Indoor Particulate Matter in Urban Households: Sources, Pathways, Characteristics, Health Effects, and Exposure Mitigation
Abstract
1. Introduction
2. Sources and Distribution of Indoor PM
2.1. Major Sources of Indoor PM
2.2. Distribution Characteristics of Indoor PM
2.3. Factors Influencing the Distribution of Indoor PM
2.4. Unique Characteristics and Spatial-Temporal Distribution of Indoor PM
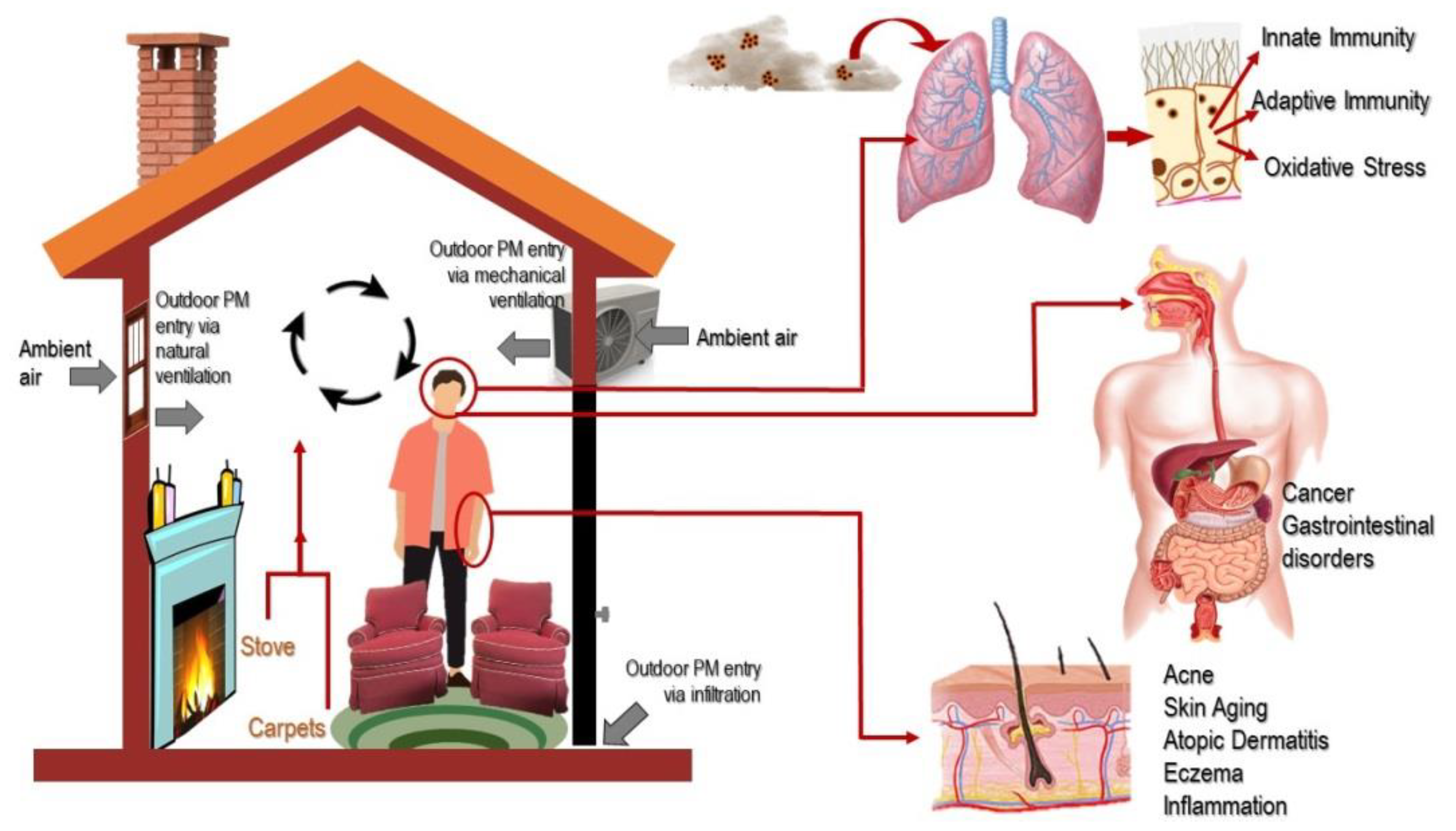
3. Pathways of Exposure to Indoor PM
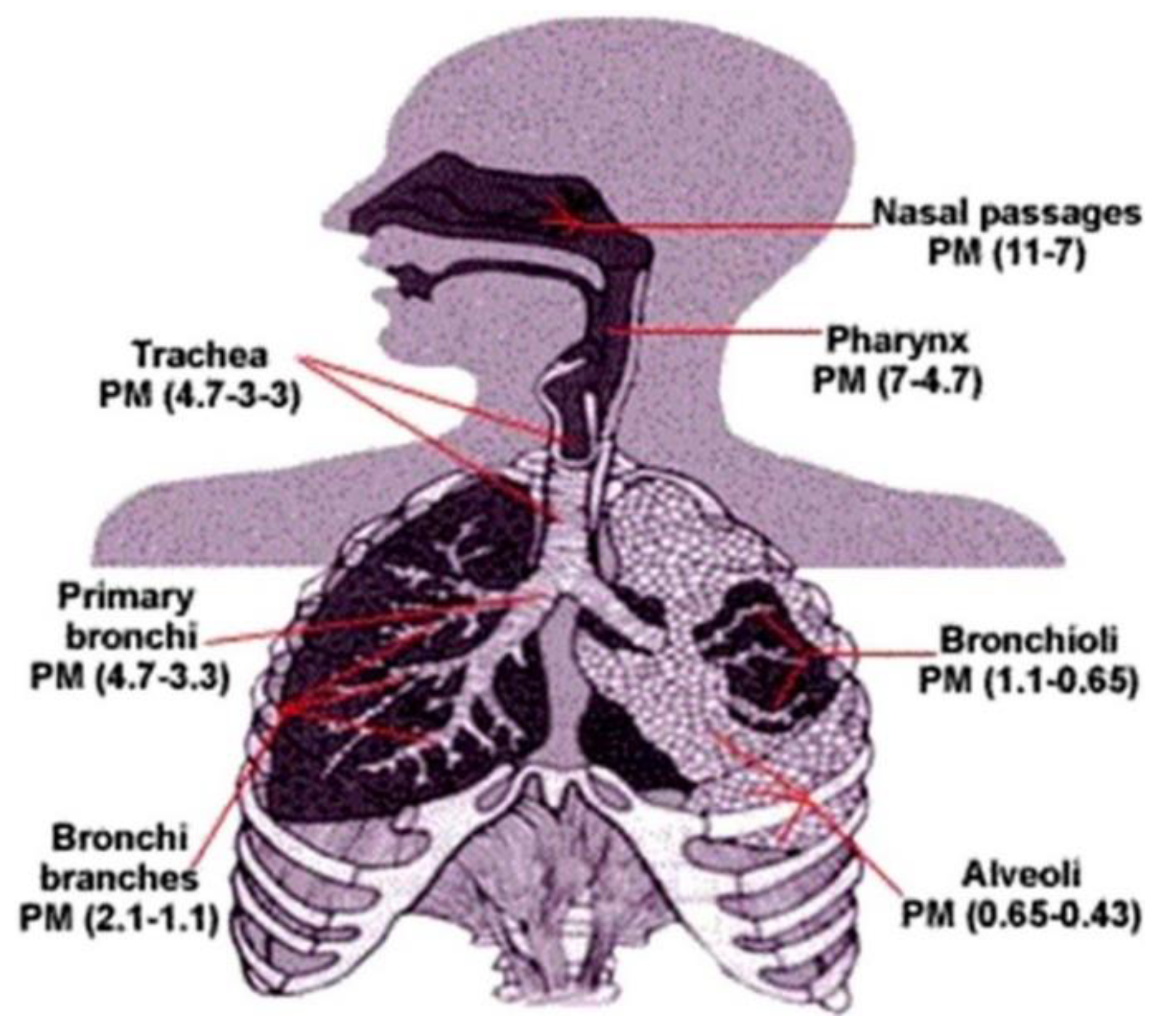
3.1. Respiratory Absorption
3.2. Cutaneous Absorption
3.3. Hand-to-Mouth Behavior
3.4. Digestive System Absorption
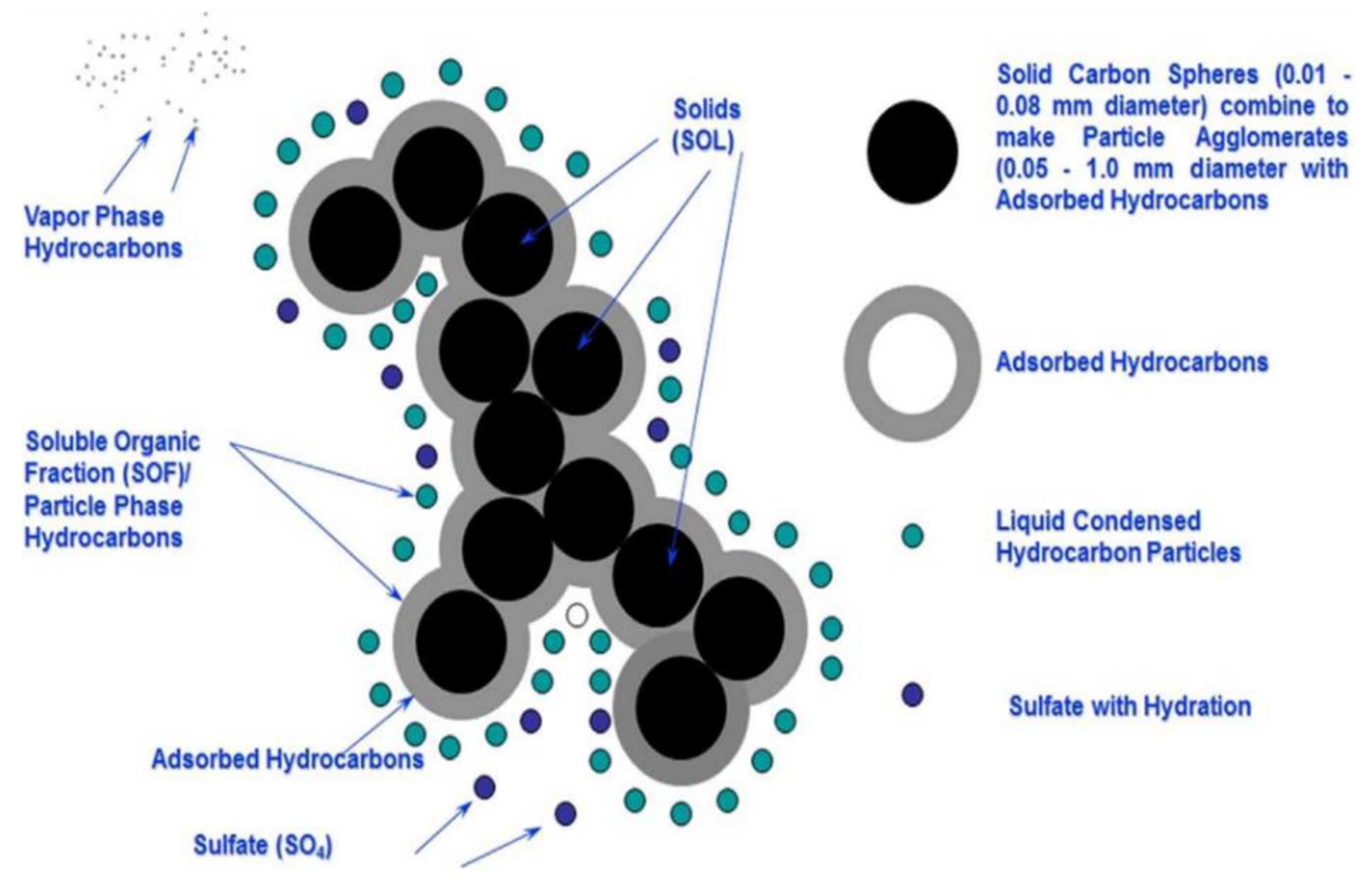
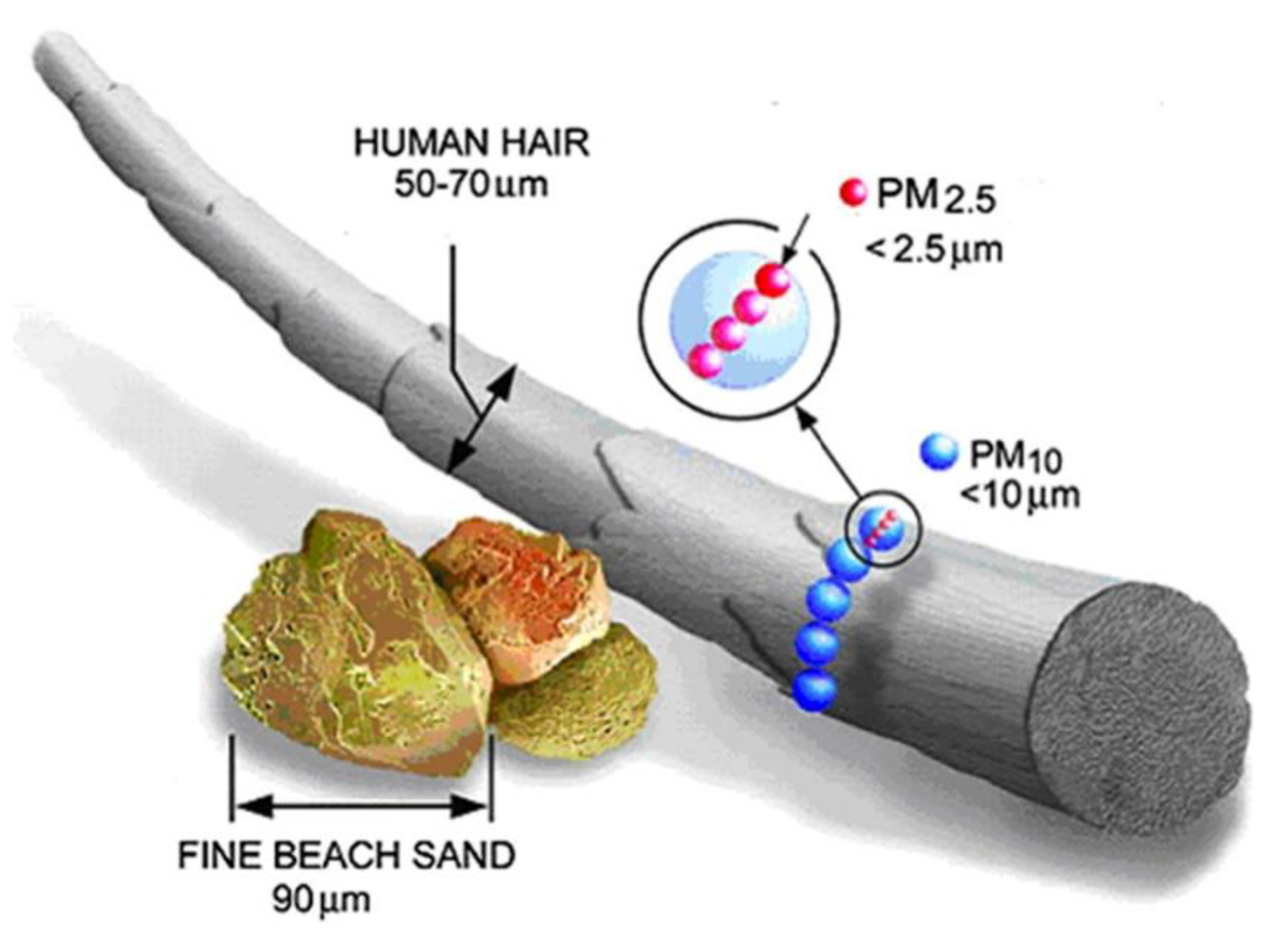
4. Characteristics of Indoor PM
5. Health Effects of Indoor PM
5.1. Overall Impact
5.2. Harm of Main Components of PM to the Human Body
5.3. Harm to Different Groups
6. Mitigation of Exposure to Indoor PM
6.1. Standards of Indoor PM
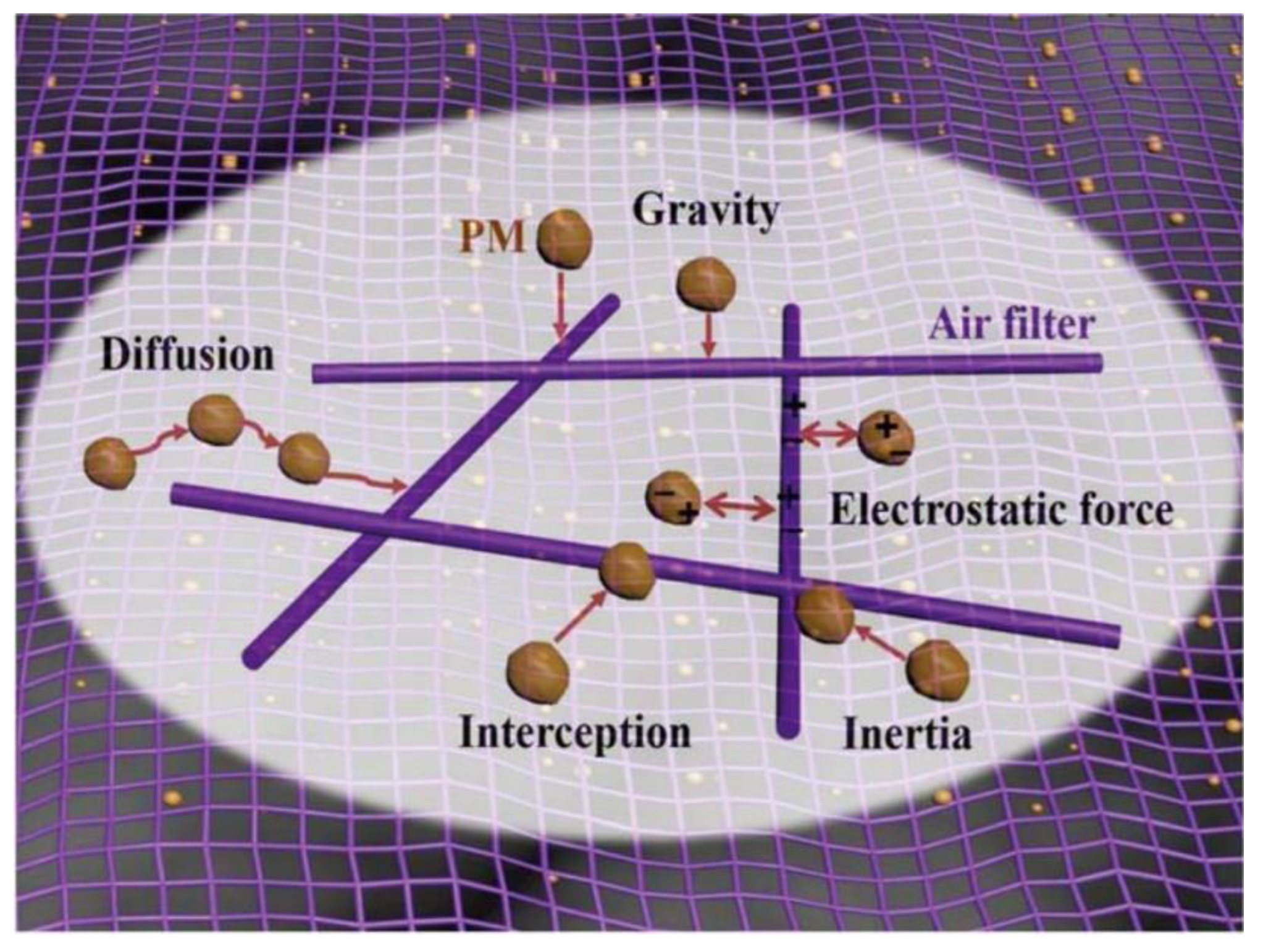
6.2. Effective Removal Technologies of Indoor PM
6.3. Strategies to Reduce Exposure to Indoor PM
6.3.1. Control Strategies for Ambient PM
- (1)
- Building ventilation
- (2)
- Climate and season
- (3)
- Traffic and industries
6.3.2. Control Strategies for Indoor PM
- (1)
- Smoking
- (2)
- Cooking
- (3)
- Indoor activities
- (4)
- Indoor layout
7. Conclusions
- Development and adoption of advanced technologies, such as the tapered element oscillating microbalance (TEOM), X-ray fluorescence (XRF), and inductively coupled plasma mass spectrometry (ICP-MS), to quantify and fingerprint sources of indoor PM.
- Characterization and monitoring of bioaccessibility of inorganic and organic contaminants in indoor PM.
- Studies on mucosal interactions of indoor PM and associated contaminants concerning their toxicity.
- Development and evaluation of advanced PM removal technologies involving electrostatic precipitation to mitigate the health impacts of indoor PM.
- As soon as possible, the government and industry should formulate detailed and uniform indoor PM control standards, based on many investigations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.; Greenbaum, D.S.; Shaikh, R.; van Erp, A.M.; Russell, A.G. Particulate matter components, sources, and health: Systematic approaches to testing effects. J. Air Waste Manag. Assoc. 2015, 65, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Nasir, Z.A.; Colbeck, I. Particulate pollution in different housing types in a UK suburban location. Sci. Total Environ. 2013, 445, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, S.; Senthilkumar, P. Particulate matter formation and its control methodologies for diesel engine: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238. [Google Scholar] [CrossRef]
- Bootdee, S.; Chantara, S.; Prapamontol, T. Determination of pm2.5 and polycyclic aromatic hydrocarbons from incense burning emission at shrine for health risk assessment. Atmos. Pollut. Res. 2016, 7, 680–689. [Google Scholar] [CrossRef]
- Coombs, K.C.; Chew, G.L.; Schaffer, C.; Ryan, P.H.; Brokamp, C.; Grinshpun, S.A.; Adamkiewicz, G.; Chillrud, S.; Hedman, C.; Colton, M.; et al. Indoor air quality in green-renovated vs. non-green low-income homes of children living in a temperate region of US (Ohio). Sci. Total Environ. 2016, 554, 178–185. [Google Scholar] [CrossRef]
- McNamara, M.; Thornburg, J.; Semmens, E.; Ward, T.; Noonan, C. Coarse particulate matter and airborne endotoxin within wood stove homes. Indoor Air 2013, 23, 498–505. [Google Scholar] [CrossRef]
- Rivas, I.; Viana, M.; Moreno, T.; Pandolfi, M.; Amato, F.; Reche, C.; Bouso, L.; Alvarez-Pedrerol, M.; Alastuey, A.; Sunyer, J.; et al. Child exposure to indoor and outdoor air pollutants in schools in Barcelona, Spain. Environ. Int. 2014, 69, 200–212. [Google Scholar] [CrossRef]
- Russo, E.T.; Hulse, T.E.; Adamkiewicz, G.; Levy, D.E.; Bethune, L.; Kane, J.; Reid, M.; Shah, S.N. Comparison of indoor air quality in smoke-permitted and smoke-free multiunit housing: Findings from the Boston Housing Authority. Nicotine Tob. Res. 2015, 17, 316–322. [Google Scholar] [CrossRef]
- Jones, R.R.; Hogrefe, C.; Fitzgerald, E.F.; Hwang, S.A.; Ozkaynak, H.; Garcia, V.C.; Lin, S. Respiratory hospitalizations in association with fine PM and its components in New York State. J. Air Waste Manag. Assoc. 2015, 65, 559–569. [Google Scholar] [CrossRef]
- Ostro, B.; Roth, L.; Malig, B.; Marty, M. The Effects of Fine Particle Components on Respiratory Hospital Admissions in Children. Environ. Health Perspect. 2009, 117, 475–480. [Google Scholar] [CrossRef]
- Wichmann, F.A.; Muller, A.; Busi, L.E.; Cianni, N.; Massolo, L.; Schlink, U.; Porta, A.; Sly, P.D. Increased asthma and respiratory symptoms in children exposed to petrochemical pollution. J. Allergy Clin. Immun. 2009, 123, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Dunea, D.; Iordache, S.; Pohoata, A. A Review of Airborne Particulate Matter Effects on Young Children’s Respiratory Symptoms and Diseases. Atmosphere 2018, 9, 150. [Google Scholar] [CrossRef]
- Reiss, R.; Anderson, E.L.; Cross, C.E.; Hidy, G.; Hoel, D.; McClellan, R.; Moolgavkar, S. Evidence of health impacts of sulfate- and nitrate-containing particles in ambient air. Inhal. Toxicol. 2007, 19, 419–449. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Moebus, S.; Mohlenkamp, S.; Stang, A.; Lehmann, N.; Dragano, N.; Schmermund, A.; Memmesheimer, M.; Mann, K.; Erbel, R.; et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation 2007, 116, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution-Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). 9 out of 10 People Worldwide Breathe Polluted Air, but More Countries are Taking Action. Available online: http://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 6 September 2018).
- Jeon, Y.M.; Lee, M.Y. Airborne nanoparticles (PM0.1) induce autophagic cell death of human neuronal cells. J. Appl. Toxicol. 2016, 36, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 2001, 74, 1–8. [Google Scholar] [CrossRef]
- Lai, H.K.; Kendall, M.; Ferrier, H.; Lindup, I.; Alm, S.; Hanninen, O.; Jantunen, M.; Mathys, P.; Colvile, R.; Ashmore, M.R.; et al. Personal exposures and microenvironment concentrations of PM2.5, VOC, NO2 and CO in Oxford, UK. Atmos. Environ. 2004, 38, 6399–6410. [Google Scholar] [CrossRef]
- Zaidi, S.M.A.; Moin, O.; Khan, J.A. Second-hand smoke in indoor hospitality venues in Pakistan. Int. J. Tuberc. Lung Dis. 2011, 15, 972–977. [Google Scholar] [CrossRef]
- Smith, K.R. National burden of disease in India from indoor air pollution. Proc. Natl. Acad. Sci. USA 2000, 97, 13286–13293. [Google Scholar] [CrossRef]
- Simoni, M.; Carrozzi, L.; Baldacci, S.; Scognamiglio, A.; di Pede, F.; Sapigni, T.; Viegi, G. The Po River Delta (north Italy) indoor epidemiological study: Effects of pollutant exposure on acute respiratory symptoms and respiratory function in adults. Arch. Environ. Health 2002, 57, 130–136. [Google Scholar] [CrossRef]
- Yamamoto, S.S.; Phalkey, R.; Malik, A.A. A systematic review of air pollution as a risk factor for cardiovascular disease in South Asia: Limited evidence from India and Pakistan. Int. J. Hyg. Environ. Health 2014, 217, 133–144. [Google Scholar] [CrossRef] [PubMed]
- You, S.M.; Wan, M.P. Experimental investigation and modelling of human-walking-induced particle resuspension. Indoor Built Environ. 2015, 24, 564–576. [Google Scholar] [CrossRef]
- WHO (World Health Organization). WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Breysse, P.N.; Diette, G.B.; Matsui, E.C.; Butz, A.M.; Hansel, N.N.; McCormack, M.C. Indoor air pollution and asthma in children. Proc. Am. Thorac. Soc. 2010, 7, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, H.; Maddalena, R.L.; Singer, B.C.; Hodgson, A.T.; McKone, T.E. Indoor pollutants emitted by office equipment: A review of reported data and information needs. Atmos. Environ. 2008, 42, 1371–1388. [Google Scholar] [CrossRef]
- Holmes, H.A.; Pardyjak, E.R.; Speckart, S.O.; Alexander, D. Comparison of indoor/outdoor carbon content and time resolved PM concentrations for gas and biomass cooking fuels in Nogales, Sonora, Mexico. Atmos. Environ. 2011, 45, 7600–7611. [Google Scholar] [CrossRef]
- Waring, M.S. Secondary organic aerosol in residences: Predicting its fraction of fine particle mass and determinants of formation strength. Indoor Air 2014, 24, 376–389. [Google Scholar] [CrossRef]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Pruss-Ustun, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Paschold, H.; Li, W.W.; Morales, H.; Walton, J. Laboratory study of the impact of evaporative coolers on indoor PM concentrations. Atmos. Environ. 2003, 37, 1075–1086. [Google Scholar] [CrossRef]
- McNamara, M.L.; Thornburg, J.; Semmens, E.O.; Ward, T.J.; Noonan, C.W. Reducing indoor air pollutants with air filtration units in wood stove homes. Sci. Total Environ. 2017, 592, 488–494. [Google Scholar] [CrossRef]
- Molchanov, O.; Krpec, K.; Horak, J. Electrostatic precipitation as a method to control the emissions of particulate matter from small-scale combustion units. J. Clean. Prod. 2020, 246, 119022. [Google Scholar] [CrossRef]
- Cai, J.; Yu, W.; Li, B.Z.; Yao, R.M.; Zhang, T.J.W.; Guo, M.; Wang, H.; Cheng, Z.; Xiong, J.; Meng, Q.Y.; et al. Particle removal efficiency of a household portable air cleaner in real-world residences: A single-blind cross-over field study. Energy Build. 2019, 203, 109464. [Google Scholar] [CrossRef]
- Isiugo, K.; Jandarov, R.; Cox, J.; Ryan, P.; Newman, N.; Grinshpun, S.A.; Indugula, R.; Vesper, S.; Reponen, T. Indoor particulate matter and lung function in children. Sci. Total Environ. 2019, 663, 408–417. [Google Scholar] [CrossRef]
- Ferro, A.R.; Kopperud, R.J.; Hildemann, L.M. Source strengths for indoor human activities that resuspend particulate matter. Environ. Sci. Technol. 2004, 38, 1759–1764. [Google Scholar] [CrossRef]
- Oliveria, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef]
- Weschler, C.J.; Nazaroff, W.W. Dermal Uptake of Organic Vapors Commonly Found in Indoor Air. Environ. Sci. Technol. 2014, 48, 1230–1237. [Google Scholar] [CrossRef]
- Hassanvand, M.S.; Naddafi, K.; Faridi, S.; Arhami, M.; Nabizadeh, R.; Sowlat, M.H.; Pourpak, Z.; Rastkari, N.; Momeniha, F.; Kashani, H.; et al. Indoor/outdoor relationships of PM10, PM2.5, and PM1 mass concentrations and their water-soluble ions in a retirement home and a school dormitory. Atmos. Environ. 2014, 82, 375–382. [Google Scholar] [CrossRef]
- Morawska, L.; Afshari, A.; Bae, G.N.; Buonanno, G.; Chao, C.Y.H.; Hanninen, O.; Hofmann, W.; Isaxon, C.; Jayaratne, E.R.; Pasanen, P.; et al. Indoor aerosols: From personal exposure to risk assessment. Indoor Air 2013, 23, 462–487. [Google Scholar] [CrossRef]
- Salje, H.; Gurley, E.S.; Homaira, N.; Ram, P.K.; Haque, R.; Petri, W.; Moss, W.J.; Luby, S.P.; Breysse, P.; Azziz-Baumgartner, E. Impact of neighborhood biomass cooking patterns on episodic high indoor particulate matter concentrations in clean fuel homes in Dhaka, Bangladesh. Indoor Air 2014, 24, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, C.; Chandramohan, D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop. Med. Int. Health 2013, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Semmens, E.O.; Noonan, C.W.; Allen, R.W.; Weiler, E.C.; Ward, T.J. Indoor particulate matter in rural, wood stove heated homes. Environ. Res. 2015, 138, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Rodelsperger, K.; Bruckel, B.; Kleineberg, J.; Woitowitz, H.J. Pleural mesothelioma associated with indoor pollution of asbestos. J. Cancer Res. Clin. 2001, 127, 123–127. [Google Scholar] [CrossRef]
- Perez, A.L.; Nelson, M.L.; Cheng, T.J.; Comerford, C.E.; Scott, P.K. A meta-analysis of airborne asbestos fiber concentrations from work with or around asbestos-containing floor tile. Int. J. Occup. Environ. Health 2018, 24, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Branis, M.; Rezacova, P.; Domasova, M. The Effect of Outdoor Air and Indoor Human Activity on Mass Concentrations of PM10, PM2.5 and PM1 in a Classroom. Environ. Res. 2005, 99, 143–149. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.-C. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef]
- Yu, K.-P.; Yang, K.R.; Chen, Y.C.; Gong, J.Y.; Chen, Y.P.; Shih, H.-C.; Lung, S.-C.C. Indoor air pollution from gas cooking infive Taiwanese families. Build. Environ. 2015, 93, 258–266. [Google Scholar] [CrossRef]
- Huboyo, H.S.; Tohno, S.; Cao, R.Q. Indoor PM2.5 Characteristics and CO Concentration Related to Water-Based and Oil-Based Cooking Emissions Using a Gas Stove. Aerosol Air Qual. Res. 2011, 11, 401–411. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Gangupomu, R.H.; Ramirez, D.; Zhu, Y.F. Measurement of Ultrafine Particles and Other Air Pollutants Emitted by Cooking Activities. Int. J. Environ. Res. Public Health 2010, 7, 1744–1759. [Google Scholar] [CrossRef]
- Liao, C.M.; Shen, S.C.; Chen, J.W.; Liang, H.M. Contribution of Chinese-style cooking and incense burning to personal exposure and residential PM concentrations in Taiwan region. Sci. Total Environ. 2006, 358, 72–84. [Google Scholar] [CrossRef]
- Gurley, E.S.; Homaira, N.; Salje, H.; Ram, P.K.; Haque, R.; Petri, W.; Bresee, J.; Moss, W.J.; Breysse, P.; Luby, S.P. Indoor exposure to particulate matter and the incidence of acute lower respiratory infections among children: A birth cohort study in urban Bangladesh. Indoor Air 2013, 23, 379–386. [Google Scholar] [CrossRef]
- Li, T.; Cao, S.; Fan, D.; Zhang, Y.; Wang, B.; Zhao, X.; Leaderer, B.P.; Shen, G.; Zhang, Y.; Duan, X. Household concentrations and personal exposure of PM2.5 among urban residents using different cooking fuels. Sci. Total Environ. 2016, 548, 6–12. [Google Scholar] [CrossRef]
- Njenga, M.; Iiyama, M.; Jamnadass, R.; Helander, H.; Larsson, L.; de Leeuw, J.; Neufeldt, H.; de Nowina, K.R.; Sundberg, C. Gasifier as a cleaner cooking system in rural Kenya. J. Clean. Prod. 2016, 121, 208–217. [Google Scholar] [CrossRef]
- Holm, S.M.; Balmes, J.; Gillette, D.; Hartin, K.; Seto, E.; Lindeman, D.; Polanco, D.; Fong, E. Cooking behaviors are related to household particulate matter exposure in children with asthma in the urban East Bay Area of Northern California. PLoS ONE 2018, 13, e0197199. [Google Scholar] [CrossRef] [PubMed]
- Drago, G.; Perrino, C.; Canepari, S.; Ruggieri, S.; L’Abbate, L.; Longo, V.; Colombo, P.; Feasca, D.; Balzan, M.; Cuttitta, G.; et al. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM2.5. Environ. Res. 2018, 165, 71–80. [Google Scholar] [CrossRef]
- Braun, M.; Koger, F.; Klingelhofer, D.; Muller, R.; Gronerberg, D.A. Particulate matter emissions of four different cigarette types of one popular brand: Influence of tobacco strength and additives. Int. J. Environ. Res. Public Health 2019, 16, 263. [Google Scholar] [CrossRef]
- Lee, S.; Wang, B. Characteristics of emissions of air pollutants from burning of incense in a large environmental chamber. Atmos. Environ. 2004, 38, 941–951. [Google Scholar] [CrossRef]
- Lin, T.C.; Krishnaswamy, G.H.; Chi, D.S. Incense smoke: Clinical, structural and molecular effects on airway disease. Clin. Mol. Allergy 2008, 6, 3. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, N.; Kumar, D.; Mavi, A.K.; Singh, K.; Kumar, M. Monitoring of indoor particulate matter during burning of mosquito coil, incense sticks and dhoop. Indian J. Allergy Asthma Immunol. 2014, 28, 68–73. [Google Scholar] [CrossRef]
- Soule, E.; Maloney, S.F.; Spindle, T.; Rudy, A.; Hiler, M.M.; Cobb, C.O. Electronic cigarette use and indoor air quality in a natural setting. Tob. Control. 2017, 26, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Schober, W.; Fembacher, L.; Frenzen, A.; Fromme, H. Passive exposure to pollutants from conventional cigarettes and new electronic smoking devices (IQOS, e-cigarette) in passenger cars. Int. J. Hyg. Environ. Health 2019, 222, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Cassee, F.R.; Héroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Ayoko, G.A.; Bae, G.N.; Buonanno, G.; Chao, C.Y.H.; Clifford, S.; Fu, S.C.; Hanninen, O.; He, C.; Isaxon, C.; et al. Airborne particles in indoor environment of homes, schools, offices and aged care facilities: The main routes of exposure. Environ. Int. 2017, 108, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Thornton, C.; Mark, D.; Harrison, R. Indoor/outdoor relationships of particulate matter in domestic homes with roadside, urban and rural locations. Atmos. Environ. 2000, 34, 2603–2612. [Google Scholar] [CrossRef]
- Kuo, H.W.; Shen, H.Y. Indoor and outdoor PM2.5 and PM10 concentrations in the air during a dust storm. Build. Environ. 2010, 45, 610–614. [Google Scholar] [CrossRef]
- Goudie, A.S. Dust Storms and Human Health. In Extreme Weather Events and Human Health; Akhtar, R., Ed.; Springer: Cham, Switzerland, 2020; pp. 13–24. [Google Scholar]
- Baklanov, A.; Molina, L.T.; Gauss, M. Megacities, air quality and climate. Atmos. Environ. 2016, 126, 235–249. [Google Scholar] [CrossRef]
- Chithra, V.S.; Shiva Nagendra, S.M. Particulate Matter Mass and Number Concentrations Inside a Naturally Ventilated School Building Located Adjacent to an Urban Roadway. J. Inst. Eng. (India) Ser. A 2014, 95, 143–149. [Google Scholar] [CrossRef]
- Putaud, J.P.; Raes, F.; Van Dingenen, R.; Bruggemann, E.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Gehrig, R.; Huglin, C.; Laj, P.; et al. A European aerosol phenomenology-2 chemical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos. Environ. 2004, 38, 2579–2595. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, J.; Wang, Q.Y.; Qin, Q.D.; Ye, J.H.; Han, Y.M.; Li, L.; Zhen, W.; Zhi, Q.; Zhang, Y.; et al. Increased Secondary Aerosol Contribution and Possible Processing on Polluted Qinter Days in China. Environ. Int. 2019, 127, 78–84. [Google Scholar] [CrossRef]
- Morakinyo, O.M.; Mokgobu, M.I.; Mukhola, M.S.; Godobedzha, T. Biological Composition of Respirable Particulate Matter in an Industrial Vicinity in South Africa. Int. J. Environ. Res. Public Health 2019, 16, 629. [Google Scholar] [CrossRef]
- Vu, T.V.; Ondracek, J.; Zdímal, V.; Schwarz, J.; Delgado-Saborit, J.M.; Harrison, R.M. Physical properties and lung deposition of particles emitted from five major indoor sources. Air Qual. Atmos. Health 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Nazaro, W.W. Indoor particle dynamics. Indoor Air 2004, 14, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Parker, S.; Edwards, K.; Finch, J.; Jeanjean, A.; Leigh, R.; Gonem, S. Effects of indoor particulate matter exposure on daily asthma control. Ann. Allergy Asthma Immunol. 2019, 123, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Abbing-Karahagopian, V.; van der Gugten, A.C.; van der Ent, C.K.; Uiterwaal, C.; de Jongh, M.; Oldenwening, M.; Brunekreef, B.; Gehring, U. Effect of endotoxin and allergens on neonatal lung function and infancy respiratory symptoms and eczema. Pediatr. Allergy Immunol. 2012, 23, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Mercorio, R.; Bonzini, M.; Angelici, L.; Iodice, S.; Delbue, S.; Mariani, J.; Apostoli, P.; Pesatori, A.C.; Bollati, V. Effects of metal-rich particulate matter exposure on exogenous and endogenous viral sequence methylation in healthy steel-workers. Environ. Res. 2017, 159, 452–457. [Google Scholar] [CrossRef]
- Hulin, M.; Simoni, M.; Viegi, G.; Annesi-Maesano, I. Respiratory health and indoor air pollutants based on quantitative exposure assessments. Eur. Respir. J. 2012, 40, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; Weel, C.V.; et al. Allergic Rhinitis and its impact on Asthma (ARIA) 2008. Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. The diversity and distribution of fungi on residential surfaces. PLoS ONE 2013, 8, e78866. [Google Scholar] [CrossRef]
- Barberán, A.; Dunn, R.R.; Reich, B.J.; Pacifici, K.; Laber, E.B.; Menninger, H.L.; Morton, J.M.; Henley, J.B.; Leff, J.W.; Miller, S.L.; et al. The ecology of microscopic life in household dust. Proc. R. Soc. B Biol. Sci. 2015, 282, 1139. [Google Scholar] [CrossRef] [PubMed]
- Weikl, F.; Tischer, C.; Probst, A.J.; Heinrich, J.; Markevych, I.; Jochner, S.; Pritsch, K. Fungal and Bacterial Communities in Indoor Dust Follow Different Environmental Determinants. PLoS ONE 2016, 11, e0154131. [Google Scholar] [CrossRef]
- Megaritis, A.; Fountoukis, C.; Charalampidis, P.; Pilinis, C.; Pandis, S.N. Response of fine particulate matter concentrations to changes of emissions and temperature in europe. Atmos. Chem. Phys. 2013, 13, 3423–3443. [Google Scholar] [CrossRef]
- Kapwata, T.; Language, B.; Piketh, S.; Wright, C.Y. Variation of Indoor Particulate Matter Concentrations and Association with Indoor/Outdoor Temperature: A Case Study in Rural Limpopo, South Africa. Atmosphere 2018, 9, 124. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Mou, J.; Du, W.; Yu, Y.; Wang, L. The influence of relative humidity and ground material on indoor walking-induced particle resuspension. J. Environ. Sci. Heal. A 2019, 54, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Morawska, L.; He, C.; Gilbert, D. Impact of ventilation scenario on air exchange rates and on indoor particle number concentrations in an air-conditioned classroom. Atmos. Environ. 2008, 42, 757–768. [Google Scholar] [CrossRef]
- Han, Y.; Qi, M.; Chen, Y.; Shen, H.; Liu, J.; Huang, Y.; Chen, H.; Liu, W.X.; Wang, X.L.; Liu, J.F. Influences of ambient air PM2.5 concentration and meteorological condition on the indoor PM2.5 concentrations in a residential apartment in Beijing using a new approach. Environ. Pollut. 2015, 205, 307–314. [Google Scholar] [CrossRef]
- Zhu, C.S.; Cao, J.J.; Shen, Z.X.; Liu, S.X.; Zhang, T.; Zhao, Z.Z.; Xu, H.M.; Zhang, E.K. Indoor and outdoor chemical components of PM2.5 in the rural areas of Northwestern China. Aerosol Air Qual. Res. 2012, 12, 1157–1165. [Google Scholar] [CrossRef]
- Huang, T.; Yu, Y.; Wei, Y.; Wang, H.; Huang, W.; Chen, X. Spatial-seasonal characteristics and critical impact factors of PM2.5 concentration in the Beijing-Tianjin-Hebei urban agglomeration. PLoS ONE 2018, 13, e0201364. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide; WHO: Geneva, Switzerland, 2005; Available online: https://apps.who.int/iris/bitstream/handle/10665/107823/9789289021920-eng.pdf?sequence=1&isAllowed=y (accessed on 15 July 2021).
- Deng, X.J.; Li, F.; Li, Y.H.; Li, J.Y.; Huang, H.Z.; Liu, X.T. Vertical distribution characteristics of PM in the surface layer of Guangzhou. Particuology 2015, 20, 3–9. [Google Scholar] [CrossRef]
- Chen, T.Z.; Liu, Y.C.; Ma, Q.X.; Chu, B.W.; Zhang, P.; Liu, C.G.; Liu, J.; He, H. Significant source of secondary aerosol: Formation from gasoline evaporative emissions in the presence of SO2 and NH3. Atmos. Chem. Phys. 2019, 19, 8063–8081. [Google Scholar] [CrossRef]
- Lim, J.M.; Jeong, J.H.; Lee, J.H.; Moon, J.H.; Chung, Y.S.; Kim, K.H. The analysis of PM2.5 and associated elements and their indoor/outdoor pollution status in an urban area. Indoor Air 2011, 21, 145–155. [Google Scholar] [CrossRef]
- Kong, S.; Lu, B.; Ji, Y.; Zhao, X.; Bai, Z.; Xu, Y.; Liu, Y.; Jiang, H. Risk assessment of heavy metals in road and soil dusts within PM2.5, PM10 and PM100 fractions in Dongying city, Shandong Province. China J. Environ. Monit. 2012, 14, 791–803. [Google Scholar] [CrossRef]
- Hsu, S.I.; Ito, K.; Kendall, M.; Lippmann, M. Factors affecting personal exposure to thoracic and fine particles and their components. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 439–447. [Google Scholar] [CrossRef]
- Hung, P.S.; Shen, H.Y.; Kuo, H.W. Factors affecting the indoor concentrations of PM2.5 and PM10 in a high-rise office building. Epidemiology 2006, 17, S355–S356. [Google Scholar] [CrossRef]
- Wang, J.; Xie, X.; Fang, C.S. Temporal and Spatial Distribution Characteristics of Atmospheric Particulate Matter (PM10 and PM2.5) in Changchun and Analysis of Its Influencing Factors. Atmosphere 2019, 10, 651. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Spatial distribution of particulate matter (PM10 and PM2.5) in Seoul Metropolitan Subway stations. J. Hazard. Mater. 2008, 154, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Cartenì, A.; Cascetta, F.; Campana, S. Underground and ground-level particulate matter concentrations in an Italian metro system. Atmos. Environ. 2015, 101, 328–337. [Google Scholar] [CrossRef]
- Guo, E.; Shen, H.; He, L.; Zhang, J. Investigation of air pollution of Shanghai subway stations in ventilation seasons in terms of PM2.5 and PM10. Toxicol. Ind. Health 2017, 33, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.; Hughson, W.G. Health effects of indoor fungal bioaerosol exposure. Appl. Occup. Environ. Hyg. 2003, 18, 535–544. [Google Scholar]
- Morman, S.A.; Plumlee, G.S. The role of airborne mineral dusts in human disease. Aeolian Res. 2013, 9, 203–212. [Google Scholar] [CrossRef]
- Srithawirat, T.; Latif, M.T.; Sulaiman, F.R. Indoor PM10 and its heavy metal composition at a roadside residential environment, Phitsanulok, Thailand. Atmósfera 2016, 29, 311–322. [Google Scholar] [CrossRef][Green Version]
- Lv, Y.; Wang, H.F.; Wei, S.S.; Zhang, L.; Zhao, Q. The Correlation between Indoor and Outdoor Particulate Matter of Different Building Types in Daqing, China. Procedia Eng. 2017, 205, 360–367. [Google Scholar] [CrossRef]
- Patel, S.; Sankhyan, S.; Boedicker, E.K.; DeCarlo, P.F.; Farmer, D.K.; Goldstein, A.H.; Katz, E.F.; Nazaroff, W.W.; Tian, Y.; Vanhanen, J.; et al. Indoor Particulate Matter during HOMEChem: Concentrations, Size Distributions, and Exposures. Environ. Sci. Technol. 2020, 54, 7107–7116. [Google Scholar] [CrossRef] [PubMed]
- Taner, S.; Pekey, B.; Pekey, H. Fine particulate matter in the indoor air of barbeque restaurants: Elemental compositions, sources and health risks. Sci. Total Environ. 2013, 454, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, M.M.M. Indoor particulate matter in urban residences of Alexandria, Egypt. J. Air Waste Manag. Assoc. 2013, 63, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.J.; Kling, K.I.; Hanninen, O.; Jayjock, M.; Londahl, J.; Wierzbicka, A.; Fonseca, A.S.; Uhrbrand, K.; Boor, B.E.; Jimenez, A.S.; et al. Source specific exposure and risk assessment for indoor aerosols. Sci. Total Environ. 2019, 668, 13–24. [Google Scholar] [CrossRef]
- Thompson, J.E. Airborne Particulate Matter: Human Exposure and Health Effects. J. Occup. Environ. Med. 2018, 60, 392–423. [Google Scholar] [CrossRef] [PubMed]
- Hodas, N.; Loh, M.; Shin, H.M.; Li, D.; Bennett, D.; McKone, T.E.; Jolliet, O.; Weschler, C.J.; Jantunen, M.; Lioy, P.; et al. Indoor inhalation intake fractions of fine particulate matter: Review of influencing factors. Indoor Air 2016, 26, 836–856. [Google Scholar] [CrossRef]
- Madureira, J.; Slezakova, K.; Silva, A.I.; Lage, B.; Mendes, A.; Aguiar, L.; Pereira, M.C.; Teixeira, J.P.; Costa, C. Assessment of indoor air exposure at residential homes: Inhalation dose and lung deposition of PM10, PM2.5 and ultrafine particles among newborn children and their mothers. Sci. Total Environ. 2020, 717, 137293. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Levesque, C.; Chénier, M.; Gardner, H.D. Contribution of metals in resuspended dust to indoor and personal inhalation exposures: Relationships between PM10 and settled dust. Build. Environ. 2018, 143, 513–522. [Google Scholar] [CrossRef]
- Brown, J.S.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 2013, 10, 12. [Google Scholar] [CrossRef]
- Kastury, F.; Smith, E.; Juhasz, A.L. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Sci. Total Environ. 2017, 574, 1054–1074. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency EPA). Exposure and Human Health Evaluation of Airborne Pollution from the World Trade Center Disaster; USEPA: Washington, DC, USA, 2002. [Google Scholar]
- Almeida-Silva, M.; Pilou, M.; Housiadas, C.; Almeida, S.M. Internal dose of particles in the elderly—Modeling based on aerosol measurements. Environ. Sci. Pollut. Res. 2018, 25, 23645–23656. [Google Scholar] [CrossRef] [PubMed]
- Segalin, B.; Kumar, P.; Micadei, K.; Fornaro, A.; Gonçalves, F.L.T. Size–segregated particulate matter inside residences of elderly in the Metropolitan Area of São Paulo, Brazil. Atmos. Environ. 2017, 148, 139–151. [Google Scholar] [CrossRef]
- Blais-Lecours, P.; Perrott, P.; Duchaine, C. Non-culturable bioaerosols in indoor settings: Impact on health and molecular approaches for detection. Atmos. Environ. 2015, 110, 45–53. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Bao, L.J.; Tao, S.; Zeng, E.Y. Dermal Uptake from Airborne Organics as an Important Route of Human Exposure to E-Waste Combustion Fumes. Environ. Sci. Technol. 2016, 50, 6599–6605. [Google Scholar] [CrossRef]
- Garrido, J.A.; Parthasarathy, S.; Moschet, C.; Young, T.M.; McKone, T.E.; Bennett, D.H. Exposure Assessment for Air-To-Skin Uptake of Semivolatile Organic Compounds (SVOCs) Indoors. Environ. Sci. Technol. 2019, 53, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Aston, L.S.; Krieger, R.I. Perspiration increased human pesticide absorption following surface contact during an indoor scripted activity program. J. Expo. Sci. Environ. Epidemiol. 2004, 14, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, Y.; Chirino, Y.I.; Osornio-Vargas, A.R.; Herrera, L.A.; Morales-Bárcenas, R.; López-Saavedra, A.; González-Ramírez, I.; Miranda, J.; García-Cuellar, C.M. Cytoplasmic p21CIP1/WAF1, ERK1/2 activation, and cytoskeletal remodeling are associated with the senescence-like phenotype after airborne particulate matter (PM10) exposure in lung cells. Toxicol. Lett. 2014, 225, 12–19. [Google Scholar] [CrossRef]
- Lademann, J.; Schaefer, H.; Otberg, N.; Teichmann, A.; Blume-Peytavi, U.; Sterry, W. Penetration of microparticles into human skin. Hautarzt 2004, 55, 1117–1119. [Google Scholar] [CrossRef]
- Wilson, R.; Jones-Otazo, H.; Petrovic, S.; Mitchell, I.; Bonvalot, Y.; Williams, D.; Richardson, G.M. Revisiting Dust and Soil Ingestion Rates Based on Hand-to-Mouth Transfer. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 158–188. [Google Scholar] [CrossRef]
- De Brouwere, K.; Buekers, J.; Cornelis, C.; Schlekat, C.E.; Oller, A.R. Assessment of indirect human exposure to environmental sources of nickel: Oral exposure and risk characterization for systemic effects. Sci. Total Environ. 2012, 419, 25–36. [Google Scholar] [CrossRef]
- Beamish, L.A.; Osornio-Vargas, A.R.; Wine, E. Air pollution: An environmental factor contributing to intestinal disease. J. Crohn’s Colitis 2011, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, Z.; Li, Y.; Wang, H.; Fan, W.; Dong, Z. Estimating the dietary exposure and risk of persistent organic pollutants in China: A national analysis. Environ. Pollut. 2021, 288, 117764. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Fan, X.; Li, Y.; Wang, Z.; Chen, L.; Wang, Y.; Zhao, X.; Fan, W.; Wu, F. A Web-Based Database on Exposure to Persistent Organic Pollutants in China. Environ. Health Perspect. 2021, 129, 057701. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.B.; et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 2015, 83, 183–191. [Google Scholar] [CrossRef]
- Xu, H.M.; Guinot, B.; Cao, J.J.; Li, Y.Q.; Niu, X.Y.; Ho, K.F.; Shen, Z.X.; Liu, S.X.; Zhang, T.; Lei, Y.L.; et al. Source, health risk and composition impact of outdoor very fine particles (VFPs) to school indoor environment in Xi’an, Northwestern China. Sci. Total Environ. 2018, 612, 238–246. [Google Scholar] [CrossRef]
- Avigo, D.; Godoi, A.F.L.; Janissek, P.R.; Makarovska, Y.; Krata, A.; Potgieter-Vermaak, S.; Alfoldy, B.; Van Grieken, R.; Godoi, R.H.M. Particulate matter analysis at elementary schools in Curitiba, Brazil. Anal. Bioanal. Chem. 2008, 391, 1459–1468. [Google Scholar] [CrossRef]
- Ali, M.Y.M.; Hanafiah, M.M.; Latif, M.T. Composition and Distribution of Particulate Matter (PM10) in a Mechanically Ventilated University Building. AIP Conf. Proc. 2016, 1784, 060017. [Google Scholar]
- Sysoltseva, M.; Winterhalter, R.; Wolf, J.; Berlin, K.; Eckert, S.; Fembacher, L.; Matzen, W.; Nitschke, L.; Scheu, C.; Fromme, H. Particulate matter in air at indoor go-kart facilities in Bavaria, Germany. Atmos. Environ. 2018, 193, 118–126. [Google Scholar] [CrossRef]
- Horemans, B.; Worobiec, A.; Buczynska, A.; Van Meel, K.; Van Grieken, R. Airborne particulate matter and BTEX in office environments. J. Environ. Monit. 2008, 10, 867–876. [Google Scholar] [CrossRef]
- Huang, R.J.; Duan, J.; Li, Y.J.; Chen, Q.; Chen, Y.; Tang, M.J.; Yang, L.; Ni, H.Y.; Lin, C.S.; Xu, W.; et al. Effects of NH3 and alkaline metals on the formation of particulate sulfate and nitrate in wintertime Beijing. Sci. Total Environ. 2020, 717, 137190. [Google Scholar] [CrossRef] [PubMed]
- Buczynska, A.J.; Krata, A.; Van Grieken, R.; Brown, A.; Polezer, G.; De Wael, K.; Potgieter-Vermaak, S. Composition of PM2.5 and PM1 on high and low pollution event days and its relation to indoor air quality in a home for the elderly. Sci. Total Environ. 2014, 490, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Marcoccia, M.; Tofful, L.; Simonetti, G.; Perrino, C.; Canepari, S. Influence of advanced wood-fired appliances for residential heating on indoor air quality. Chemosphere 2018, 211, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Salameh, D.; Detournay, A.; Pey, J.; Perez, N.; Liguori, F.; Saraga, D.; Bove, M.C.; Brotto, P.; Cassola, F.; Massabo, D.; et al. PM2.5 chemical composition in five European Mediterranean cities: A 1-year study. Atmos. Res. 2015, 155, 102–117. [Google Scholar] [CrossRef]
- Saraga, D.; Maggos, T.; Sadoun, E.; Fthenou, E.; Hassan, H.; Tsiouri, V.; Karavoltsos, S.; Sakellari, A.; Vasilakos, C.; Kakosimos, K. Chemical Characterization of Indoor and Outdoor Particulate Matter (PM2.5, PM10) in Doha, Qatar. Aerosol Air Qual. Res. 2017, 17, 1156–1168. [Google Scholar] [CrossRef]
- Biester, H.; Hermanns, Y.M.; Cortizas, A.M. The influence of organic matter decay on the distribution of major and trace elements in ombrotrophic mires-a case study from the Harz Mountains. Geochim. Cosmochim. Acta 2012, 84, 126–136. [Google Scholar] [CrossRef]
- Wang, X.H.; Bi, X.H.; Sheng, G.Y.; Fu, J.M. Chemical Composition and Sources of PM10 and PM2.5 Aerosols in Guangzhou, China. Environ. Monit. Assess. 2006, 119, 425–439. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Lam, K.S.; Chan, L.Y.; Wang, T.; Cheng, K.K. Chemical characteristics of aerosols at coastal station in Hong Kong. I. Seasonal variation of major ions, halogens and mineral dusts between 1995 and 1996. Atmos. Environ. 2000, 34, 2771–2783. [Google Scholar] [CrossRef]
- Mahfouz, M.M.; Yigiterhan, O.; Elnaiem, A.E.; Hassan, H.M.; Alfoldy, B. Elemental compositions of particulate matter retained on air condition unit’s filters at Greater Doha, Qatar. Environ. Geochem. Health 2019, 41, 2533–2548. [Google Scholar] [CrossRef]
- Monsalve, S.M.; Martinez, L.; Vasquez, K.Y.; Orellana, S.A.; Vergara, J.K.; Mateo, M.M.; Salazar, R.C.; Alburquenque, M.F.; Lillo, D.D.C. Trace element contents in fine particulate matter (PM2.5) in urban school microenvironments near a contaminated beach with mine tailings, Chaaral, Chile. Environ. Geochem. Health 2018, 40, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kopiec, P.; Pastuszka, J.; Mathews, B.; Widziewicz, K. Factors determining the concentration and chemical composition of particulate matter in the air of selected service facilities. E3S Web Conf. 2018, 28, 01032. [Google Scholar] [CrossRef]
- Ali, M.Y.; Hanafiah, M.M.; Khan, M.F.; Latif, M.T. Quantitative source apportionment and human toxicity of indoor trace metals at university buildings. Build. Environ. 2017, 121, 238–246. [Google Scholar] [CrossRef]
- Alves, C.A.; Vicente, E.D.; Evtyugina, M.; Vicente, A.M.; Nunes, T.; Lucarelli, F.; Calzolai, G.; Nava, S.; Calvo, A.I.; Alegre, C.D.; et al. Indoor and outdoor air quality: A university cafeteria as a case study. Atmos. Pollut. Res. 2020, 11, 531–544. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, Z.X.; Cao, J.J.; Ho, K.F.; Zhang, R.J.; Bie, Z.J.; Chang, H.R.; Liu, S.X. Chemical profiles of urban fugitive dust over Xi’an in the south margin of the Loess Plateau. China Atmos. Pollut. Res. 2014, 5, 421–430. [Google Scholar] [CrossRef]
- Choppala, G.; Bolan, N.; Kunhikrishnan, A.; Skinner, W.; Seshadri, B. Concomitant reduction and immobilization of chromium in relation to its bioavailability in soils. Environ. Sci. Pollut. Res. 2015, 22, 8969–8978. [Google Scholar] [CrossRef]
- Yadav, S.; Rajamani, V. Air quality and trace metal chemistry of different size fractions of aerosols in N-NW India-implications for source diversity. Atmos. Environ. 2006, 40, 698–712. [Google Scholar] [CrossRef]
- Lowther, S.D.; Jones, K.C.; Wang, X.M.; Whyatt, J.D.; Wild, O.; Booker, D. Particulate Matter Measurement Indoors: A Review of Metrics, Sensors, Needs, and Applications. Environ. Sci. Technol. 2019, 53, 11644–11656. [Google Scholar] [CrossRef]
- Sakellaris, I.; Saraga, D.; Mandin, C.; de Kluizenaar, Y.; Fossati, S.; Spinazzè, A.; Cattaneo, A.; Szigeti, T.; Mihucz, V.; de Oliveira Fernandes, E.; et al. Personal Control of the Indoor Environment in Offices: Relations with Building Characteristics, Influence on Occupant Perception and Reported Symptoms Related to the Building—The Officair Project. Appl. Sci. 2019, 9, 3227. [Google Scholar] [CrossRef]
- Abdel-Salam, M.M.M. Indoor PM in Different Residential Areas of Alexandria City, Egypt. Indoor Built Environ. 2012, 21, 857–862. [Google Scholar] [CrossRef]
- Brauer, M.; Freedman, G.; Frostad, J.; van Donkelaar, A.; Martin, R.V.; Dentener, F.; van Dingenen, R.; Estep, K.; Amini, H.; Apte, J.S.; et al. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environ. Sci. Technol. 2012, 45, 652–660. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhao, A.; Chen, H.L.; Zhao, Z.H.; Cai, J.; Wang, C.C.; Yang, C.Y.; Li, H.C.; Xu, X.H.; Ha, S.D.; et al. Cardiopulmonary Benefits of Reducing Indoor Particles of Outdoor Origin: A Randomized, Double-Blind Crossover Trial of Air Purifiers. J. Am. Coll. Cardiol. 2015, 65, 2279–2287. [Google Scholar] [CrossRef]
- Salimifard, P.; Rim, D.; Gomes, C.; Kremer, P.; Freihaut, J.D. Resuspension of Biological Particles from Indoor Surfaces: Effects of Humidity and Air Swirl. Sci. Total Environ. 2017, 583, 241–247. [Google Scholar] [CrossRef]
- Dadvand, P.; Parker, J.; Bell, M.L.; Bonzini, M.; Brauer, M.; Darrow, L.A.; Gehring, U.; Glinianaia, S.V.; Gouveia, N.; Ha, E.H.; et al. Maternal exposure to particulate air pollution and term birth weight: A multi-country evaluation of effect and heterogeneity. Environ. Health Persp. 2013, 121, 267–373. [Google Scholar] [CrossRef]
- Neuberger, M.; Moshammer, H.; Rabczenko, D. Acute and Subacute Effects of Urban Air Pollution on Cardiopulmonary Emergencies and Mortality: Time Series Studies in Austrian Cities. Int. J. Environ. Res. Public Health 2013, 10, 4728–4751. [Google Scholar] [CrossRef]
- Yorifuji, T.; Kashima, S.; Doi, H. Associations of acute exposure to fine and coarse particulate matter and mortality among older people in Tokyo, Japan. Sci. Total Environ. 2016, 542, 354–359. [Google Scholar] [CrossRef]
- COMEAP (The Committee on the Medical Effects of Air Pollutants). Long-Term Exposure to Air Pollution: Effect on Mortality; COMEAP: Oxfordshire, UK, 2009. [Google Scholar]
- Health Matters: Air Pollution. 2018. Available online: https://elkssl0654429fdf150efe5e4fdae07ab4ffa3lib.v.ntu.edu.cn:4443/government/publications/health-matters-air-pollution/health-matters-air-pollution (accessed on 8 April 2020).
- Samoli, E.; Stafoggia, M.; Rodopoulou, S.; Ostro, B.; Declercq, C.; Alessandrini, E.; Díaz, J.; Karanasiou, A.; Kelessis, A.G.; Tertre, A.L.; et al. Associations between fine and coarse particles and mortality in Mediterranean cities: Results from the MED-PARTICLES project. Environ. Health Perspect. 2013, 121, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Rice, M.B.; Gold, D.R. Air pollution and lung function in children. J. Allergy Clin. Immun. 2021, 148, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Morawska, L.; Birmili, W.; Paasonen, P.; Hu, M.; Kulmala, M.; Harrison, R.M.; Norford, L.; Britter, R. Ultra-fine particles in cities. Environ. Int. 2014, 66, 1–10. [Google Scholar] [CrossRef]
- Xu, H.M.; Guinot, B.; Niu, X.Y.; Cao, J.J.; Ho, K.F.; Zhao, Z.H.; Ho, S.S.H.; Liu, S.X. Concentrations, particle-size distributions, and indoor/outdoor differences of polycyclic aromatic hydrocarbons (PAHs) in a middle school classroom in Xi’an, China. Environ. Geochem. Health 2015, 37, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, K.; Castro, D.; Pereira, M.C.; Morais, S.; Delerue-Matos, C.; Alvim-Ferraz, M.C. Influence of Tobacco Smoke on Carcinogenic PAH Composition in Indoor PM10 and PM2.5. Atmos. Environ. 2009, 43, 6376–6382. [Google Scholar] [CrossRef]
- Da Silva, C.S.; Rossato, J.M.; Vaz Rocha, J.A.; Vargas, V.M.F. Characterization of an area of reference for inhalable particulate matter (PM2.5) associated with genetic biomonitoring in children. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2015, 778, 44–55. [Google Scholar] [CrossRef]
- Yousef, A.O.S.; Fahad, A.A.; Moneim, A.E.A.; Metwally, D.M.; El-khadragy, M.F.; Kassab, R.B. The Neuroprotective Role of Coenzyme Q10 Against Lead Acetate-Induced Neurotoxicity Is Mediated by Antioxidant, Anti-Inflammatory and Anti-Apoptotic Activities. Int. J. Environ. Res. Public Health 2019, 16, 2895. [Google Scholar] [CrossRef] [PubMed]
- Rundell, K.W.; Hoffman, J.R.; Caviston, R.; Bulbulian, R.; Hollenbach, A.M. Inhalation of ultrafine and fine particulate matter disrupts systemic vascular function. Inhal. Toxicol. 2007, 19, 133–140. [Google Scholar] [CrossRef]
- Dacunto, P.J.; Cheng, K.C.; Acevedo-Bolton, V.; Jiang, R.T.; Klepeis, N.E.; Repace, J.L.; Ott, W.R.; Hildemann, L.M. Identifying and quantifying secondhand smoke in source and receptorrooms: Logistic regression and chemical mass balance approaches. Indoor Air 2014, 24, 59–70. [Google Scholar] [CrossRef]
- Quezada-Maldonado, E.M.; Sánchez-Pérez, Y.; Chirino, Y.I.; García-Cuellar, C.M. Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways. Environ. Pollut. 2021, 287, 117313. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, C.; Hofer, I.; Kaltschmitt, M. Additives as a fuel-oriented measure to mitigate inorganic particulate matter (PM) emissions during small-scale combustion of solid biofuels. Biomass Convers. Biorefinery 2019, 9, 3–20. [Google Scholar] [CrossRef]
- Gemenetzis, P.; Moussas, P.; Arditsoglou, A.; Samara, C. Mass concentration and elemental composition of indoor PM2.5 and PM10 in university rooms in Thessaloniki, northern Greece. Atmos. Environ. 2006, 40, 3195–3206. [Google Scholar] [CrossRef]
- Wolfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef]
- Nanayakkara, G.K.; Wang, H.; Yang, X.F. Proton leak regulates mitochondrial reactive oxygen species generation in endothelial cell activation and inflammation—A novel concept. Arch. Biochem. Biophys. 2019, 662, 68–74. [Google Scholar] [CrossRef]
- Peng, J.J.; Zhang, L.L.; Meng, Q.Q.; Zhang, F.; Mao, X.N.; Liu, J.M.; Chen, Y.H.; Zou, H.F.; Shi, B.Y.; Wu, R.J.; et al. Adverse impact of ambient PM2.5 on expression and trafficking of surfactant protein A through reactive oxygen species damage to lamellar bodies. Toxicol. Lett. 2019, 315, 47–54. [Google Scholar] [CrossRef]
- Gilli, G.; Traversi, D.; Rovere, R.; Pignata, C.; Schiliro, T. Chemical characteristics and mutagenic activity of PM10 in Torino, a Northern Italian City. Sci. Total Environ. 2007, 385, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Magnani, N.D.; Marchini, T.; Garces, M.; Mebert, A.; Caceres, L.; Diaz, L.; Desimone, M.; Evelson, P.A. Role of transition metals present in air particulate matter on lung oxygen metabolism. Int. J. Biochem. Cell B 2016, 81, 419–426. [Google Scholar] [CrossRef]
- Suh, H.H.; Zanobetti, A.; Schwartz, J.; Coull, B.A. Chemical Properties of Air Pollutants and Cause-Specific Hospital Admissions among the Elderly in Atlanta, Georgia. Environ. Health Persp. 2011, 119, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Lipfert, F.W.; Baty, J.D.; Miller, J.P.; Wyzga, R.E. PM2.5 constituents and related air quality variables as predictors of survival in a cohort of U.S. military veterans. Inhal. Toxicol. 2006, 18, 645–657. [Google Scholar] [CrossRef]
- Ho, C.C.; Chen, Y.C.; Yet, S.F.; Weng, C.Y.; Tsai, H.T.; Hsu, J.F.; Lin, P.P. Identification of ambient fine PM components related to vascular dysfunction by analyzing spatiotemporal variations. Sci. Total Environ. 2020, 719, 137243. [Google Scholar] [CrossRef]
- Ostro, B.D. Associations between morbidity and alternative measures of particulate matter. Risk Anal. 1990, 10, 421–427. [Google Scholar] [CrossRef]
- Rich, D.Q.; Zhang, W.J.; Lin, S.; Squizzato, S.; Thurston, S.W.; van Wijngaarden, E.; Croft, D.; Masiol, M.; Hopke, P.K. Triggering of cardiovascular hospital admissions by source specific fine particle concentrations in urban centers of New York State. Environ. Int. 2019, 126, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Lin, Y.K. Mortality and emergency room visits associated with ambient PM constituents in metropolitan Taipei. Sci. Total Environ. 2016, 569, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Feng, W.Y.; Broadwin, R.; Green, S.; Lipsett, M. The effects of components of fine particulate air pollution on mortality in California: Results from CALFINE. Environ. Health Persp. 2007, 115, 13–19. [Google Scholar] [CrossRef]
- Fine, P.M.; Sioutas, C.; Solomon, P.A. Secondary particulate matter in the United States: Insights from the particulate matter supersites program and related studies. J. Air Waste Manag. Assoc. 2008, 58, 234–253. [Google Scholar] [CrossRef]
- Goodsell, D.S. The Molecular Perspective: Polycyclic Aromatic Hydrocarbons. Stem Cells 2004, 22, 873–874. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Kwon, H.O.; Lammel, G.; Jung, K.S.; Lee, S.J.; Choi, S.D. Spatially high-resolved monitoring and risk assessment of polycyclic aromatic hydrocarbons in an industrial city. J. Hazard. Mater. 2020, 393, 122409. [Google Scholar] [CrossRef]
- Li, J.; Fan, H.Z.; Liu, K.P.; Li, X.Y.; Fan, D.P.; Lu, X.C.; Xia, Y.; Cao, Y.; Xiao, C. Associations of urinary polycyclic aromatic hydrocarbons with albuminuria in US adults, NHANES 2003–2014. Ecotoxicol. Environ. Safe 2020, 195, 110445. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Q.; Pan, W.Y.; Li, C.M.; Ma, X.C.; Yin, S.S.; Zhou, J.H.; Liu, J. Exposure to polycyclic aromatic hydrocarbons and risk for premature ovarian failure and reproductive hormones imbalance. J. Environ. Sci. 2020, 91, 1–9. [Google Scholar] [CrossRef]
- Hien, V.T.D.; Lin, C.; Thanh, V.C.; Oanh, N.T.K.; Thanh, B.X.; Weng, C.E.; Yuan, C.S.; Rene, E.R. An overview of the development of vertical sampling technologies for ambient volatile organic compounds (VOCs). J. Environ. Manag. 2019, 247, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.C.; Chan, L.Y.; Li, W.M. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ. Res. 2004, 94, 57–66. [Google Scholar] [CrossRef]
- Ramadan, A.; Yassin, M.F.; Alshammari, B.Z. Health risk assessment associated with volatile organic compounds in a parking garage. Int. J. Environ. Sci. Technol. 2019, 16, 2549–2564. [Google Scholar] [CrossRef]
- Wolkoff, P. Indoor air pollutants in office environments: Assessment of comfort, health, and performance. Int. J. Hyg. Environ. Health 2013, 216, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.R.; Wang, X.M. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Safe. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Widziewicz, K.; Loska, K. Metal induced inhalation exposure in urban population: A probabilistic approach. Atmos. Environ. 2016, 128, 198–207. [Google Scholar] [CrossRef]
- . Hadnagy, W.; Stiller-Winkler, R.; Kainka, E.; Ranft, U.; Idel, H. Influence of urban particulate air pollution (PM10; PM2.5) on the immune system of children. J. Aerosol Sci. 1998, 29, S997–S998. [Google Scholar] [CrossRef]
- DeFranco, E.; Moravec, W.; Xu, F.; Hall, E.; Hossain, M.; Haynes, E.N.; Muglia, L.; Chen, A. Exposure to airborne particulate matter during pregnancy is associated with preterm birth: A population-based cohort study. Environ. Health 2016, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.P.; Salemi, J.L.; Stuart, A.L.; Yu, H.; Jordan, M.M.; DuClos, C.; Cavicchia, P.; Correia, J.A.; Watkins, S.M.; Kirby, R.S. Associations between exposure to ambient benzene and PM2.5 during pregnancy and the risk of selected birth defects in offspring. Environ. Res. 2015, 142, 345–353. [Google Scholar] [CrossRef]
- Siddika, N.; Balogun, H.A.; Amegah, A.K.; Jaakkola, J.J. Prenatal ambient air pollution exposure and the risk of stillbirth: Systematic review and meta-analysis of the empirical evidence. Occup. Environ. Med. 2016, 73, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.A.; Perera, F.P.; Spengler, J.D.; Mroz, E.; Stigter, L.; Flak, E.; Majewska, R.; Klimaszewska-Rembiasz, M.; Jacek, R. Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int. J. Hyg. Environ. Health 2013, 216, 395–401. [Google Scholar] [CrossRef]
- Burtscher, H.; Schuepp, K. The occurrence of ultrafine particles in the specific environment of children. Paediatr. Respir. Rev. 2012, 13, 89–94. [Google Scholar] [CrossRef]
- Mendell, M.J. Indoor residential chemical emissions as risk factors for-respiratory and allergic effects in children: A review. Indoor Air 2007, 17, 259–277. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Sundell, J.; Weschler, C.J.; Sigsgaard, T.; Lundgren, B.; Hasselgren, M.; Hagerhed-Engman, L. The association between asthma and allergic symptoms in children and phthalates in house dust: A nested case-control study. Environ. Health Persp. 2004, 112, 1393–1397. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Heydarpour, P.; Reis, J.; Amiri, M.; Sahraian, M.A. Multiple sclerosis and air pollution exposure: Mechanisms toward brain autoimmunity. Med. Hypotheses 2017, 100, 23–30. [Google Scholar] [CrossRef]
- Chapman, R.S.; Watkinson, W.P.; Dreher, K.L.; Costa, D.L. Ambient particulate matter and respiratory and cardiovascular illness in adults: Particle-borne transition metals and the heart-lung axis. Environ. Toxicol. Phar. 1997, 4, 331–338. [Google Scholar] [CrossRef]
- Chang, L.T.; Tang, C.S.; Pan, Y.Z.; Chan, C.C. Association of heart rate variability of the elderly with personal exposure to PM1, PM1–2.5, and PM2.5–10. Bull. Environ. Contam. Toxicol. 2007, 79, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wu, C.D.; Chiang, H.C.; Chu, D.C.; Lee, K.Y.; Lin, W.Y.; Yeh, J.I.; Tsai, K.W.; Guo, Y.L.L. The effects of fine and coarse PM on lung function among the elderly. Sci. Rep. 2019, 9, 14790. [Google Scholar] [CrossRef] [PubMed]
- Prada, D.; Lopez, G.; Solleiro-Villavicencio, H.; Garcia-Cuellar, C.; Baccarelli, A.A. Molecular and cellular mechanisms linking air pollution and bone damage. Environ. Res. 2020, 185, 109465. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Serrano-Sierra, A.; Torres-Jardón, R.; Zhu, H.; Yuan, Y.; Smith, D.; Delgado-Chavez, R.; Cross, J.V.; Medina-Cortina, H.; Kavanaugh, M.; et al. The impact of environmental metals in young urbanites’ brains. Exp. Toxicol. Pathol. 2013, 65, 503–511. [Google Scholar] [CrossRef]
- Pohl, H.R.; Roney, N.; Abadin, H.G. Metal ions affecting the neurological system. Met. Ions Life Sci. 2011, 8, 247–262. [Google Scholar]
- Li, Y.C.; Shu, M.; Ho, S.S.H.; Wang, C.; Cao, J.J.; Wang, G.H.; Wang, X.X.; Wang, K.; Zhao, X.Q. Characteristics of PM2.5 emitted from different cooking activities in China. Atmos. Res. 2015, 166, 83–91. [Google Scholar] [CrossRef]
- Wan, M.P.; Wu, C.L.; Szeto, G.N.; Chan, T.C.; Chao, C.Y.H. Ultrafine particles, and PM2.5 generated from cooking in homes. Atmos. Environ. 2011, 45, 6141–6148. [Google Scholar] [CrossRef]
- Wong, T.W.; Wong, A.H.S.; Lee, F.S.C.; Qiu, H. Respiratory health and lung function in Chinese restaurant kitchen workers. Occup. Environ. Med. 2011, 68, 746–752. [Google Scholar] [CrossRef]
- Cheng, J.H.; Lee, Y.S.; Chen, K.S. Carbonyl compounds in dining areas, kitchens and exhaust streams in restaurants with varying cooking methods in Kaohsiung, Taiwan. J. Environ. Sci. 2016, 41, 218–226. [Google Scholar] [CrossRef] [PubMed]
- See, S.W.; Balasubramanian, R. Risk assessment of exposure to indoor aerosols associated with Chinese cooking. Environ. Res. 2006, 102, 197–204. [Google Scholar] [CrossRef]
- Pan, C.H.; Chan, C.C.; Wu, K.Y. Effects on Chinese Restaurant Workers of Exposure to Cooking Oil Fumes: A Cautionary Note on Urinary 8-Hydroxy-2-Deoxyguanosine. Cancer Epidemiol. Prev. Biomark. 2008, 17, 3351–3357. [Google Scholar] [CrossRef]
- Bigert, C.; Lonn, M.; Feychting, M.; Sjogren, B.; Lewne, M.; Gustavsson, P. Incidence of myocardial infarction among cooks and other restaurant workers in Sweden 1987–2005. Scand. J. Work. Environ. Health 2013, 39, 204–211. [Google Scholar] [CrossRef]
- Zimmer, A.T.; Baron, P.A.; Biswas, P. The influence of operating parameters on number-weighted aerosol size distribution generated from a gas metal arc welding process. J. Aerosol Sci. 2002, 33, 519–531. [Google Scholar] [CrossRef]
- Racette, B.A.; McGee-Minnich, L.; Moerlein, S.M.; Mink, J.W.; Videen, T.O.; Perlmutter, J.S. Welding-related parkinsonism. Clinical features, treatment, and pathophysiology. Neurology 2001, 56, 8–13. [Google Scholar] [CrossRef]
- Sears, C.G.; Sears, L.; Zierold, K.M. Sex differences in the association between exposure to indoor particulate matter and cognitive control among children (age 6–14 years) living near coal-fired power plants. Neurotoxicol. Teratol. 2020, 78, 106855. [Google Scholar] [CrossRef]
- Gregory, A.C.; Shendell, D.G.; Okosun, I.S.; Gieseker, K.E. Multiple Sclerosis disease distribution and potential impact of environmental air pollutants in Georgia. Sci. Total Environ. 2008, 396, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.H.; Lin, J.S.; Chan, C.C. Office Workers’ Sick Building Syndrome and Indoor Carbon Dioxide Concentrations. J. Occup. Environ. Hyg. 2012, 9, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Jouzizadeh, M.; Tabesh, M.; Malekipirbazari, M.; Gabdrashova, R.; Nurzhan, S.; Farrokhi, H.; Khanbabaie, R.; Mehri-Dehnavi, H.; Bekezhankyzy, Z.; et al. The impact of frying aerosol on human brain activity. Neurotoxicology 2019, 74, 149–161. [Google Scholar] [CrossRef]
- Weichenthal, S.; Villeneuve, P.J.; Burnett, R.T.; Van Donkelaar, A.; Martin, R.V.; Jones, R.R.; DellaValle, C.T.; Sandler, D.P.; Ward, M.H.; Hoppin, J.A. Long-Term Exposure to Fine Particulate Matter: Association with Nonaccidental and Cardiovascular Mortality in the Agricultural Health Study Cohort. Environ. Health Persp. 2014, 122, 609–615. [Google Scholar] [CrossRef]
- Reynolds, S.J.; Black, D.W.; Borin, S.S.; Breuer, G.; Burmeister, L.F.; Fuortes, L.J.; Smith, T.F.; Stein, M.A.; Subramanian, P.; Thorne, P.S.; et al. Indoor environmental quality in six commercial office buildings in the midwest United States. Appl. Occup. Environ. Hyg. 2001, 16, 1065–1077. [Google Scholar] [CrossRef]
- Dai, X.L.; Liu, J.J.; Li, X.D.; Zhao, L. Long-term monitoring of indoor CO2 and PM2.5 in Chinese homes: Concentrations and their relationships with outdoor environments. Build. Environ. 2018, 144, 238–247. [Google Scholar] [CrossRef]
- Maleki, H.; Sorooshian, A.; Goudarzi, G.; Baboli, Z.; Birgani, Y.T.; Rahmati, M. Air pollution prediction by using an artificial neural network model. Clean Technol. Environ. 2019, 21, 1341–1352. [Google Scholar] [CrossRef]
- Administration of Quality Supervision, Inspection and Quarantine; Ministry of Environmental Protection; Ministry of Health. Indoor Air Quality Standard, Standard No: GB/T 18883-2002. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/dqhjbh/dqhjzlbz/200303/t20030301_67375.shtml (accessed on 15 July 2021).
- Institute of Environmental Epidemiology. Guidelines for Good Indoor Air Quality in Office Premises; Ministry of the Environment: Singapore, 1996.
- TEC Green Office. Indoor Air Quality Guidelines for Sydney Olympic Facilities; TEC Green Office: Sydney, Australia, 1997. [Google Scholar]
- Health Canada. Exposure Guidelines for Residential Indoor Air Quality: A Report of the Federal-Provincial Advisory Committee on Environmental and Occupational Health; Health Canada: Ottawa, ON, Canada, 1989. [Google Scholar]
- ANSI/ASHRAE Standard 62.1. Ventilation for Acceptable Indoor Air Quality; American Society of Heating. Refrigerating and Air-Conditioning Engineers, Inc.: Atlanta, GA, USA, 2007; Available online: https://doc.wendoc.com/ba5824c3fc5ebb69eb1225b67.html (accessed on 15 July 2021).
- Abdul-Wahab, S.A.; En, S.C.F.; Elkamel, A.; Ahmadi, L.; Yetilmezsoy, K. A review of standards and guidelines set by international bodies for the parameters of indoor air quality. Atmos. Pollut. Res. 2015, 5, 751–767. [Google Scholar] [CrossRef]
- Dales, R.; Liu, L.; Wheeler, A.J.; Gilbert, N.L. Quality of indoor residential air and health. Can. Med. Assoc. J. 2008, 179, 147–152. [Google Scholar] [CrossRef]
- ACGIH (American Conference of Governmental Industrial Hygienists). Threshol Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2005; pp. 4524–16340. [Google Scholar]
- Charles, K.; Magee, R.J.; Won, D.; Lusztyk, E. Indoor Air Quality Guidelines and Standards: Final Report 5.1. Achiev. Gains 2005, 4, 206–207. [Google Scholar]
- Bluyssen, P. Product Policy and Indoor Air Quality. Indoor Sources and Health Effects: Background Information and Ways to Go; Directorate General for Environment: Brussels, Belgium, 2010. [Google Scholar]
- Salthammer, T. Critical evaluation of approaches in setting indoor air quality guidelines and reference values. Chemosphere 2011, 82, 1507–1517. [Google Scholar] [CrossRef]
- Wen, C.; Liu, S.; Yao, X.; Peng, L.; Li, X.; Hu, Y.; Chi, T.H. A novel spatiotemporal convolutional long short-term neural network for air pollution prediction. Sci. Total Environ. 2019, 654, 1091–1099. [Google Scholar] [CrossRef]
- Liu, H.; Cao, C.Y.; Huang, J.Y.; Chen, Z.; Chen, G.Q.; Lai, Y.K. Progress on particulate matter filtration technology: Basic concepts, advanced materials, and performances. Nanoscale 2020, 12, 437–453. [Google Scholar] [CrossRef]
- Zheng, C.H.; Kanaoka, C. Recent advances in dust collection technology and ISO standardization in bag filtration. J. Zhejiang Univ. Sci. A 2018, 19, 21–33. [Google Scholar] [CrossRef]
- Simon, X.; Chazelet, S.; Thomas, D.; Bemer, D.; Regnier, R. Experimental study of pulse-jet cleaning of bag filters supported by rigid rings. Powder Technol. 2007, 172, 67–81. [Google Scholar] [CrossRef]
- Bai, Y.; Han, B.; He, C.; Gu, G.Q.; Nie, J.H.; Shao, J.J.; Xiao, T.X.; Deng, C.R.; Wang, Z.L. Washable Multilayer Triboelectric Air Filter for Efficient Particulate Matter PM2.5 Removal. Adv. Funct. Mater. 2018, 28, 1706680. [Google Scholar] [CrossRef]
- Leman, A.M.; Zakaria, S.; Salleh, M.N.M.; Feriyanto, D.; Sunar, N.M.; Misdan, N. Indoor Air Contaminant Adsorption by Palm Shell Activated Carbon Filter-A Proposed Study, 2nd ed.; International Conference on Green Design and Manufacture: Phuket, Thailand, 2016. [Google Scholar]
- Bologa, A.; Paur, H.R.; Seifert, H.; Wascher, T.; Woletz, K. Novel wet electrostatic precipitator for collection of fine aerosol. J. Electrostat. 2009, 67, 150–153. [Google Scholar] [CrossRef]
- Tan, Z.C.; Zhang, Y.H. A Review of effects and control methods of particulate matter in animal indoor environments. J. Air Waste Manag. Assoc. 2004, 54, 845–854. [Google Scholar] [CrossRef]
- Gu, G.Q.; Han, C.B.; Lu, C.X.; He, C.; Jiang, T.; Gao, Z.L.; Li, C.J.; Wang, Z.L. Triboelectric Nanogenerator Enhanced Nanofiber Air Filters for Efficient Particulate Matter Removal. ACS Nano 2017, 11, 6211–6217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, R.N.; Zhang, X.; Han, Y.W.; Niu, W.F.; Xue, Z.Y.; Wang, L.F. Controlling fine particles in flue gas from lead-zinc smelting by plasma technology. Plasma Sci. Technol. 2020, 22, 044004. [Google Scholar] [CrossRef]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review of indoor air treatment technologies. Rev. Environ. Sci. Bio/Technol. 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Xie, B.; Li, S.H.; Jin, H.; Hu, S.D.; Wang, F.; Zhou, F.B. Analysis of the performance of a novel dust collector combining cyclone separator and cartridge filter. Powder Technol. 2018, 339, 695–701. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Yang, C.H. Granular-activated carbon adsorption followed by annular-type photocatalytic system for control of indoor aromatic compounds. Sep. Purif. Technol. 2009, 66, 438–442. [Google Scholar] [CrossRef]
- Liu, R.L.; Zhou, G.; Wang, C.M.; Jiang, W.J.; Wei, X. Preparation and performance characteristics of an environmentally-friendly agglomerant to improve the dry dust removal effect for filter material. J. Hazard. Mater. 2020, 397, 122734. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liang, J.C.; Zhang, C.; Tao, Y.; Ling, G.W.; Yang, Q.H. Advanced Materials for Capturing Particulate Matter: Progress and Perspectives. Small Methods. 2018, 2, 1800012. [Google Scholar] [CrossRef]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef]
- Schmid, S.; Meier, L.; Berchtold, C.; Zenobi, R. Online Monitoring of Molecular Processes in a Plasma Air Purifying System. Environ. Sci. Technol. 2012, 46, 4067–4073. [Google Scholar] [CrossRef]
- Li, Z.; Wen, Q.; Zhang, R. Sources, health effects and control strategies of indoor fine particulate matter (PM2.5): A review. Sci. Total Environ. 2017, 586, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Luo, Z.W.; Liu, J.; Wang, Y.W.; Lin, Y.Y. Health and economic benefits of building ventilation for reducing indoor PM2.5 exposure from both indoor and outdoor origins in urban Beijing. China. Sci. Total Environ. 2018, 626, 546–554. [Google Scholar] [CrossRef]
- Davis, J.; Benner, R. Seasonal trends in the abundance, composition and bioavailability of particulate and dissolved organic matter in the Chukchi/Beaufort Seas and western Canada Basin. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 3396–3410. [Google Scholar] [CrossRef]
- Kume, K.; Ohura, T.; Noda, T.; Amagai, T.; Fusaya, M. Seasonal and spatial trends of suspended-particle associated polycyclic aromatic hydrocarbons in urban Shizuoka, Japan. J. Hazard. Mater. 2007, 144, 513–521. [Google Scholar] [CrossRef]
- Shao, Z.J.; Bi, J.; Ma, Z.W.; Wang, J.N. Seasonal trends of indoor fine particulate matter and its determinants in urban residences in Nanjing, China. Build. Environ. 2017, 125, 319–325. [Google Scholar] [CrossRef]
- Mueller, W.; Steinle, S.; Parkka, J.; Parmes, E.; Liedes, H.; Kuijpers, E.; Pronk, A.; Sarigiannis, D.; Karakitsios, S.; Chapizanis, D.; et al. Urban greenspace and the indoor environment: Pathways to health via indoor particulate matter, noise, and road noise annoyance. Environ. Res. 2019, 180, 108850. [Google Scholar] [CrossRef]
- Li, Y.; He, J.H.; Sun, Q.L.; Wang, P. High Temperature Resistant Nanofiber by Bubbfil-Spinning. Therm. Sci. 2015, 19, 1461–1462. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.C.; Lee, H.W.; Ye, M.; Zheng, G.Y.; Liu, N.A.; Li, W.Y.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef]
- Repace, J.; Zhang, B.; Bondy, S.J.; Benowitz, N.; Ferrence, R. Air quality, mortality, and economic benefits of a smoke-free workplace law for non-smoking Ontario bar workers. Indoor Air 2013, 23, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.M.; Ramphul, M.; Bush, A.P. An update on controversies in e-cigarettes. Paediatr. Respir. Rev. 2020, 36, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Wu, D.M.; Ma, Y.; Ma, X.M.; Wang, S.L.; Li, F.X.; Li, M.H.; Zhang, T. Toxicity of electronic cigarettes: A general review of the origins, health hazards, and toxicity mechanisms. Sci. Total Environ. 2021, 772, 145475. [Google Scholar] [CrossRef]
- Ng, T.P.; Hui, K.P.; Tan, W.C. Respiratory Symptoms and lungs function effects of domestic exposure to tobacco smoke and cooking by gas in non-smoking women in Singapore. J. Epidemiol. Commun. Health 1993, 47, 454–458. [Google Scholar] [CrossRef]
- Dennekamp, M.; Howarth, S.; Dick, C.A.; Cherrie, J.W.; Donaldson, K.; Seaton, A. Ultrafine particles and nitrogen oxides generated by gas and electric cooking. Occup. Environ. Med. 2001, 58, 511–516. [Google Scholar] [CrossRef]
- He, C.R.; Morawska, L.D.; Hitchins, J.; Gilbert, D. Contribution from indoor sources to particle number and mass concentrations in residential houses. Atmos. Environ. 2004, 38, 3405–3415. [Google Scholar] [CrossRef]
- See, S.W.; Karthikeyana, S.; Balasubramanian, R. Health risk assessment of occupational exposure to particulate-phase polycyclic aromatic hydrocarbons associated with Chinese, Malay and Indian cooking. J. Environ. Monitor. 2006, 8, 369–376. [Google Scholar] [CrossRef]
- Torkmahalleh, M.A.; Gorjinezhad, S.; Keles, M.; Unluevcek, H.S.; Azgin, C.; Cihan, E.; Tanis, B.; Soy, N.; Ozaslan, N.; Ozturk, F.; et al. A controlled study for the characterization of PM2.5 emitted during grilling ground beef meat. J. Aerosol Sci. 2017, 103, 132–140. [Google Scholar] [CrossRef]
- Hartinger, S.M.; Commodore, A.A.; Hattendorf, J.; Lanata, C.F.; Gil, A.I.; Verastegui, H.; Aguilar-Villalobos, M.; Mausezahl, D.; Naeher, L.P. Chimney stoves modestly improved Indoor Air Quality measurements compared with traditional open fire stoves: Results from a small-scale intervention study in rural Peru. Indoor Air 2013, 23, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.T.B.S.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Sousa, S.I.V. Indoor air quality in urban nurseries at Porto city: Particulate matter assessment. Atmos. Environ. 2013, 84, 133–143. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Hoehn, R. Modeled PM2.5 removal by trees in ten U.S. cities and associated health effects. Environ. Pollut. 2013, 178, 395–402. [Google Scholar] [CrossRef]
- Little, P.; Wiffen, R.D. Emission and deposition of petrol engine exhaust Pb-I. Deposition of exhaust Pb to plant and soil surface. Atmos. Environ. 1977, 11, 437–447. [Google Scholar] [CrossRef]
- Langner, M.; Kull, M.; Endlicher, W.R. Determination of PM10 deposition based on antimony flux to selected urban surfaces. Environ. Pollut. 2011, 159, 2028–2034. [Google Scholar] [CrossRef]
- Wang, Y.; Akbari, H. The effects of street tree planting on urban heat island mitigation in Montreal. Sustain. Cities Soc. 2016, 27, 121–128. [Google Scholar] [CrossRef]
- Du, L.; Batterman, S.; Parker, E.; Godwin, C.; Chin, J.Y.; O’Toole, A.; Robins, T.; Brakefield-Caldwell, W.; Lewis, T. Particle concentrations and effectiveness of freestanding air filters in bedrooms of children with asthma in Detroit, Michigan. Build. Environ. 2011, 46, 2303–2313. [Google Scholar] [CrossRef]
| Region | Level (μg/m3) | Composition | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Carbon (μg/m3) | Non-Sea Salt Sulfate (NSS-SO42–) (μg/m3) | Nitrate (NO3–) (μg/m3) | Ammonium (NH4+) (μg/m3) | Sea-Salt (μg/m3) | Mineral Dust (μg/m3) | Non-Dust Elements (μg/m3) | |||
| A classroom in Xi’an, Northwestern China | VFPs = 35.4 | 11.94 | 3.60 | 0.94 | 0.20 | 0.52 | 0.38 | 0.15 | [132] |
| Elementary schools in Curitiba, Brazil | 7.41 | 0.18 | [133] | ||||||
| Biology Department Building, University Kebangsaan Malaysia | PM10 = 271 | 3224.84 | [134] | ||||||
| Indoor go-kart facilities | PM10 = 4.9 − 34.9 PM2.5 = 2.3 − 29.2 | 2.10 | 0.16 | [135] | |||||
| Nine offices in the province of Antwerp, Belgium | PM2.5 = 0.09 | 2.33 | 0.82 | 0.76 | 0.73 | 0.073 | [136] | ||
| Haidian district is close to the fourth ring road of Beijing | PM2.5 | 20.54 | 27.51 | 18.73 | 0.43 | 4.78 | [137] | ||
| Broechem is a village (12 km2) located in the province of Antwerp | PM2.5 = 24.8 PM1 = 15.7 | 36.4 | 45.7 | 22.1 | 4.7 | [138] | |||
| A peri-urban area about 40 km from Rome | PM2.5 = 16.7 PM10 = 27.6 | 12.72 | 4 | 1.32 | 0.61 | 0.88 | 4.4 | 230.56 | [139] |
| Located in the NE of the Iberian Peninsula | PM2.5 = 37 | 11.3 | 1.4 | 0.72 | 0.48 | 0.34 | 9.76 | 0.075 | [8] |
| Region | EF | Source | References | |
|---|---|---|---|---|
| Earth Crust/Soil | Non-Earth Crust | |||
| Universiti Kebangsaan Malaysia, Building 1 | <1 | 2–5 | Undefined sources, Crustal sources, Indoor-induced sources, urban origin sources and Earth’s crust | [148] |
| Universiti Kebangsaan Malaysia, Building 2 | <1 | 2–5 | Undefined sources, Combustion sources, biogenic sources, anthropogenic sources, crustal source | [148] |
| León (Spain) university cafeteria | <5 | >10 | Building materials, consumer products, and human activities | [149] |
| Broechem is a village located in the province of Antwerp, Belgium | 0.1–2.4 | >100 | Traffic and domestic heating, the harbour of Antwerp, a large petrochemical plant, a municipal waste incinerator, and a nonferrous plant to the south of Antwerp | [138] |
| Nine offices in the province of Antwerp, Belgium | <10 | 10–1000 | Outdoor influences, indoor respirable suspended particulates | [136] |
| Six schools located in Chañaral, Chile | 5–20 | <2 | Industrial and mining activities | [136] |
| Residential and commercial buildings of Doha city, state of Qatar | 1.04–3.03 | 1.94–63 | Outdoor mineral particles, non-exhaust traffic emission, industrial sources, the influence of indoor activity such as smoking. | [135] |
| Guangzhou city, China | <5 | 10–1000 | Coal combustion and sewage sludge incineration | [133] |
| Xian city, China | <5 | 10–30 | Building construction, paved road dust, fresh soil dust | [150] |
| Country | Value | Organization | Reference |
|---|---|---|---|
| China | 0.15 mg/m3 of PM10; 75 µg/m3 of PM2.5 | AQSIQ and CABR | [232] and (JGJ/T 309-2013) |
| Singapore | 150 µg/m3(in office) 1a of PM10 | Institute of Environmental Epidemiology | [233] |
| Australia | N/A of PM2.5 90 µg/m3 of PM10 | N/A The National Health and Medical Research Council | [234] |
| Canada | 100 µg/m3 as 1 h average (Short-Term Exposure) 40 µg/m3 as 8 h average (Long-Term Exposure) | Health Canada | [235,236,237,238] |
| US | 3 mg/m3 as 8 h average (Ceiling Level) 1b 35 µg/m3 as 24 h average of PM2.5 15 µg/m3 as 1 y average of PM2.5 150 µg/m3 as 24 h average (Exposure) 2a | American Conference of Governmental Industrial Hygienist, 2005. NAAQS/EPA ASHRAE | [236,237,239] |
| Finland | <20 µg/m3 as 8 h average of PM10 4 mg/m3 as 8 h average of PM10 | FiSIAQ | [237] |
| Germany | 50 µg/m3 as 24 h average of PM10 | FiSIAQ | [236,240] |
| Worldwide | 25 µg/m3 as 24 h average of PM2.5 10 µg/m3 as 1 y average of PM2.5 20 µg/m3 as 1 y average of PM10 | WHO | [241,242] |
| Theory | Application | References |
|---|---|---|
| Filtration | Bag type dust collector Ultralow penetration air filters Pulse-jet cleaning of bag filters Triboelectric air filter | [244] [245] [246] [247] |
| Adsorption | Carbon-based materials | [248] |
| Electrostatic | Wet electrostatic precipitators Tube electrostatic precipitator (R-TENG)-enhanced PI-nanofiber air filter | [249] [250] [251] |
| NIP Technology | Plasma dust collector | [252] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ou, C.; Magana-Arachchi, D.; Vithanage, M.; Vanka, K.S.; Palanisami, T.; Masakorala, K.; Wijesekara, H.; Yan, Y.; Bolan, N.; et al. Indoor Particulate Matter in Urban Households: Sources, Pathways, Characteristics, Health Effects, and Exposure Mitigation. Int. J. Environ. Res. Public Health 2021, 18, 11055. https://doi.org/10.3390/ijerph182111055
Zhang L, Ou C, Magana-Arachchi D, Vithanage M, Vanka KS, Palanisami T, Masakorala K, Wijesekara H, Yan Y, Bolan N, et al. Indoor Particulate Matter in Urban Households: Sources, Pathways, Characteristics, Health Effects, and Exposure Mitigation. International Journal of Environmental Research and Public Health. 2021; 18(21):11055. https://doi.org/10.3390/ijerph182111055
Chicago/Turabian StyleZhang, Ling, Changjin Ou, Dhammika Magana-Arachchi, Meththika Vithanage, Kanth Swaroop Vanka, Thava Palanisami, Kanaji Masakorala, Hasintha Wijesekara, Yubo Yan, Nanthi Bolan, and et al. 2021. "Indoor Particulate Matter in Urban Households: Sources, Pathways, Characteristics, Health Effects, and Exposure Mitigation" International Journal of Environmental Research and Public Health 18, no. 21: 11055. https://doi.org/10.3390/ijerph182111055
APA StyleZhang, L., Ou, C., Magana-Arachchi, D., Vithanage, M., Vanka, K. S., Palanisami, T., Masakorala, K., Wijesekara, H., Yan, Y., Bolan, N., & Kirkham, M. B. (2021). Indoor Particulate Matter in Urban Households: Sources, Pathways, Characteristics, Health Effects, and Exposure Mitigation. International Journal of Environmental Research and Public Health, 18(21), 11055. https://doi.org/10.3390/ijerph182111055







