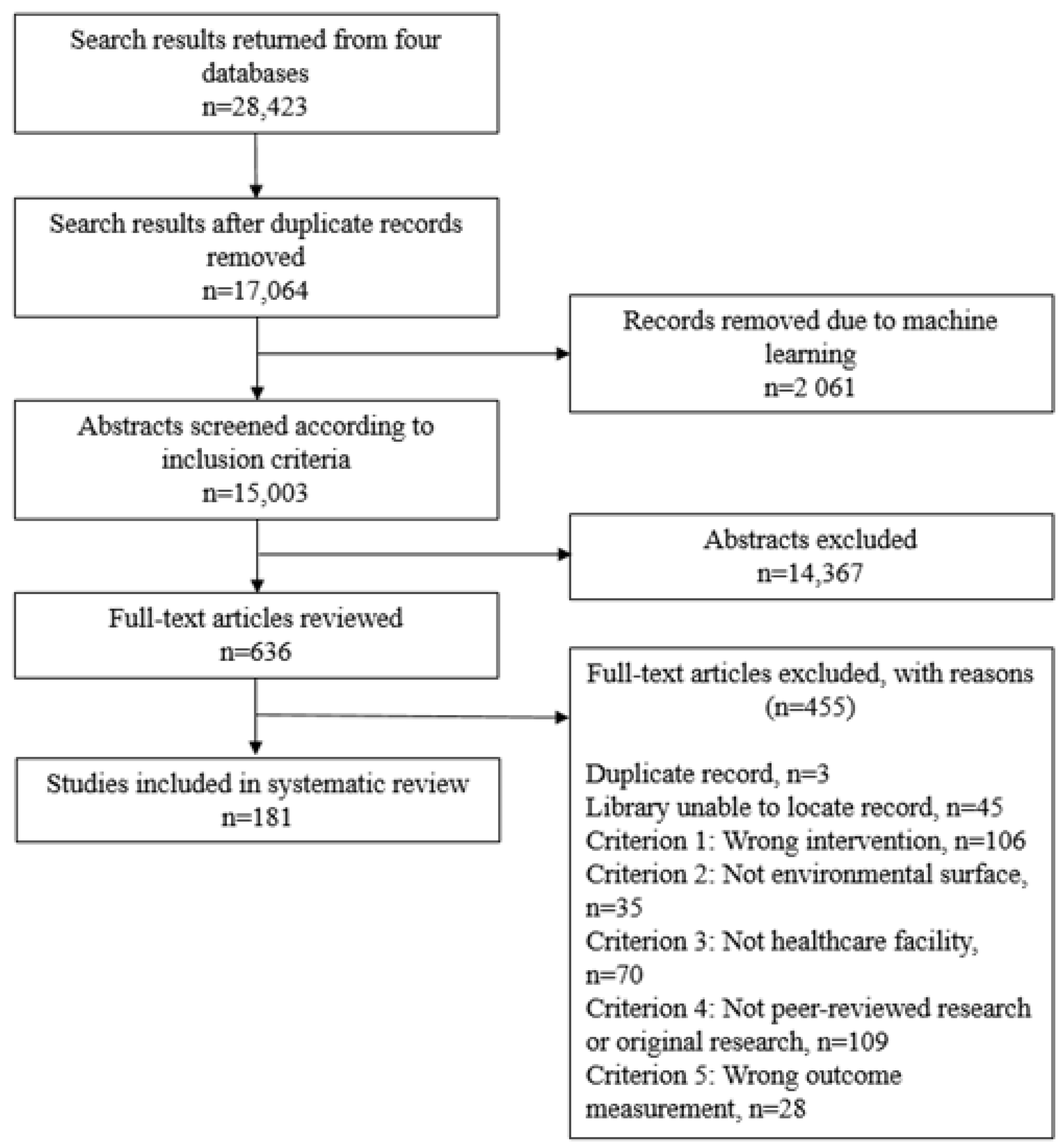
The initial literature search identified 17,064 studies, of which 2061 were eliminated through machine learning (Figure 2). Of the remaining 15,003 articles, 635 articles were selected for full text review, and were 181 included for data extraction. The included studies are listed in Supplementary Material 2, Table S6. Characteristics of the included studies with respect to disinfection intervention type, outcome HAI or organism assessed, outcome measurement, study design, and World Bank country income group for country of study location are listed in Table 1.
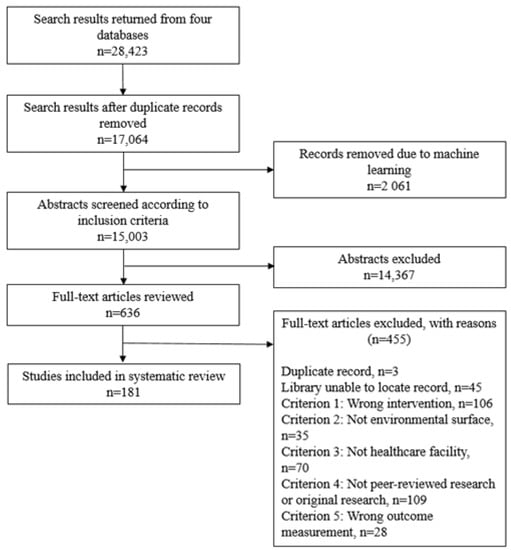
Figure 2.
PRISMA flowchart of systematic review.

Table 1.
Study characteristics.
Manually applied interventions included alcohol, peroxygen, quaternary ammonium compounds (QACs), sodium hypochlorite, and other chlorine; surface interventions included copper and other non-copper surface applications or coatings; and vapor interventions included hydrogen peroxide interventions. We identified the target pathogens and/or HAIs measured due to each disinfection intervention and presented an evidence map and summary of the data relating to study design, organism outcome, and disinfection intervention.
Most studies (86%) were conducted in high income countries such as the USA, UK, Italy, and Japan. Studies from upper-middle income countries (10%) were conducted in Turkey, Brazil, South Africa, Russia, Mexico, Indonesia, China, and Bosnia and Herzegovina. Studies from lower-middle income countries (3%) comprised India, Sri Lanka, Pakistan, and Morocco. One study was conducted in a low-income country (Sierra Leone).
3.1. Disinfection Type
Manually applied disinfectant application methods included mopping, wiping, pouring, or spraying, using, e.g., cotton, microfiber, or pre-moistened cloths, wipes, mops. Alcohol disinfection, including some disinfectants with multiple active ingredients (e.g., chlorhexidine gluconate, QAC), was identified in 11% of studies [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Peroxygen disinfection, including hydrogen peroxide, peracetic acid, or peroxymonosulfate, was identified in 9% of studies [50,56,57,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. QAC disinfection, which included diverse active ingredients such as primarily didecyl dimethyl ammonium chloride and benzyl ammonium chloride, was identified in 25% of studies [20,31,44,49,52,59,63,64,69,73,75,79,80,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113]. Sodium hypochlorite disinfection, which comprised any disinfection method specified as bleach or sodium hypochlorite, was identified in 19% of studies [20,44,60,61,75,89,95,97,100,104,109,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135]. Other chlorine disinfectants were identified in 14% of studies [65,70,78,101,118,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154]. Other chlorines included demand-release chlorines such as sodium dichloroisocyanurate, chloramine, chlorine-dioxide, and bromo-chloro-dimethyl-hydantoin, as well as electrolyzed water, hypochlorous acid, and any unspecified chlorine-based disinfectant. All other manually applied disinfectants, which included phenols, hydrochlorides, aldehydes, copper, glucopratamin, triethylene glycol, and grapefruit seed extract, were identified in 10% of studies [49,55,57,84,136,145,155,156,157,158,159,160,161,162,163,164,165,166].
For surface interventions, we found that copper surfaces were in 9% of studies [90,132,152,154,167,168,169,170,171,172,173,174,175,176,177,178,179]. Other non-copper surface applications or coatings comprised 8% of studies [87,102,180,181,182,183,184,185,186,187,188,189,190,191,192]. Other non-copper surfaces included coatings incorporating metals such as titanium oxide and silver ions as well as other coatings comprised of polymers, isopropyl alcohol and organofunctional silane, organosilane products, silicon nano-coating inorganic metal and organic quaternary ammonium, silicone quaternary amine, quaternary ammonium silyl oxide, and titanyl oxide moieties. One study used a probiotic-based cleaning product.
Vapor disinfection includes systems described as producing and dispersing vapors, aerosols, or droplets of disinfectants through spray, mist, or fogging machines. Hydrogen peroxide vapor was identified in 18% of studies [70,72,77,85,86,99,111,117,122,125,129,130,141,142,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211]. Other vaporized disinfection methods, which included chlorine dioxide, sodium hypochlorite, essential oils, formalin, QACs, glutaral, beta propiolactone, steam, acidic electrolytic water, ozone, and steam, were identified in 10% of studies [70,84,137,203,212,213,214,215,216,217,218,219,220,221,222,223].
3.2. Outcomes
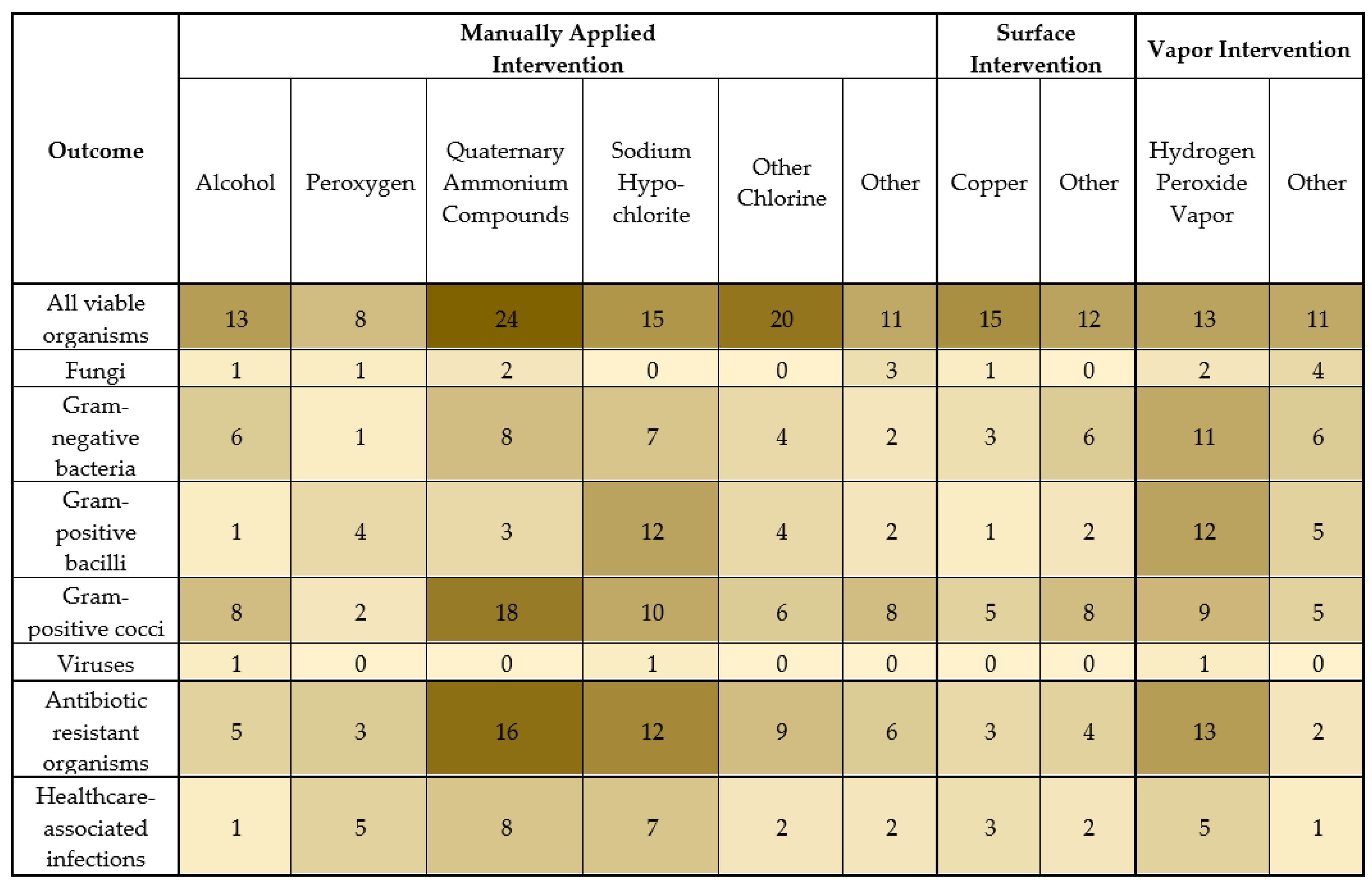
Of the 181 studies included, 168 (93%) assessed organisms on environmental surfaces (Figure 3). Many studies described multiple outcomes and multiple intervention types. Of the included studies, the outcome organisms that were reported were usually all viable organisms (66%) or Gram-positive cocci (38%), followed by Gram-negative bacteria (25%), Gram-positive bacilli (20%), and fungi (7%). Three studies (2%) assessed the disinfectant efficacy on environmental surfaces for viruses in situ. Antibiotic-resistant organisms were assessed in 33% of the studies, most commonly MRSA, VRE, carbapenem-resistant Acitenobacter baumannii, extended-spectrum beta-lactamase (ESBL)-producing organisms, and other antibiotic-resistant Gram-negative organisms.
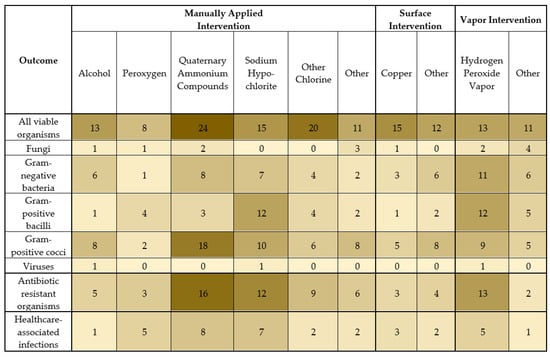
Figure 3.
Number of studies with the indicated outcome organism or healthcare-associated infection and the indicated intervention type. A darker shade indicates more studies with the indicated outcome and intervention type.
Most studies assessing all viable bacteria measured concentration, though when assessing specific organisms, the outcome was more commonly percent surface positive. Overall, 63% of studies reported concentration outcomes, 43% reported percent surface positive, 6% reported ATP or qualitative outcomes, and 2% reported outcomes related to gene abundance.
Of the 181 studies included, 28 (15%) reported HAI outcomes due to an environmental surface disinfection intervention, and 11 of the 28 HAI studies assessed drug-resistant organisms.
3.3. Disinfection Efficacy
Efficacy was defined differently among the included studies and was reported by comparing reduction, prevalence ratio, mean, median, range, and/or qualitative assessment. The intervention was not always compared to a control or another intervention with respect to statistical significance nor with respect to measures of variance and confidence intervals. Outcome measurements included concentration, gene abundance, percent surfaces positive, and ATP bioluminescence (Table 1). Studies used different comparators, with some studies comparing a disinfectant to a control without disinfectant and others to another disinfectant.
Efficacy for each of the ten disinfection interventions is presented by different outcome (Gram-positive organisms (bacilli and cocci), Gram-negative organisms, fungi, all viable organisms, and HAIs) in Supplementary Material 4. The study setting, intervention methods, and results for all studies organized by disinfection type, and outcome organisms are listed in Supplementary Material 5.
3.4. Proposed Framework for Ideal Disinfection
In this review we catalogued studies that assessed the in situ efficacy of disinfectants on environmental surfaces. However, the disinfectant efficacy on target organisms is not the only consideration for the effective disinfection of environmental surfaces. Building on the framework identifying properties for the ideal disinfectant [41], we propose an updated framework for ideal disinfection that includes all disinfection types and not only chemical disinfectants. The proposed decision-making framework for the ideal disinfectant includes nine criteria categorized under three themes: fit for purpose, safety, and implementation (Table 2).

Table 2.
Proposed framework for ideal disinfection as part of a larger infection prevention and control strategy.
The fit for purpose criteria allow the healthcare facility to identify disinfection needs based on, for example, critical care setting or pathogen. This systematic review rigorously catalogues evidence concerning the first question regarding disinfection efficacy. Other questions include the persistence or residual effect of the disinfectants that are more commonly studied among surface and vapor disinfectant interventions than among manually applied disinfectants (see, e.g., [63,64,102,103,132,166,180], the efficacy of the disinfectant when in the presence of increased biofilm or organic material (see, e.g., [49,56,110,145,197,201]), and whether pre-cleaning is needed (see, e.g., [123,141,193]).
Safety criteria ensure that the disinfectant does not have unintended side effects. We identified themes around disinfectants contributing to chemical or antimicrobial resistance (e.g., [44,62,69,110,158]) and toxicity or discomfort to healthcare workers and patients (see, e.g., [49,134,135,137,145,164,165,182,196,213,219,220,222]) as well as the compatibility of the disinfectant on surfaces and clothing (see, e.g., [61,69,83,85,139,145,172,189,220]).
Many articles included themes around the implementation of disinfection interventions. Specific themes were related to the adherence to the protocol, the appropriate application of the disinfectant, and the costs. Adherence was discussed as being related to monitoring and training. Studies assessing disinfection implementation found that objective measurements of disinfection (e.g., ATP fluorescence or environmental samples rather than visual inspection) improved disinfection practices [28,153].
Monitoring for disinfection compliance was primarily conducted through biological indicators for HPV interventions [194,199,201,209] and by using fluorescent markers or random audits [62,69,114,135,153]. Implementation may be affected by the inappropriate application of the protocol related to disinfectant contact time or improper disinfectant concentration (see, e.g., [61,78,153]) or whether implementation improved or worsened due to the method of application (e.g., wipes vs. mop; cotton vs. microfiber; one cloth vs. two cloths; see, e.g., [62,92,104,107,153,165]). Some antimicrobial coatings may not bind appropriately to target surfaces, and this may decrease the apparent efficacy. Training environmental services staff before and during interventions were identified as important for both adherence to protocol and to the appropriate application of the disinfectant (see, e.g., [20,78,120,125]). Few studies mentioned costs although some reported monetary or time costs associated with a disinfectant type (see, e.g., [69,70,92,114,126,131,137,141,160,197,201,223]).
3.5. Study Quality
Studies primarily used a before-after design without a simultaneous control (48%) or controlled cohort/controlled before-after study designs (46%). Few studies had crossover designs (5%) (Table 1).
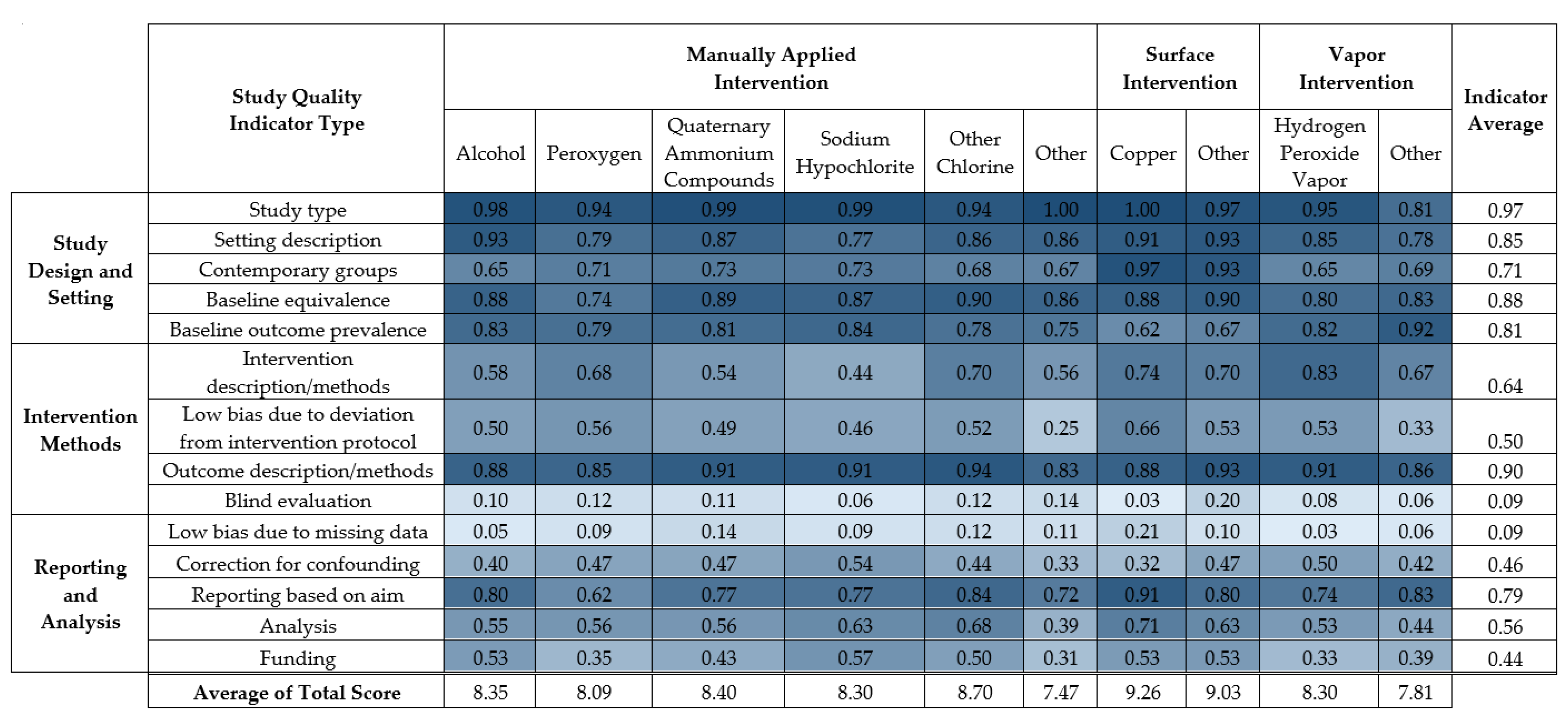
The average score for each of fourteen study quality indicators is displayed in Figure 4. Results of the 14-point study quality assessment for each study are listed in Supplementary Material 2, Table S7. A summary table of the proportions of studies that received each study quality criterion for each study quality indicator appears in Supplementary Material 2, Table S5.
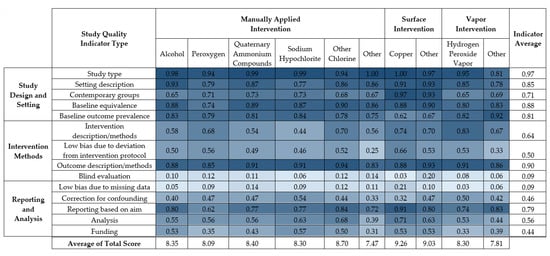
Figure 4.
Average score for fourteen study quality indicators individually and in sum for each disinfection intervention type.
The strengths of the disinfection interventions were primarily indicated in study description and study design. The majority (93%) of the studies had natural study designs, with 6% having seeded study designs. Most (73%) studies described the healthcare setting and environmental surfaces, 77% had clearly defined and equivalent healthcare settings for the control and intervention groups, 76% measured the initial burden before disinfection intervention, 85% had well-defined outcome methods, and 62% reported results based on the aim of the study.
Frequent weaknesses in study quality concerned implementation, reporting, and analysis. Most (90%) studies did not report whether there were missing data in the analyses, and 85% did not report blind evaluation of both healthcare workers and microbiologists. Only 13% reported blind evaluation in either group. Half (52%) of the studies did not sufficiently identify the disinfectant (e.g., product active ingredients and concentration), 67% of studies did not report measures of variance nor conduct a statistical test, 38% of studies measured the implementation of the disinfection intervention through, e.g., ATP assays, 23% indicated that the staff were trained but that intervention was not monitored, and 38% did not discuss monitoring or training during the intervention. Finally, 45% of the studies had funding other than academic or government sources and did not include a statement of influence or conflicts of interest regarding funding contributions to study design, implementation, decision to publish, etc.
The validation of the study quality instrument revealed a Cohen’s kappa coefficient of 0.75 (95% confidence interval 0.70–0.80) for agreeability between scoring by initial reviewers compared to scoring by the second independent reviewer (i.e., 70–80% of the scores can be attributed to reliable scoring by instrument users, and 20–30% can be attributed to random chance, error, or other factors). The raw percent agreement was calculated since the reviewers were trained, and low randomness due to guessing was expected. The raw percent agreement was 84%. The Cohen’s kappa suggests moderate inter-rater reliability, and the raw percent agreement suggests strong inter-rater reliability for scoring [47]. We interpreted the variability among indicator score variability as the degree to which the indicator could be easily interpreted for the study. Cohen’s kappa and raw percent agreement for each study quality indicator are in Supplementary Material 2, Table S8.
4. Discussion
In this evidence map and systematic review, we identified 181 studies that described disinfection interventions on environmental surfaces across ten types of disinfection groups. We compared disinfectant interventions with respect to study design, outcome organism, and study quality; however, comparing disinfectant efficacy was difficult due to the heterogeneity in the study design and the unmeasured variability in disinfection implementation.
4.1. Strengths and Weaknesses
This systematic review identified important gaps in study design and study reporting for studies describing the efficacy of disinfection on environmental surfaces. Studies from low- and lower-middle income countries comprised only 4% of the included studies. Study design flaws affecting many studies included the omission of contemporary controls and only used a historical control. For the studies that did use a contemporary control (e.g., cohort study or controlled before-after), many did not report the initial concentration when comparing reductions or disinfection efficacy across two experimental groups. Among studies reporting initial concentration, few assessed and corrected for different initial concentrations between groups (see, e.g., [126]). Confounders identified in the studies included the differential use of cleaning or disinfection by the experimental group (e.g., researchers vs. healthcare services; trained nurses vs. outsourced cleaning team), differential implementation of disinfection strategy (no monitoring of implementation), differential or unclear sample collection time relative to routine or standard cleaning/disinfection, and no baseline equivalence of the outcome (initial burden not measured on control compared to intervention surfaces). The lack of monitoring and the audit of environmental services and disinfection implementation is a determinant that was not measured in most studies and has been identified in other systematic reviews of IPC as an important determinant for effective disinfection [19,28]. The best study designs compared the concentration of the outcome organism before and after disinfection intervention and before and after a contemporary control in equivalent healthcare settings.
4.2. Disinfection Efficacy
Many studies inadequately described the disinfection intervention (active ingredient, contact times, and final dilutions for disinfectants used in intervention studies). The method of application is important. Contact time may be affected by different methods of implementation (e.g., wet mopping vs. spray mopping; cotton vs. microfiber cloths) (see, e.g., [62,92,107,153,165]).
The outcomes that were measured were primarily on all viable organisms, specifically bacteria; only three studies assessed viruses, and eleven assessed fungi. Many studies did not assess concentration but rather the prevalence of surfaces that were positive for an organism. For pathogens of concern, most studies reported prevalence rather than concentration, and as a result, many may not have observed reductions, which is probably due to the low initial burden of the pathogen. More studies that reported all of the viable bacteria outcomes found significant effects compared to studies that reported other outcome organisms, which is possibly due to fewer studies assessing concentration among specific pathogens (see, e.g., [182]). Large sample sizes are necessary to assess significant reductions of low-prevalence pathogens; alternatively, studies that inoculate high concentrations of pathogens may elicit a better understanding of disinfectant efficacy.
4.3. Healthcare-Associated Infections
The identified studies have provided extensive evidence that environmental surfaces can be colonized with HAI-related pathogens after disinfection and that these surfaces could be an important transmission pathway, with some pathogens surviving prescribed disinfection. HAIs caused by antimicrobial-resistant organisms were assessed less often. It is estimated that 426,277 healthcare-associated infections are caused by antimicrobial-resistant microorganisms every year in the European Union [224]. Antimicrobial-resistant organisms present a challenge for treatment and can lead to increased morbidity and mortality, as they have a higher burden in low and middle income countries due to delayed presentation, low access to microbiological diagnostics and testing, and the low availability of second-line antibiotics [225]. Disinfection interventions on environmental surfaces may reduce HAIs; however, disinfection efficacy is only one component in a larger system of IPC strategies that are applicable to environmental surfaces.
5. Conclusions
Comparing disinfection efficacy was impeded by study heterogeneity and study quality. As such, we conclude that guidelines for disinfectant use are primarily based on laboratory data rather than on a systematic review of in situ disinfection efficacy. We built upon the framework of the criteria for the selection of the ideal disinfectant to review important components for system-level disinfection efficacy as part of infection prevention and control (IPC) strategies.
In addition to disinfection efficacy, bundled interventions, including monitoring and implementation interventions such as measuring environmental bioburden, audit and feedback, training/re-education of environmental services staff, the addition of more cleaning staff or supervisors, and/or the use of implementation or quality checklists can improve IPC efficacy [226]. Monitoring/audit and feedback programs can prevent and control HAIs and antimicrobial resistance by supporting behavior changes during IPC implementation to create a monitoring and learning culture (as recommended in WHO 2018 [226]). Evidence deemed as being high-quality is reported to indicate that surveillance with active feedback may reduce HAIs [25]. A separate systematic review found intermediate-level evidence that standardizing audits and feedback reduces HAIs [227]. Studies reporting the sustainability of implementation interventions highlight the importance of ongoing education, direct feedback, and fiscal commitment to the monitoring/audit and feedback program from administrators [28].
Contextual factors for successful disinfection implementation include placing environmental services within the administrative hierarchy of the hospital, the outsourcing of environmental services, and a positive patient safety culture between clinical and environmental services staff and between supervisors and front-line personnel [28]. Multimodal strategies, including team-based, task-oriented, positive, and hands-on training, were considered to be more effective than formal training for IPC program adherence [227]. While implementation research has found that training, monitoring, and feedback of IPC implementation increases adherence to IPC programs, evidence about the long-term efficacy of IPC interventions is still needed [228]. As such, a complex of factors determines IPC effectiveness. While the choice of disinfectant and its efficacy have been dominant considerations in research and IPC programs, it is critically important for practitioners and researchers to consider system-level efficacy in reducing organism load and reducing HAIs in healthcare settings.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph182111100/s1, Supplementary Material 1: Database Search Strategies; Supplementary Material 2: List of Included Studies and Study Quality Assessment; Supplementary Material 3: PRISMA Checklists; Supplementary Material 4: Summary of Disinfection Intervention Efficacy; Supplementary Material 5: Tables for Disinfection Efficacy by Disinfection Intervention and Outcome
Author Contributions
Conceptualization, J.B. and R.C.; methodology, E.C.C., C.K.C., M.C., K.G., G.P., D.F., and R.C.; software, K.G., M.C., and G.P.; validation, E.C.C. and D.F.; formal analysis, E.C.C.; investigation, H.A., A.B., E.B., E.C.C., G.C., C.H., K.H., E.J.G., T.J., S.M., and Y.S.; resources, J.B.; data curation, H.A., A.B., E.B., E.C.C., G.C., C.H., K.H., E.J.G., T.J., S.M., and Y.S.; writing—original draft preparation, E.C.C.; writing—review and editing, E.C.C., R.C., M.C., C.K.C., and J.B.; visualization, E.C.C.; supervision, J.B., R.C., and E.C.C.; project administration, E.C.C.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a gift from Kersia Group to the Water Institute at the University of North Carolina for research related to disinfection in health care settings. Collin Knox Coleman was supported in part by a grant from the National Institute of Environmental Health Sciences (T32ES007018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All supporting data are in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. Kersia Group contributed to the scope of the research project and study design but had no role in study implementation, data collection, analyses, interpretation of the data, preparation of the manuscript, or decision to publish.
References
- Magill, S.S.; O’Leary, S.; Thompson, D.; Dumyati, G.; Nadle, J.; Wilson, L.; Kainer, M.; Lynfield, R.; Greissman, S.; Ray, S.; et al. Changes in Prevalence of Health Care–Associated Infections. N. Engl. J. Med. 2018, 380, 1085–1086. [Google Scholar]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.-P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Curcio, D.; Cane, A.; Fernández, F.; Correa, J. Surgical site infection in elective clean and clean-contaminated surgeries in developing countries. Int. J. Infect. Dis. 2019, 80, 34–45. [Google Scholar] [CrossRef]
- Bonell, A.; Azarrafiy, R.; Huong, V.T.L.; Le Viet, T.; Phu, V.D.; Dat, V.Q.; Wertheim, H.; Van Doorn, H.R.; Lewycka, S.; Nadjm, B. A Systematic Review and Meta-analysis of Ventilator-associated Pneumonia in Adults in Asia: An Analysis of National Income Level on Incidence and Etiology. Clin. Infect. Dis. 2019, 68, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Vickery, K.; Deva, A.; Jacombs, A.; Allan, J.; Valente, P.; Gosbell, I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 2012, 80, 52–55. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile Infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Donskey, C.J. Beyond high-touch surfaces: Portable equipment and floors as potential sources of transmission of health care–associated pathogens. Am. J. Infect. Control. 2019, 47, A90–A95. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Sattar, S.A.; Springthorpe, V.S.; Wells, G.A.; Tostowaryk, W. Rotavirus survival on human hands and transfer of infectious virus to animate and nonporous inanimate surfaces. J. Clin. Microbiol. 1988, 26, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Springthorpe, V.S.; Sattar, S.A.; Rivard, S.; Rahman, M. Potential role of hands in the spread of respiratory viral infections: Studies with human parainfluenza virus 3 and rhinovirus 14. J. Clin. Microbiol. 1991, 29, 2115–2119. [Google Scholar] [CrossRef]
- Mbithi, J.N.; Springthorpe, V.S.; Boulet, J.R.; Sattar, S.A. Survival of hepatitis A virus on human hands and its transfer on contact with animate and inanimate surfaces. J. Clin. Microbiol. 1992, 30, 757–763. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Yang, X.-Y.; Ding, X.-X.; Li, R.-J.; Pan, M.-S.; Zhao, X.; Hu, X.-Q.; Zhang, J.-J.; Yang, L.-Q.; Yang, X.-Y. Exposure to infected/colonized roommates and prior room occupants increases the risks of healthcare-associated infections with the same organism. J. Hosp. Infect. 2019, 101, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Cohen, C.C.; Løyland, B.; Larson, E.L. Transmission of health care-associated infections from roommates and prior room occupants: A systematic review. Clin. Epidemiol. 2017, 9, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Datta, R.; Platt, R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch. Intern. Med. 2006, 166, 1945–1951. [Google Scholar] [CrossRef]
- Shaughnessy, M.K.; Micielli, R.L.; DePestel, D.D.; Arndt, J.; Strachan, C.L.; Welch, K.B.; Chenoweth, C.E. Evaluation of Hospital Room Assignment and Acquisition of Clostridium difficile Infection. Infect. Control Hosp. Epidemiol. 2011, 32, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Nseir, S.; Blazejewski, C.; Lubret, R.; Wallet, F.; Courcol, R.; Durocher, A. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the intensive care unit. Clin. Microbiol. Infect. 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Carling, P.C.; Parry, M.M.; Rupp, M.E.; Po, J.L.; Dick, B.; Von Beheren, S. Improving Cleaning of the Environment Surrounding Patients in 36 Acute Care Hospitals. Infect. Control Hosp. Epidemiol. 2008, 29, 1035–1041. [Google Scholar] [CrossRef]
- Donskey, C.J. Does improving surface cleaning and disinfection reduce health care-associated infections? Am. J. Infect. Control 2013, 41, S12–S19. [Google Scholar] [CrossRef]
- Eckstein, B.C.; Adams, D.A.; Eckstein, E.C.; Rao, A.; Sethi, A.K.; Yadavalli, G.K.; Donskey, C.J. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect. Dis. 2007, 7, 61. [Google Scholar] [CrossRef]
- Hota, B.; Blom, D.W.; Lyle, E.A.; Weinstein, R.A.; Hayden, M.K. Interventional evaluation of environmental contamination by vancomycin-resistant enterococci: Failure of personnel, product, or procedure? J. Hosp. Infect. 2009, 71, 123–131. [Google Scholar] [CrossRef]
- Po, J.L.; Burke, R.; Sulis, C.; Carling, P.C. Dangerous cows: An analysis of disinfection cleaning of computer keyboards on wheels. Am. J. Infect. Control. 2009, 37, 778–780. [Google Scholar] [CrossRef] [PubMed]
- Creamer, E.; Dorrian, S.; Dolan, A.; Sherlock, O.; Fitzgerald-Hughes, D.; Thomas, T.; Walsh, J.; Shore, A.; Sullivan, D.; Kinnevey, P.; et al. When are the hands of healthcare workers positive for meticillin-resistant Staphylococcus aureus? J. Hosp. Infect. 2010, 75, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef]
- Price, L.; MacDonald, J.; Melone, L.; Howe, T.; Flowers, P.; Currie, K.; Curran, E.; Ness, V.; Waddell, D.; Manoukian, S.; et al. Effectiveness of national and subnational infection prevention and control interventions in high-income and upper-middle-income countries: A systematic review. Lancet Infect. Dis. 2018, 18, e159–e171. [Google Scholar] [CrossRef]
- Louh, I.K.; Greendyke, W.G.; Hermann, E.A.; Davidson, K.W.; Falzon, L.; Vawdrey, D.K.; Shaffer, J.A.; Calfee, D.P.; Furuya, E.Y.; Ting, H.H.; et al. Clostridium Difficile Infection in Acute Care Hospitals: Systematic Review and Best Practices for Prevention. Infect. Control Hosp. Epidemiol. 2017, 38, 476–482. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Arduino, M. Editorial commentary: Climbing the evidentiary hierarchy for environmental infection control. Clin. Infect. Dis. 2013, 56, 36–39. [Google Scholar] [CrossRef]
- Han, J.H.; Sullivan, N.; Leas, B.F.; Pegues, D.A.; Kaczmarek, J.L.; Umscheid, C.A. Cleaning hospital room surfaces to prevent health care-associated infections: A technical brief. Ann. Intern. Med. 2015, 163, 598–607. [Google Scholar] [CrossRef]
- ASTM International. ASTM E2197—02 Standard Quantitative Disk Carrier Test Method for Determining the Bactericidal, Virucidal, Fungicidal, Mycobactericidal and Sporicidal Activities of Liquid Chemical Germicides; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Sattar, S.A.; Springthorpe, V.S.; Adegbunrin, O.; Zafer, A.A.; Busa, M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J. Virol. Methods 2003, 112, 3–12. [Google Scholar] [CrossRef]
- Le Coutour, C.; Oblin, I. Disinfection of surfaces in hospital: Comparison between theoric and real efficiency of three commercial products. Tech. Hosp. Med.-Soc. Sanit. 1991, 46, 49–50. [Google Scholar]
- Neely, A.N.; Maley, M.P. Survival of enterococci and staphylococci on hospital fabrics and plastic. J. Clin. Microbiol. 2000, 38, 724–726. [Google Scholar] [CrossRef]
- Noskin, G.A.; Stosor, V.; Cooper, I.; Peterson, L.R. Recovery of Vancomycin-Resistant Enterococci on Fingertips and Environmental Surfaces. Infect. Control Hosp. Epidemiol. 1995, 16, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Traoré, O.; Springthorpe, V.S.; Sattar, S.A. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J. Appl. Microbiol. 2002, 92, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.; Campbell, L.; Harvey, R.; Bischoff, K.; Alali, W.; Barling, K.; Anderson, R. Patterns of Antimicrobial Resistance among Commensal Escherichia coli Isolated from Integrated Multi-Site Housing and Worker Cohorts of Humans and Swine. Foodborne Pathog. Dis. 2005, 2, 24–37. [Google Scholar] [CrossRef]
- Leas, B.; Sullivan, N.; Han, J.; Pegues, D.; Kaczmarek, J.; Umscheid, C. Environmental Cleaning for the Prevention of Healthcare-Associated Infections. Agency Healthc. Res. Qual. 2015, 22, 121. [Google Scholar]
- Schreiber, P.W.; Sax, H.; Wolfensberger, A.; Clack, L.; Kuster, S.P. The preventable proportion of healthcare-associated infections 2005–2016: Systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 1277–1295. [Google Scholar] [CrossRef] [PubMed]
- Doll, M.; Stevens, M.; Bearman, G. Environmental cleaning and disinfection of patient areas. Int. J. Infect. Dis. 2018, 67, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Selection of the Ideal Disinfectant. Hosp. Epidemiol. 2014, 35, 855–865. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Centers for Disease Control and Prevention (CDC). Guidelines for Environmental Infection Control in Health-Care Facilities Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC); CDC: Atlanta, GA, USA, 2019.
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available online: https://stacks.cdc.gov/view/cdc/47378 (accessed on 15 December 2019).
- World Health Organization. Prevention of Hospital-Acquired Infections: A Practical Guide, 2nd ed.; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Varghese, A.; Cawley, M.; Hong, T. Supervised clustering for automated document classification and prioritization: A case study using toxicological abstracts. Environ. Syst. Decis. 2018, 38, 398–414. [Google Scholar] [CrossRef]
- Yuen, J.W.M.; Chung, T.W.K.; Loke, A.Y. Methicillin-Resistant Staphylococcus aureus (MRSA) contamination in bedside surfaces of a hospital ward and the potential effectiveness of enhanced disinfection with an antimicrobial polymer surfactant. Int. J. Environ. Res. Public Health. 2015, 12, 3026–3041. [Google Scholar] [CrossRef]
- World Bank. World Bank Country and Lending Groups—World Bank Data Help Desk. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 11 January 2021).
- NIH Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 11 January 2021).
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, n71. [Google Scholar] [CrossRef]
- Fujii, M. Prevention of MRSA infection in neurosurgery: Examination from the patient environment. No Shinkei Geka. 1996, 24, 241–245. [Google Scholar]
- Fukada, T.; Tsuchiya, Y.; Iwakiri, H.; Ozaki, M. Adenosine triphosphate bioluminescence assay for monitoring contamination of the working environment of anaesthetists and cleanliness of the operating room. J. Infect. Prev. 2015, 16, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Iwakiri, H.; Ozaki, M. Anaesthetists’ role in computer keyboard contamination in an operating room. J. Hosp. Infect. 2008, 70, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.W.; Breshears, J.; Campbell, A.; Husbands, C.; Rupert, R. Assessment and risk reduction of infectious pathogens on chiropractic treatment tables. Chiropr. Osteopat. 2007, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; de Andrade, D.; Rigotti, M.A.; de Almeida, M.T.G.; Guerra, O.G.; dos Santos, A.G., Jr. Assessment of disinfection of hospital surfaces using different monitoring methods. Rev. Lat. Am. Enfermagem. 2015, 23, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Dramowski, A.; Whitelaw, A.; Cotton, M.F. Assessment of terminal cleaning in pediatric isolation rooms: Options for low-resource settings. Am. J. Infect. Control. 2016, 44, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Oie, S.; Yanagi, C.; Matsui, H.; Nishida, T.; Tomita, M.; Kamiya, A. Contamination of environmental surfaces by Staphylococcus aureus in a dermatological ward and its preventive measures. Biol. Pharm. Bull. 2005, 28, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Doidge, M.; Allworth, A.M.; Woods, M.; Marshall, P.; Terry, M.; Brien, K.O.; Goh, H.M.; George, N.; Nimmo, G.R.; Schembri, M.A.; et al. Control of an Outbreak of Carbapenem—Resistant Acinetobacter baumannii in Australia after Introduction of Environmental Cleaning with a Commercial Oxidizing Disinfectant. Infect. Control Hosp. Epidemiol. 2010, 31, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.; Rudramurthy, S.; Jain, N.; Shamanth, A.; Sharma, D.; Jain, K.; Yaddanupudi, L.; Chakrabarti, A. Controlling a possible outbreak of Candida auris infection: Lessons learnt from multiple interventions. J. Hosp. Infect. 2017, 97, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.S.; Wan, G.H.; Chen, Y.W.; Ku, H.L.; Li, L.P.; Liu, C.H.; Mau, H.S. Effectiveness of bacterial disinfectants on surfaces of mechanical ventilator systems. Respir. Care 2012, 57, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Codish, S.; Toledano, R.; Novack, V.; Sherf, M.; Borer, A. Effectiveness of stringent decontamination of computer input devices in the era of electronic medical records and bedside computing: A randomized controlled trial. Am. J. Infect. Control. 2015, 43, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Casini, B.; Selvi, C.; Cristina, M.L.; Totaro, M.; Costa, A.L.; Valentini, P.; Barnini, S.; Baggiani, A.; Tagliaferri, E.; Privitera, G. Evaluation of a modified cleaning procedure in the prevention of carbapenem-resistant Acinetobacter baumannii clonal spread in a burn intensive care unit using a high-sensitivity luminometer. J. Hosp. Infect. 2017, 95, 46–52. [Google Scholar] [CrossRef]
- Alhmidi, H.; Koganti, S.; Cadnum, J.L.; Rai, H.; Jencson, A.L.; Donskey, C.J. Evaluation of a novel alcohol-based surface disinfectant for disinfection of hard and soft surfaces in healthcare facilities. Open Forum Infect. Dis. 2017, 4, 8–10. [Google Scholar] [CrossRef]
- Andersen, B.M.; Rasch, M.; Kvist, J.; Tollefsen, T.; Lukkassen, R.; Sandvik, L.; Welo, A. Floor cleaning: Effect on bacteria and organic materials in hospital rooms. J. Hosp. Infect. 2008, 71, 57–65. [Google Scholar] [CrossRef]
- Schmidt, M.G.; Fairey, S.E.; Attaway, H.H. In situ evaluation of a persistent disinfectant provides continuous decontamination within the clinical environment. Am. J. Infect. Control 2019, 47, 732–734. [Google Scholar] [CrossRef]
- Attaway, H.H.; Fairey, S.; Steed, L.L.; Salgado, C.D.; Michels, H.T.; Schmidt, M.G. Intrinsic bacterial burden associated with intensive care unit hospital beds: Effects of disinfection on population recovery and mitigation of potential infection risk. Am. J. Infect. Control. 2012, 40, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Hutton, A.; Mariyaselvam, M.; Hodges, E.; Wong, K.; Blunt, M.; Young, P. Keyboard cleanliness: A controlled study of the residual effect of chlorhexidine gluconate. Am. J. Infect. Control. 2015, 43, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.A.; Sexton, J.D.; Pivo, T.; Humphrey, K.; Leslie, R.A.; Gerba, C.P. Microbial transmission in an outpatient clinic and impact of an intervention with an ethanol-based disinfectant. Am. J. Infect. Control. 2019, 47, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Imtiaz, S.; Zafar, A.; Javed, H.; Atif, M.; Abosalif, K.O.A.A.; Ejaz, H. Role of hospital surfaces in transmission of infectious diseases. Pak. J. Med. Health Sci. 2018, 12, 857–859. [Google Scholar]
- Bokulich, N.A.; Mills, D.A.; Underwood, M.A. Surface microbes in the neonatal intensive care unit: Changes with routine cleaning and over time. J. Clin. Microbiol. 2013, 51, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Botha, S.L.; Weaving, P.; Satta, G. A pilot study to assess the effectiveness and cost of routine universal use of peracetic acid sporicidal wipes in a real clinical environment. Am. J. Infect. Control 2016, 44, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Doan, L.; Forrest, H.; Fakis, A.; Craig, J.; Claxton, L.; Khare, M. Clinical and cost effectiveness of eight disinfection methods for terminal disinfection of hospital isolation rooms contaminated with Clostridium difficile 027. J. Hosp. Infect. 2012, 82, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Armellino, D.; Goldstein, K.; Thomas, L.; Walsh, T.J.; Petraitis, V. Comparative evaluation of operating room terminal cleaning by two methods: Focused multivector ultraviolet (FMUV) versus manual-chemical disinfection. Am. J. Infect. Control. 2020, 48, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.G.; Digney, W.; Locket, P.; Dancer, S.J. Controlling methicillin-resistant Staphylococcus aureus (MRSA) in a hospital and the role of hydrogen peroxide decontamination: An interrupted time series analysis. BMJ Open 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Carling, P.C.; Perkins, J.; Ferguson, J.; Thomasser, A. Evaluating a New Paradigm for Comparing Surface Disinfection in Clinical Practice. Infect. Control Hosp. Epidemiol. 2014, 35, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L. Evaluation of a New Hydrogen Peroxide Wipe Disinfectant. Infect. Control Hosp. Epidemiol. 2013, 34, 521–523. [Google Scholar] [CrossRef]
- Deshpande, A.; Mana, T.S.; Cadnum, J.L.; Jencson, A.C.; Sitzlar, B.; Fertelli, D.; Hurless, K.; Kundrapu, S.; Sunkesula, V.C.; Donskey, C.J.; et al. Evaluation of a Sporicidal Peracetic Acid/Hydrogen Peroxide—Based Daily Disinfectant Cleaner. Soc. Healthc. Epidemiol. Am. 2014, 35, 5–8. [Google Scholar] [CrossRef]
- Wiemken, T.L.; Curran, D.R.; Kelley, R.R.; Pacholski, E.B.; Carrico, R.M.; Peyrani, P.; Khan, M.S.S.; Ramirez, J.A. Evaluation of the effectiveness of improved hydrogen peroxide in the operating room. Am. J. Infect. Control 2014, 42, 1004–1005. [Google Scholar] [CrossRef]
- Yui, S.; Ali, S.; Muzslay, M.; Jeanes, A.; Wilson, A.P.R. Identification of Clostridium difficile Reservoirs in the Patient Environment and Efficacy of Aerial Hydrogen Peroxide Decontamination. Infect. Control Hosp. Epidemiol. 2017, 38, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Siani, H.; Wesgate, R.; Maillard, J.Y. Impact of antimicrobial wipes compared with hypochlorite solution on environmental surface contamination in a health care setting: A double-crossover study. Am. J. Infect. Control 2018, 46, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Guercia, K.A.; Sullivan, L.; Havill, N.L.; Fekieta, R.; Kozakiewicz, J.; Goffman, D. Prospective cluster controlled crossover trial to compare the impact of an improved hydrogen peroxide disinfectant and a quaternary ammonium-based disinfectant on surface contamination and health care outcomes. Am. J. Infect. Control 2017, 45, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Dharan, S.; Mourouga, P.; Copin, P.; Bessmer, G.; Tschanz, B.; Pittet, D. Routine disinfection of patients’ environmental surfaces. Myth or reality? J. Hosp. Infect. 1999, 42, 113–117. [Google Scholar] [CrossRef]
- Sjöberg, M.; Eriksson, M.; Andersson, J.; Norén, T. Transmission of Clostridium difficile spores in isolation room environments and through hospital beds. Apmis 2014, 122, 800–803. [Google Scholar] [CrossRef]
- Alfa, M.J.; Lo, E.; Olson, N.; Macrae, M.; Buelow-Smith, L. Use of a daily disinfectant cleaner instead of a daily cleaner reduced hospital—Acquired infection rates. Am. J. Infect. Control 2015, 43, 141–146. [Google Scholar] [CrossRef]
- Gonzalez, S.; Illescas, A.; Escarzaga, E. Reduction of Bacterial Contamination of the Environment of a General Hospital, by the Use of a new Germicide Biomet 66. Rev. Med. del Hosp. Gen. 1963, 26, 873–878. [Google Scholar]
- Strat, E. Research on the effectiveness of disinfectants in the surfactant group o the degeneration of the hospital environment. Rev. Med. Chir. Soc. Med. Nat. Iasi. 1971, 75, 957–966. [Google Scholar]
- Passaretti, C.L.; Otter, J.A.; Reich, N.G.; Myers, J.; Shepard, J.; Ross, T.; Carroll, K.C.; Lipsett, P.; Perl, T.M. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin. Infect. Dis. 2013, 56, 27–35. [Google Scholar] [CrossRef]
- Otter, J.A.; Cummins, M.; Ahmad, F.; van Tonder, C.; Drabu, Y.J. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2007, 67, 182–188. [Google Scholar] [CrossRef]
- Lewis, B.D.; Spencer, M.; Rossi, P.J.; Lee, C.J.; Brown, K.R.; Malinowski, M.; Seabrook, G.R.; Edmiston, C.E. Assessment of an innovative antimicrobial surface disinfectant in the operating room environment using adenosine triphosphate bioluminescence assay. Am. J. Infect. Control 2015, 43, 283–285. [Google Scholar] [CrossRef]
- Suzuki, A.; Namba, Y.; Matsuura, M.; Horisawa, A. Bacterial contamination of floors and other surfaces in operating rooms: A five-year survey. J. Hyg. 1984, 93, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Roux, D.; Aubier, B.; Cochard, H.; Quentin, R.; Van Der Mee-Marquet, N. Contaminated sinks in intensive care units: An underestimated source of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the patient environment. J. Hosp. Infect. 2013, 85, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Hinsa-Leasure, S.M.; Nartey, Q.; Vaverka, J.; Schmidt, M.G. Copper alloy surfaces sustain terminal cleaning levels in a rural hospital. Am. J. Infect. Control 2016, 44, e195–e203. [Google Scholar] [CrossRef] [PubMed]
- Furlan, M.C.R.; Ferreira, A.M.; Rigotti, M.A.; Guerra, O.G.; Frota, O.P.; De Sousa, A.F.L.; De Andrade, D. Correlation among monitoring methods of surface cleaning and disinfection in outpatient facilities. Acta Paul. Enferm. 2019, 32, 282–289. [Google Scholar] [CrossRef]
- Byers, K.E.; Durbin, L.J.; Simonton, B.M.; Anglim, A.M.; Adal, A.; Farr, B.M.; Control, S.I.; Epidemiology, H.; Apr, N. Disinfection of Hospital Rooms Contaminated with Vancomycin-Resistant Enterococcus faecium. Infect. Control Hosp. Epidemiol. 2020, 19, 261–264. [Google Scholar]
- Panknin, H. Diversity of the ambient flora and effectiveness of surface disinfection measures in the neonatal unit. Hyg. Med. 2014, 39, 245–247. [Google Scholar]
- Kitagawa, H.; Mori, M.; Kashiyama, S.; Sasabe, Y.; Ukon, K.; Shimokawa, N.; Shime, N.; Ohge, H. Effect of pulsed xenon ultraviolet disinfection on methicillin-resistant Staphylococcus aureus contamination of high-touch surfaces in a Japanese hospital. Am. J. Infect. Control 2020, 48, 139–142. [Google Scholar] [CrossRef]
- Styaningsih, N.; Suwundo, A.; Adi, M.S. Effectiveness of Disinfectant A and B on the Growth of Bacteria in the Area of Central Surgical Installation of Hospital X in Kudus City. Indian J. Public Health Res. Dev. 2019, 10, 795–804. [Google Scholar] [CrossRef]
- Santos, A.G., Jr.; Ferreira, A.M.; Frota, O.P.; Rigotti, M.A.; Barcelos, L.d.S.; Lopes de Sousa, A.F.; de Andrade, D.; Guerra, O.G.; Furlan, M.C.R. Effectiveness of Surface Cleaning and Disinfection in a Brazilian Healthcare Facility. Open Nurs. J. 2018, 12, 36–44. [Google Scholar] [CrossRef][Green Version]
- Anderson, D.J.; Moehring, R.W.; Weber, D.J.; Lewis, S.S.; Chen, L.F.; Schwab, J.C.; Becherer, P.; Blocker, M.; Triplett, P.F.; Knelson, L.P.; et al. Effectiveness of targeted enhanced terminal room disinfection on hospital-wide acquisition and infection with multidrug-resistant organisms and Clostridium difficile: A secondary analysis of a multicentre cluster randomised controlled trial with crossover. Lancet Infect. Dis. 2018, 18, 845–853. [Google Scholar] [CrossRef]
- Frota, O.P.; Ferreira, A.M.; Guerra, O.G.; Rigotti, M.A.; de Andrade, D.; Borges, N.M.A.; de Almeida, M.T. Efficiency of cleaning and disinfection of surfaces: Correlation between assessment methods. Rev. Bras. Enferm. 2017, 70, 1176–1183. [Google Scholar] [CrossRef]
- Blazejewski, C.; Wallet, F.; Rouzé, A.; Le Guern, R.; Ponthieux, S.; Salleron, J.; Nseir, S. Efficiency of hydrogen peroxide in improving disinfection of ICU rooms. Crit. Care. 2015, 19, 30. [Google Scholar] [CrossRef]
- Rutala, W.A.; Kanamori, H.; Gergen, M.F.; Knelson, L.P.; Sickbert-Bennett, E.E.; Chen, L.F.; Anderson, D.J.; Sexton, D.J.; Weber, D.J. Enhanced disinfection leads to reduction of microbial contamination and a decrease in patient colonization and infection. Infect. Control Hosp. Epidemiol. 2018, 39, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.L.; Leet, T.; Miller, J.; Mundy, L.M. Environmental Control to Reduce Transmission of Clostridium difficile. Clin. Infect. Dis. 2000, 31, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L.; Guercia, K.A.; Schweon, S.J.; Moore, B.A. Evaluation of two organosilane products for sustained antimicrobial activity on high-touch surfaces in patient rooms. Am. J. Infect. Control 2014, 42, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Stewart, M.; Hunter, J.; Yip, B.; Reid, D.; Robertson, C.; Dancer, S.J. How quickly do hospital surfaces become contaminated after detergent cleaning? Healthc. Infect. 2013, 18, 3–9. [Google Scholar] [CrossRef]
- Casini, B.; Righi, A.; De Feo, N.; Totaro, M.; Giorgi, S.; Zezza, L.; Valentini, P.; Tagliaferri, E.; Costa, A.L.; Barnini, S.; et al. Improving cleaning and disinfection of high-touch surfaces in intensive care during carbapenem-resistant Acinetobacter baumannii endemo-epidemic situations. Int. J. Environ. Res. Public Health. 2018, 15, 2305. [Google Scholar] [CrossRef]
- Fitton, K.; Barber, K.R.; Karamon, A.; Zuehlke, N.; Atwell, S.; Enright, S. Long-acting water-stable organosilane agent and its sustained effect on reducing microbial load in an intensive care unit. Am. J. Infect. Control 2017, 45, 1214–1217. [Google Scholar] [CrossRef]
- Vesley, D.; Klapes, N.A.; Benzow, K.; Le, C.T. Microbiological evaluation of wet and dry floor sanitization systems in hospital patient rooms. Appl. Environ. Microbiol. 1987, 53, 1042–1045. [Google Scholar] [CrossRef]
- Sigler, V.; Hensley, S. Persistence of mixed Staphylococci assemblages following disinfection of hospital room surfaces. J. Hosp. Infect. 2013, 83, 253–256. [Google Scholar] [CrossRef]
- Fattorini, M.; Buonocore, G.; Lenzi, D.; Burgassi, S.; Cardaci, R.M.R.; Biermann, K.P.; Cevenini, G.; Messina, G. Public Health since the beginning: Neonatal incubators safety in a clinical setting. J. Infect. Public Health 2018, 11, 788–792. [Google Scholar] [CrossRef]
- Hacek, D.M.; Ascp, M.T.; Ogle, A.M.; Fisher, A.; Ascp, M.T.; Robicsek, A. Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. Am. J. Infect. Control 2010, 38, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Butin, M.; Dumont, Y.; Monteix, A.; Raphard, A.; Roques, C.; Martins Simoes, P.; Picaud, J.C.; Laurent, F. Sources and reservoirs of Staphylococcus capitis NRCS-A inside a NICU. Antimicrob. Resist. Infect. Control 2019, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Mepham, S.; Athan, B.; Mack, D.; Smith, R.; Jacobs, M.; Hopkins, S. Terminal decontamination of the Royal Free London’s high-level isolation unit after a case of Ebola virus disease using hydrogen peroxide vapor. Am. J. Infect. Control. 2016, 44, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Strassle, P.; Thom, K.A.; Johnsonm, J.K.; Leekha, S.; Lissauer, M.; Zhu, J.; Harris, A.D. The effect of terminal cleaning on environmental contamination rates of multidrug-resistant Acinetobacter baumannii. Am. J. Infect. Control 2012, 40, 1005–1007. [Google Scholar] [CrossRef][Green Version]
- Garvey, M.I.; Wilkinson, M.A.C.; Bradley, C.W.; Holden, K.L.; Holden, E. Wiping out MRSA: Effect of introducing a universal disinfection wipe in a large UK teaching hospital. Antimicrob. Resist. Infect. Control 2018, 7, 155. [Google Scholar] [CrossRef]
- Orenstein, R.; Aronhalt, K.C.; McManus, J.E., Jr.; Fedraw, L.A. A Targeted Strategy to Wipe Out Clostridium difficile. Infect. Control. Hosp. Epidemiol. 2011, 32, 1137–1139. [Google Scholar]
- Kaatz, G.W.; Gitlin, S.D.; Schaberg, D.R.; Wilson, K.H.; Kauffman, C.A.; Seo, S.M.; Fekety, R. Acquisition of Clostridium difficile from the hospital environment. Am. J. Epidemiol. 1988, 127, 1289–1294. [Google Scholar] [CrossRef]
- Youkee, D.; Brown, C.S.; Lilburn, P.; Shetty, N.; Brooks, T.; Simpson, A.; Bentley, N.; Lado, M.; Kamara, T.B.; Walker, N.F.; et al. Assessment of Environmental Contamination and Environmental Decontamination Practices within an Ebola Holding Unit, Freetown, Sierra Leone. PLoS ONE 2015, 10, e0145167. [Google Scholar] [CrossRef]
- Mosci, D.; Marmo, G.W.; Sciolino, L.; Zaccaro, C.; Antonellini, R.; Accogli, L.; Lazzarotto, T.; Mongardi, M.; Landini, M.P. Automatic environmental disinfection with hydrogen peroxide and silver ions versus manual environmental disinfection with sodium hypochlorite: A multicentre randomized before-and-after trial. J. Hosp. Infect. 2017, 97, 175–179. [Google Scholar] [CrossRef]
- Galván Contreras, R.-; Tapia, R.A.R.; Cervantes, E.S.; Aguilar, R.M.A.C. Comparative study on the effectiveness of 6% sodium hypochlorite solution vs a bromine-chloro-dimethylhydantoin solution for disinfecting hospital environments. Perinatol. Reprod. Hum. 2017, 30, 145–150. [Google Scholar]
- Huang, Y.S.; Chen, Y.C.; Chen, M.L.; Cheng, A.; Hung, I.C.; Wang, J.T.; Sheng, W.H.; Chang, S.C. Comparing visual inspection, aerobic colony counts, and adenosine triphosphate bioluminescence assay for evaluating surface cleanliness at a medical center. Am. J. Infect. Control 2015, 43, 882–886. [Google Scholar] [CrossRef]
- Patel, S.S.; Pevalin, D.J.; Prosser, R.; Couchman, A. Comparison of detergent-based cleaning, disinfectant-based cleaning, and detergent-based cleaning after enhanced domestic staff training within a source isolation facility. Br. J. Infect. Control 2007, 8, 20–25. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Fawley, W.N.; Wigglesworth, N.; Parnell, P.; Verity, P.; Freeman, J. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J. Hosp. Infect. 2003, 54, 109–114. [Google Scholar] [CrossRef]
- Barbut, F.; Menuet, D.; Verachten, M.; Girou, E. Comparison of the Efficacy of a Hydrogen Peroxide Dry-Mist Disinfection System and Sodium Hypochlorite Solution for Eradication of Clostridium difficile Spores. Infect. Control Hosp. Epidemiol. 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Nerandzic, M.M.; Kundrapu, S.; Donskey, C.J. Does Organic Material on Hospital Surfaces Reduce the Effectiveness of Hypochlorite and UV Radiation for Disinfection of Clostridium difficile? Infect. Control Hosp. Epidemiol. 2013, 34, 1106–1108. [Google Scholar] [CrossRef]
- Anderson, D.J.; Chen, L.F.; Weber, D.J.; Moehring, R.W.; Lewis, S.S.; Triplett, P.F.; Blocker, M.; Becherer, P.; Schwab, J.C.; Knelson, L.P.; et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): A cluster-randomised, multicentre, crossover study. Lancet 2017, 389, 805–814. [Google Scholar] [CrossRef]
- Lerner, A.O.; Abu-Hanna, J.; Carmeli, Y.; Schechner, V. Environmental contamination by carbapenem-resistant Acinetobacter baumannii: The effects of room type and cleaning methods. Infect. Control Hosp. Epidemiol. 2019, 41, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Jinadatha, C.; Quezada, R.; Huber, T.W.; Williams, J.B.; Zeber, J.E.; Copeland, L.A. Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infect. Dis. 2014, 14, 187. [Google Scholar] [CrossRef]
- Casini, B.; Tuvo, B.; Cristina, M.L.; Spagnolo, A.M.; Totaro, M.; Baggiani, A.; Privitera, G.P. Evaluation of an Ultraviolet C (UVC) Light-Emitting Device for Disinfection of High Touch Surfaces in Hospital Critical Areas. Int. J. Environ. Res. Public Health 2019, 16, 3572. [Google Scholar] [CrossRef]
- Simon Garcia, M.J.; Gonzalez Sanchez, J.A.; Alcudia Perez, F.; Sanchez Sanchez, C.; Gomez Mayoral, B.; Merino Martinez, M.R. Evaluation of the effect of a cleaning disinfection intervention on the rate of multiresistant microorganism infections in the Intensive Care Unit. Enferm. Intensiva 2009, 20, 27–34. [Google Scholar] [PubMed]
- Manian, F.A.; Griesnauer, S.; Bryant, A. Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. Am. J. Infect. Control 2013, 41, 537–541. [Google Scholar] [CrossRef]
- Manian, F.A.; Griesenauer, S.; Senkel, D.; Setzer, J.M.; Doll, S.A.; Perry, A.M.; Wiechens, M. Isolation of Acinetobacter baumannii Complex and Methicillin-Resistant Staphylococcus aureus from Hospital Rooms Following Terminal Cleaning and Disinfection: Can We Do Better? Infect. Control Hosp. Epidemiol. 2011, 32, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Ghantoji, S.S.; Stibich, M.; Stachowiak, J.; Cantu, S.; Adachi, J.A.; Raad, I.I.; Chemaly, R.F. Non-inferiority of pulsed xenon UV light versus bleach for reducing environmental Clostridium difficile contamination on high-touch surfaces in Clostridium difficile infection isolation rooms. J. Med. Microbiol. 2015, 64, 191–194. [Google Scholar] [CrossRef]
- Coppin, J.D.; Villamaria, F.C.; Williams, M.D.; Copeland, L.A.; Zeber, J.E.; Jinadatha, C. Self-sanitizing copper-impregnated surfaces for bioburden reduction in patient rooms. Am. J. Infect. Control 2017, 45, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Aucella, F.; Vigilante, M.; Valente, G.L.; Stallone, C. Systematic monitor disinfection is effective in limiting HCV spread in hemodialysis. Blood Purif. 2000, 18, 110–114. [Google Scholar] [CrossRef]
- Rathod, S.N.; Beauvais, K.; Sullivan, L.K.; Sudikoff, S.N.; Peaper, D.R.; Martinello, R.A. The effectiveness of a novel colorant additive in the daily cleaning of patient rooms. Infect. Control Hosp. Epidemiol. 2019, 40, 721–723. [Google Scholar] [CrossRef]
- Ho, Y.H.; Wang, L.S.; Jiang, H.L.; Chang, C.H.; Hsieh, C.J.; Chang, D.C.; Tu, H.Y.; Chiu, T.Y.; Chao, H.J.; Tseng, C.C. Use of a sampling area-adjusted adenosine triphosphate bioluminescence assay based on digital image quantification to assess the cleanliness of hospital surfaces. Int. J. Environ. Res. Public Health 2016, 13, 576. [Google Scholar] [CrossRef]
- Hall, T.J.; Jeanes, A.; McKain, L.W.; Jepson, M.J.; Coen, P.G.; Hickok, S.S.; Gant, V.A. A UK district general hospital cleaning study: A comparison of the performance of ultramicrofibre technology with or without addition of a novel copper-based biocide with standard hypochlorite-based cleaning. J. Infect. Prev. 2011, 12, 232–236. [Google Scholar] [CrossRef]
- Oztoprak, N.; Kizilates, F.; Percin, D. Comparison of steam technology and a two-step cleaning (water/detergent) and disinfecting (1000 resp. 5000 ppm hypochlorite) method using microfiber cloth for environmental control of multidrug-resistant organisms in an intensive care unit. GMS Hyg. Infect. Control. 2019, 14, Doc15. [Google Scholar]
- Andersen, B.M.; Bånrud, H.; Bøe, E.; Bjordal, O.; Drangsholt, F. Comparison of UV C Light and Chemicals for Disinfection of Surfaces in Hospital Isolation Units. Infect. Control Hosp. Epidemiol. 2006, 27, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.G.; Hill, C.; Higgins, M.M.; Craddock, J.G.; Nc, C.H. Disinfection of Immersion Tanks (Hubbard) in a Hospital Burn Unit. Arch Env. Health 1974, 28, 101–105. [Google Scholar] [CrossRef]
- Chen, C.H.; Tu, C.C.; Kuo, H.Y.; Zeng, R.F.; Yu, C.S.; Lu, H.H.S.; Liou, M.L. Dynamic change of surface microbiota with different environmental cleaning methods between two wards in a hospital. Appl. Microbiol. Biotechnol. 2017, 101, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Best, E.L.; Parnell, P.; Thirkell, G.; Verity, P.; Copland, M.; Else, P.; Denton, M.; Hobson, R.P.; Wilcox, M.H. Effectiveness of deep cleaning followed by hydrogen peroxide decontamination during high Clostridium difficile infection incidence. J. Hosp. Infect. 2014, 87, 25–33. [Google Scholar] [CrossRef]
- Garvey, M.I.; Bradley, C.W.; Jumaa, P. Environmental decontamination following occupancy of a burns patient with multiple carbapenemase-producing organisms. J. Hosp. Infect. 2016, 93, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Bogusz, A.; Hunter, J.; Devanny, I.; Yip, B.; Reid, D.; Robertson, C.; Dancer, S.J. Evaluating Use of Neutral Electrolyzed Water for Cleaning Near-Patient Surfaces. Infect. Control Hosp. Epidemiol. 2014, 35, 1505–1510. [Google Scholar] [CrossRef]
- Hosein, I.; Madeloso, R.; Nagaratnam, W.; Villamaria, F.; Stock, E.; Jinadatha, C. Evaluation of a pulsed xenon ultraviolet light device for isolation room disinfection in a United Kingdom hospital. Am. J. Infect. Control. 2016, 44, e157–e161. [Google Scholar] [CrossRef]
- Ojajärvi, J.; Mäkelä, P. Evaluation of Chlorine Compounds for Surface Disinfection by Laboratory and ln-use Testing. Scand. J. Infect. Dis. 1976, 8, 267–270. [Google Scholar] [CrossRef]
- Johnson, A.; Weston, L.; Grisewood, L.; Kyffin, M. Evaluation of the Ultra-VTM (ultraviolet) decontamination system as an adjunct to cleaning in a district general hospital. J. Hosp. Infect. 2016, 94, 406–407. [Google Scholar] [CrossRef]
- Frabetti, A.; Vandini, A.; Balboni, P.; Triolo, F.; Mazzacane, S. Experimental evaluation of the efficacy of sanitation procedures in operating rooms. Am. J. Infect. Control 2009, 37, 658–664. [Google Scholar] [CrossRef]
- Al-Hamad, A.; Maxwell, S. How clean is clean? Proposed methods for hospital cleaning assessment. J. Hosp. Infect. 2008, 70, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, S.D.; Patel, A.; Tucker, D.; French, G.L. Lack of enhanced effect of a chlorine dioxide-based cleaning regimen on environmental contamination with Clostridium difficile spores. J. Hosp. Infect. 2012, 82, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Shelly, M.J.; Scanlon, T.G.; Ruddy, R.; Hannan, M.M.; Murray, J.G. Meticillin-resistant Staphylococcus aureus (MRSA) environmental contamination in a radiology department. Clin. Radiol. 2011, 66, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Allen, O.; Jadkauskaite, L.; Shafi, N.T.; Jackson, A.; Athithan, V.; Chiu, Y.D.; IES, E.; Floto, R.A.; Haworth, C.S. Microbiological evaluation of UV disinfection effectiveness in a specialist cystic fibrosis clinic. J. Cyst. Fibros. 2019, 18, e37–e39. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S.J. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Xu, H.; Wu, J.; Zhu, Y.; Wang, L.; Jin, H.; Wei, L.; Shen, L.; Ni, X.; Cao, J.; et al. Sequential enhanced cleaning eliminates multidrug-resistant organisms in general intensive care unit of a traditional Chinese medicine hospital. J. Crit. Care 2017, 41, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.J.; Casey, A.L.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Elliott, T.S.J. The Antimicrobial Efficacy of Copper Alloy Furnishing in the Clinical Environment: A Crossover Study. Infect. Control Hosp. Epidemiol. 2012, 33, 3–9. [Google Scholar] [CrossRef]
- Gable, T.S. Bactericidal effectiveness of floor cleaning methods in a hospital environment. Hospitals 1966, 40, 107–111. [Google Scholar] [PubMed]
- Ogino, J.; Fujimori, I.; Goto, R.; Hisamastu, K.; Murakami, Y.; Yamada, T.; Kikushima, K. Efficacy of pyoktanin and DF-100 for prevention of nosocomial MRSA infection. Pract. Otol. Suppl. 1995, 79, 104–109. [Google Scholar]
- Smith, T.L.; Iwen, P.C.; Olson, S.B.; Rupp, M.E. Environmental contamination with vancomycin-resistant Enterococci in an outpatient setting. Infect. Control Hosp. Epidemiol. 1998, 19, 515–518. [Google Scholar] [CrossRef]
- Meinke, R.; Meyer, B.; Frei, R.; Passweg, J.; Widmer, A.F. Equal Efficacy of Glucoprotamin and an Aldehyde Product for Environmental Disinfection in a Hematologic Transplant Unit: A Prospective Crossover Trial. Infect. Control Hosp. Epidemiol. 2012, 33, 1077–1080. [Google Scholar] [CrossRef]
- Stibich, M.; Stachowiak, J.; Tanner, B.; Berkheiser, M.; Moore, L.; Raad, I.; Chemaly, R.F. Evaluation of a Pulsed-Xenon Ultraviolet Room Disinfection Device for Impact on Hospital Operations and Microbial Reduction. Infect. Control Hosp. Epidemiol. 2011, 32, 286–288. [Google Scholar] [CrossRef]
- Danforth, D.; Nicolle, L.E.; Hume, K.; Alfieri, N.; Sims, H. Nosocomial infections on nursing units with floors cleaned with a disinfectant compared with detergent. J. Hosp. Infect. 1987, 10, 229–235. [Google Scholar] [CrossRef]
- Tekin, A.; Dal, T.; Selçuk, C.T.; Deveci, Ö.; Tekin, R.; Mete, M.; Dayan, S.; Hoşoǧlu, S. Orthophenylphenol in healthcare environments: A trial related to a new administration method and a review of the literature. Turk. J. Med. Sci. 2013, 43, 805–809. [Google Scholar] [CrossRef]
- Hamilton, D.; Foster, A.; Ballantyne, L.; Kingsmore, P.; Bedwell, D.; Hall, T.J.; Hickok, S.S.; Jeanes, A.; Coen, P.G.; Gant, V.A. Performance of ultramicrofibre cleaning technology with or without addition of a novel copper-based biocide. J. Hosp. Infect. 2010, 74, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Hedin, G.; Rynbäck, J.; Loré, B. Reduction of bacterial surface contamination in the hospital environment by application of a new product with persistent effect. J. Hosp. Infect. 2010, 75, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Dunklin, E.W.; Lester, W. Residual surface disinfection ii. the effect of orthophenylphenol treatment of the floor on bacterial contamination in a recovery room. J. Infect. Dis. 1959, 104, 41–55. [Google Scholar] [CrossRef]
- Daschner, F.; Rabbenstein, G.; Langmaack, H. Surface decontamination in the control of hospital infections: Comparison of different methods. Dtsch. Med. Wochenschr. 1980, 105, 325–329. [Google Scholar] [CrossRef]
- Exner, M.; Vogel, F.; Hamann, R. Surface disinfection in a medical intensive care unit. Intensivmedizin 1982, 19, 26–29. [Google Scholar]
- Marais, F.; Mehtar, S.; Chalkley, L. Antimicrobial efficacy of copper touch surfaces in reducing environmental bioburden in a South African community healthcare facility. J. Hosp. Infect. 2010, 74, 80–82. [Google Scholar] [CrossRef]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef]
- Inkinen, J.; Mäkinen, R.; Keinänen-Toivola, M.M.; Nordström, K.; Ahonen, M. Copper as an antibacterial material in different facilities. Lett. Appl. Microbiol. 2017, 64, 19–26. [Google Scholar] [CrossRef]
- Schmidt, M.G.; Attaway, H.H.; Ms, I.I.I.; Bs, S.E.F.; Lisa, L.; Schmidt, M.G.; Iii, H.H.A.; Fairey, S.E.; Steed, L.L.; Michels, H.T.; et al. Copper Continuously Limits the Concentration of Bacteria Resident on Bed Rails within the Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2013, 34, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.G.; Von Dessauer, B.; Benavente, C.; Benadof, D.; Cifuentes, P.; Elgueta, A.; Duran, C.; Navarrete, M.S. Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a pediatric intensive care unit. Am. J. Infect. Control. 2016, 44, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.D.; Sepkowitz, K.A.; John, J.F.; Cantey, J.R.; Attaway, H.H.; Freeman, K.D.; Sharpe, P.A.; Michels, H.T.; Schmidt, M.G. Copper Surfaces Reduce the Rate of Healthcare-Acquired Infections in the Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2013, 34, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Hirsch, B.; Attaway, H.; Nadan, R.; Fairey, S.; Hardy, J.; Miller, G.; Armellino, D.; Moran, W.; Sharpe, P.; et al. Evaluation of the Antimicrobial Properties of Copper Surfaces in an Outpatient Infectious Disease Practice. Infect. Control Hosp. Epidemiol. 2012, 33, 200–201. [Google Scholar] [CrossRef]
- Von Dessauer, B.; Navarrete, M.S.; Benadof, D.; Benavente, C.; Schmidt, M.G. Potential effectiveness of copper surfaces in reducing health care–associated infection rates in a pediatric intensive and intermediate care unit: A nonrandomized controlled trial. Am. J. Infect. Control 2016, 44, e133–e139. [Google Scholar] [CrossRef]
- Sifri, C.D.; Burke, G.H.; Enfield, K.B. Reduced health care-associated infections in an acute care community hospital using a combination of self-disinfecting copper-impregnated composite hard surfaces and linens. Am. J. Infect. Control 2016, 44, 1565–1571. [Google Scholar] [CrossRef]
- Souli, M.; Antoniadou, A.; Katsarolis, I.; Mavrou, I.; Paramythiotou, E.; Papadomichelakis, E.; Drogari-Apiranthitou, M.; Panagea, T.; Giamarellou, H.; Petrikkos, G.; et al. Reduction of environmental contamination with multidrug-resistant bacteria by copper-alloy coating of surfaces in a highly endemic setting. Infect. Control Hosp. Epidemiol. 2017, 38, 765–771. [Google Scholar] [CrossRef]
- Schmidt, M.G.; Attaway, H.H.; Fairey, S.E.; Howard, J.; Mohr, D.; Craig, S. Self-disinfecting copper beds sustain terminal cleaning and disinfection effects throughout patient care. Appl. Environ. Microbiol. 2020, 86, e01886-19. [Google Scholar] [CrossRef]
- Schmidt, M.G.; Attaway, H.H.; Sharpe, P.A.; John, J.; Sepkowitz, K.A.; Morgan, A.; Fairey, S.E.; Singh, S.; Steed, L.L.; Cantey, J.R.; et al. Sustained reduction of microbial burden on common hospital surfaces through introduction of copper. J. Clin. Microbiol. 2012, 50, 2217–2223. [Google Scholar] [CrossRef]
- Esolen, L.M.; Thakur, L.; Layon, A.J.; Fuller, T.A.; Harrington, D.J.; Jha, K.; Kariyawasam, S. The efficacy of self-disinfecting bedrail covers in an intensive care unit. Am. J. Infect. Control 2018, 46, 417–419. [Google Scholar] [CrossRef]
- Prindis, V.; Michalek, J.; Kubatova, I. Application of photocatalytic nanolayers SmartCoat in health care facility. EMBEC NBC 2018, 65, 1089–1090. [Google Scholar]
- Edmiston, C.E.; Spencer, M.; Lewis, B.D.; Rossi, P.J.; Brown, K.R.; Malinowski, M.; Seabrook, G.R.; Leaper, D. Assessment of a novel antimicrobial surface disinfectant on inert surfaces in the intensive care unit environment using ATP-bioluminesence assay. Am. J. Infect. Control 2020, 48, 143–146. [Google Scholar] [CrossRef]
- Lee, W.S.; Hsieh, T.C.; Shiau, J.C.; Ou, T.Y.; Chen, F.L.; Liu, Y.H.; Yen, M.Y.; Hsueh, P.R. Bio-Kil, a nano-based disinfectant, reduces environmental bacterial burden and multidrug-resistant organisms in intensive care units. J. Microbiol. Immunol. Infect. 2017, 50, 737–746. [Google Scholar] [CrossRef]
- Özpolat, B.; Çavuşoǧlu, T.; Yilmaz, S.; Büyükkoçak, Ü.; Günaydin, S. Clinical and laboratory evaluation of anti-microbial efficacy of photocataylsts. J. Clin. Anal. Med. 2011, 2, 32–35. [Google Scholar] [CrossRef]
- Thom, K.A.; Standiford, H.C.; Johnson, J.K.; Hanna, N.; Furuno, J.P. Effectiveness of an Antimicrobial Polymer to Decrease Contamination of Environmental Surfaces in the Clinical Setting. Infect. Control Hosp. Epidemiol. 2014, 35, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Ortí-Lucas, R.M.; Muñoz-Miguel, J. Effectiveness of surface coatings containing silver ions in bacterial decontamination in a recovery unit. Antimicrob. Resist. Infect. Control. 2017, 6, 61. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, S.G.; Kim, K.S.; Heo, Y.J.; Oh, J.E.; Jeong, S.J. Environmental disinfection with photocatalyst as an adjunctive measure to control transmission of methicillin-resistant Staphylococcus aureus: A prospective cohort study in a high-incidence setting. BMC Infect. Dis. 2018, 18, 610. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.; Whatley, V.; Spooner, E.; Nevill, A.M.; Cooper, M.; Ramsden, J.J.; Dancer, S.J. How Does a Photocatalytic Antimicrobial Coating Affect Environmental Bioburden in Hospitals? Infect. Control Hosp. Epidemiol. 2018, 39, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.H.; Carlino, S.; Gerba, C.P. Long-term efficacy of a self-disinfecting coating in an intensive care unit. Am. J. Infect. Control. 2014, 42, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- De Jong, B.; Meeder, A.M.; Koekkoek, K.W.A.C.; Schouten, M.A.; Westers, P.; van Zanten, A.R.H. Pre–post evaluation of effects of a titanium dioxide coating on environmental contamination of an intensive care unit: The TITANIC study. J. Hosp. Infect. 2018, 99, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Afinogenova, A.G.; Kraeva, L.A.; Afinogenov, G.E.; Veretennikov, V.V. Probiotic-based sanitation as alternatives to chemical disinfectants. Russ. J. Infect. Immun. 2017, 7, 419–424. [Google Scholar] [CrossRef]
- Taylor, L.; Phillips, P.; Hastings, R. Reduction of bacterial contamination in a healthcare environment by silver antimicrobial technology. J. Infect. Prev. 2009, 10, 6–12. [Google Scholar] [CrossRef]
- Karunanayake, L.I.; Waniganayake, Y.C.; Nirmala Gunawardena, K.D.; Danuka Padmaraja, S.A.; Peter, D.; Jayasekera, R.; Karunanayake, P. Use of silicon nanoparticle surface coating in infection control: Experience in a tropical healthcare setting. Infect. Dis. Heal. 2019, 24, 201–207. [Google Scholar] [CrossRef]
- Shapey, S.; Machin, K.; Levi, K.; Boswell, T.C. Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J. Hosp. Infect. 2008, 70, 136–141. [Google Scholar] [CrossRef]
- Havill, N.L.; Moore, B.A.; Boyce, J.M. Comparison of the Microbiological Efficacy of Hydrogen Peroxide Vapor and Ultraviolet Light Processes for Room Decontamination. Infect. Control Hosp. Epidemiol. 2012, 33, 507–512. [Google Scholar] [CrossRef]
- Andersen, B.M.; Rasch, M.; Hochlin, K.; Jensen, F.H.; Wismar, P.; Fredriksen, J.E. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J. Hosp. Infect. 2006, 62, 149–155. [Google Scholar] [CrossRef]
- Humayun, T.; Qureshi, A.; Al Roweily, S.F.; Carig, J.; Humayun, F. Efficacy of Hydrogen Peroxide Fumigation in Improving Disinfection of Hospital Rooms and Reducing the Number of Microorganisms. J. Ayub Med. Coll. Abbottabad 2019, 31, S646–S650. [Google Scholar]
- Ali, S.; Muzslay, M.; Bruce, M.; Jeanes, A.; Moore, G.; Wilson, A.P.R. Efficacy of two hydrogen peroxide vapour aerial decontamination systems for enhanced disinfection of meticillin-resistant Staphylococcus aureus, Klebsiella pneumoniae and Clostridium difficile in single isolation rooms. J. Hosp. Infect. 2016, 93, 70–77. [Google Scholar] [CrossRef]
- Chan, H.T.; White, P.; Sheorey, H.; Cocks, J.; Waters, M.J. Evaluation of the biological efficacy of hydrogen peroxide vapour decontamination in wards of an Australian hospital. J. Hosp. Infect. 2011, 79, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, T.; Walder, M.; Uzcátegui, N.; Odenholt, I.; Lanbeck, P.; Medstrand, P.; Widell, A. Hydrogen peroxide vapor decontamination in a patient room using feline Calicivirus and Murine Norovirus as surrogate markers for human norovirus. Infect. Control Hosp. Epidemiol. 2016, 37, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Yezli, S.; Schouten, M.A.; Van Zanten, A.R.H.; Houmes-Zielman, G.; Nohlmans-Paulssen, M.K.E. Hydrogen peroxide vapor decontamination of an intensive care unit to remove environmental reservoirs of multidrug-resistant gram-negative rods during an outbreak. Am. J. Infect. Control. 2010, 38, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Biswal, M.; Kumar, A.; Edwin, A.; Sunita, T.; Emmanuel, R.; Gupta, A.K.; Sharma, M. Hydrogen peroxide vapour for decontaminating air-conditioning ducts and rooms of an emergency complex in northern India: Time to move on. J. Hosp. Infect. 2011, 78, 200–203. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Otter, J.A.; McDonald, L.C.; Adams, N.M.; Cooper, T.; Thompson, A.; Wiggs, L.; Killgore, G.; Tauman, A.; et al. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect. Control Hosp. Epidemiol. 2008, 29, 723–729. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, R.; Singh, K.; Attri, J. Infection Control in Isolation Units/Hdus/Icus—A Comparative Study Using Three Different Disinfectants with Fogger for Environmental Decontamination. J. Evol. Med. Dent. Sci. 2017, 6, 3091–3096. [Google Scholar] [CrossRef]
- Oon, A.; Reading, E.; Ferguson, J.K.; Dancer, S.J.; Mitchell, B.G. Measuring environmental contamination in critical care using dilute hydrogen peroxide (DHP) technology: An observational cross-over study. Infect. Dis. Heal. 2020, 25, 107–112. [Google Scholar] [CrossRef]
- Popov, D.A.; Anuchina, N.M. Microbiological Efficacy of Hospital Environment Decontamination by Hydrogen Peroxide Aerosol. Biomed. Eng. 2016, 50, 92–95. [Google Scholar] [CrossRef]
- Hardy, K.J.; Gossain, S.; Henderson, N.; Drugan, C.; Oppenheim, B.A.; Gao, F.; Hawkey, P.M. Rapid recontamination with MRSA of the environment of an intensive care unit after decontamination with hydrogen peroxide vapour. J. Hosp. Infect. 2007, 66, 360–368. [Google Scholar] [CrossRef]
- Barbut, F.; Yezli, S.; Mimoun, M.; Pham, J.; Chaouat, M.; Otter, J.A. Reducing the spread of Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus on a burns unit through the intervention of an infection control bundle. Burns 2013, 39, 395–403. [Google Scholar] [CrossRef]
- Mccord, J.; Prewitt, M.; Dyakova, E.; Mookerjee, S.; Otter, J.A. Reduction in Clostridium difficile infection associated with the introduction of hydrogen peroxide vapour automated room disinfection. J. Hosp. Infect. 2016, 94, 185–187. [Google Scholar] [CrossRef]
- French, G.L.; Otter, J.A.; Shannon, K.P.; Adams, N.M.T.; Watling, D.; Parks, M.J. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2004, 57, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Pearse, R. Use of hydrogen peroxide vapour for environmental control during a Serratia outbreak in a neonatal intensive care unit. J. Hosp. Infect. 2005, 61, 364–366. [Google Scholar] [CrossRef]
- Ray, A.; Perez, F.; Beltramini, A.M.; Jakubowycz, M.; Dimick, P.; Jacobs, M.R.; Roman, K.; Bonomo, R.A.; Salata, R.A. Use of Vaporized Hydrogen Peroxide Decontamination during an Outbreak of Multidrug-Resistant Acinetobacter baumannii Infection at a Long-Term Acute Care Hospital. Infect. Control Hosp. Epidemiol. 2010, 31, 1236–1241. [Google Scholar] [CrossRef]
- Aimiya, K.; Sato, T.; Hishida, H.; Yamaguchi, K. Primary Decontamination Treatments and the Control of Microbial Contamination in a New Ward. J. Antibact. Antifung. Agents 1989, 17, 53–56. [Google Scholar]
- Gelmini, F.; Belotti, L.; Vecchi, S.; Testa, C.; Beretta, G. Air dispersed essential oils combined with standard sanitization procedures for environmental microbiota control in nosocomial hospitalization rooms. Complement. Ther. Med. 2016, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.J.; Gibbs, S.G.; Iwen, P.C.; Smith, P.W.; Hewlett, A.L. Impact of chlorine dioxide gas sterilization on nosocomial organism viability in a hospital room. Int. J. Environ. Res. Public Health 2013, 10, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.D.; Tanner, B.D.; Maxwell, S.L.; Gerba, C.P. Reduction in the microbial load on high-touch surfaces in hospital rooms by treatment with a portable saturated steam vapor disinfection system. Am. J. Infect. Control 2011, 39, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, P.S.; Singh, R.N.; Shekhawat, R.; Joshi, K.R. Fumigation of neonatal nursery: How effective in reducing the environmental pathogens? Indian Pediatr. 1992, 29, 126–127. [Google Scholar] [PubMed]
- Munster, A.M.; Ostrander, W.E. Terminal disinfection of contaminated patient care areas: To fog or not to fog? Am. Surg. 1974, 40, 713–715. [Google Scholar] [PubMed]
- Lowe, J.J.; Gibbs, S.G.; Iwen, P.C.; Smith, P.W.; Hewlett, A.L. Decontamination of a hospital room using gaseous chlorine dioxide: Bacillus anthracis, Francisella tularensis, and Yersinia pestis. J. Occup. Environ. Hyg. 2013, 10, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Čamdžić, A.; Dedeić-Ljubović, A.; Madacki-Todorović, K. Using desinfection devices in intensive care units. Acta Med. Salin. 2019, 49, 191–194. [Google Scholar]
- Nakata, S.; Ikeda, T.; Nakatani, H.; Sakamoto, M.; Higashidutsumi, M.; Honda, T.; Kawayoshi, A.; Iwamura, Y. Evaluation of an automatic fogging disinfection unit. Environ. Health Prev. Med. 2001, 6, 160–164. [Google Scholar] [CrossRef]
- Nagai, I.; Kadota, M.; Matsuoka, K.; Jitsukawa, S. Evaluation of Chemical Aerosol Spray Disinfection in the Operating Room. Med. J. Osaka Univ. 1983, 34, 27–31. [Google Scholar] [PubMed]
- Dyas, A.; Boughton, B.J.; Das, B.C. Ozone killing action against bacterial and fungal species; microbiological testing of a domestic ozone generator. J. Clin. Pathol. 1983, 36, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Ory, J.; Cazaban, M.; Richaud-Morel, B.; Di Maio, M.; Dunyach-Remy, C.; Pantel, A.; Sotto, A.; Laurent, F.; Lavigne, J.P.; Butin, M. Successful implementation of infection control measure in a neonatal intensive care unit to combat the spread of pathogenic multidrug resistant Staphylococcus capitis. Antimicrob. Resist. Infect. Control 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Bernabé, K.J.; Langendorf, C.; Ford, N.; Ronat, J.B.; Murphy, R.A. Antimicrobial resistance in West Africa: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 50, 629–639. [Google Scholar] [CrossRef]
- World Health Organization. Improving Infection Prevention and Control at the Health Facility—Interim Practical Manual Supporting Implementation of the WHO Guidelines on Core Components of Infection Prevention and Control Programmes; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Zingg, W.; Holmes, A.; Dettenkofer, M.; Goetting, T.; Secci, F.; Clack, L.; Allegranzi, B.; Magiorakos, A.-P.; Pittet, D. Review Hospital organisation, management, and structure for prevention of health-care-associated infection: A systematic review and expert consensus. Lancet 2015, 15, 212–224. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, G.A.; Lee, S.H.; Park, Y.H. Effectiveness and core components of infection prevention and control programmes in long-term care facilities: A systematic review. J. Hosp. Infect. 2019, 102, 377–393. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).