Effect of Short-Term Low-Nitrogen Addition on Carbon, Nitrogen and Phosphorus of Vegetation-Soil in Alpine Meadow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and the Experimental Design
2.2. Sampling and Measurement
2.3. Data Statistical Analysis
3. Results
3.1. Community Characteristics
3.2. Vegetation Carbon, Nitrogen and Phosphorus
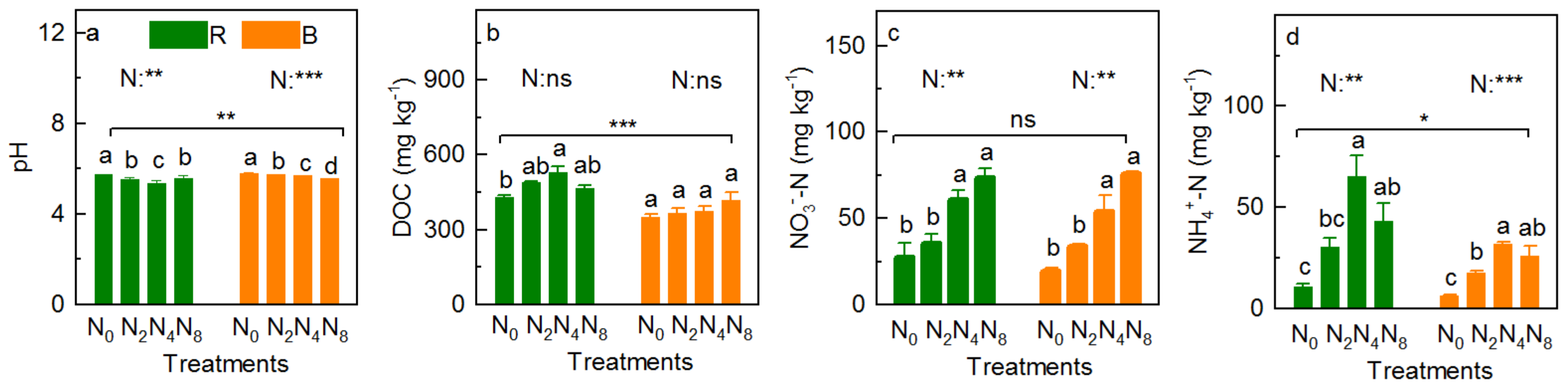
3.3. Soil Physicochemical Property
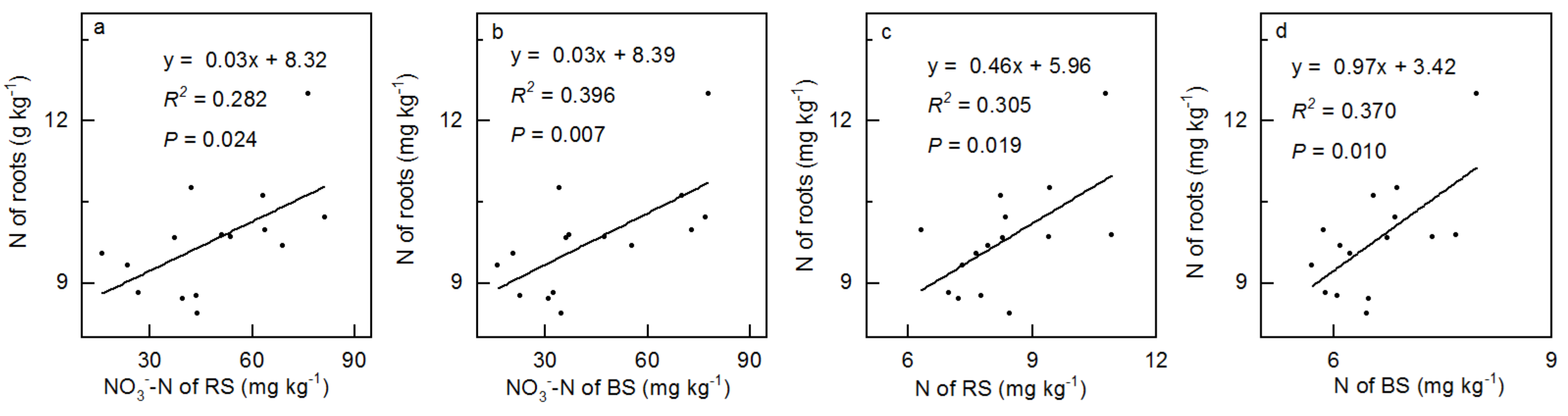
3.4. Correlation between Soil and Vegetation
4. Discussion
4.1. Effects of N Addition on Community Characteristics
4.2. Effects of N Addition on Vegetation TC, TN and TP Content
4.3. Effects of N Addition on Physicochemical Property
4.4. The Linkage between Plant and Soil
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Z.R.; Zhang, Y.; Zhang, Y. Nitrogen deposition magnifies the positive response of plant community production to precipitation: Ammonium to nitrate ratio matters. Environ. Pollut. 2021, 276, 116659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, M.; Kou, Y.; Liu, D.; Liu, Q.; Zhang, Z.; Jiang, Z.; Yin, H. Differential effects of N addition on the stoichiometry of microbes and extracellular enzymes in the rhizosphere and bulk soils of an alpine shrubland. Plant Soil 2020, 449, 285–301. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Sun, Z.; Piao, S.; Ma, Y.; Chen, Y.; Wang, J.; Qiao, C.; Wang, X.; Li, P. Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol. 2016, 212, 1019–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Cheng, S.; Yu, G.; Zheng, J.; Zhang, P.; Xu, M.; Li, Y.; Yang, X. Responses of CO2 efflux from an alpine meadow soil on the Qinghai Tibetan Plateau to multi-form and low-level N addition. Plant Soil 2012, 351, 177–190. [Google Scholar] [CrossRef]
- Fang, H.; Cheng, S.; Yu, G.; Xu, M.; Wang, Y.; Li, L.; Dang, X.; Wang, L.; Li, Y. Experimental nitrogen deposition alters the quantity and quality of soil dissolved organic carbon in an alpine meadow on the Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2014, 81, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Li, B.; Li, W.; Wang, G.; Knops, J.M.H. Effects of nitrogen and phosphorus fertilization on soil carbon fractions in alpine meadows on the Qinghai-Tibetan Plateau. PLoS ONE 2014, 9, e103266. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Dong, S.; Shen, H.; Han, Y.; Zhang, J.; Xu, Y.; Gao, X.; Yang, M.; Li, Y.; Zhao, Z.; et al. Different responses of multifaceted plant diversities of alpine meadow and alpine steppe to nitrogen addition gradients on Qinghai-Tibetan Plateau. Sci. Total Environ. 2019, 688, 1405–1412. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, F.; Quan, Q.; Song, B.; Wang, J.; Zhou, Q.; Niu, S. Common species stability and species asynchrony rather than richness determine ecosystem stability under nitrogen enrichment. Ecosystems 2021, 24, 686–698. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, D.; Weber-Grullon, L.; Fu, H. Nitrogen deposition enhances plant-microbe interactions in a semiarid grassland: The role of soil physicochemical properties. Geoderma 2020, 373, 114446. [Google Scholar] [CrossRef]
- Zong, N.; Shi, P.; Song, M.; Zhang, X.; Jiang, J.; Chai, X. Nitrogen critical loads for an alpine meadow ecosystem on the Tibetan Plateau. Environ. Manage. 2016, 57, 531–542. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Lan, Z.; Hu, S.; Bai, Y. Soil acidification exerts a greater control on soil respiration than soil nitrogen availability in grasslands subjected to long-term nitrogen enrichment. Funct. Ecol. 2016, 30, 658–669. [Google Scholar] [CrossRef]
- Long, M.; Wu, H.; Smith, M.; Kimberly, J.; Pierre, L.; Lü, X.; Zhang, H.; Han, X.; Yu, Q. Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland. Plant Soil 2016, 408, 475–484. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, W.; Ying, P.; Feng, Y.; Zhang, J. Effects of exogenous nitrogen addition on soil nutrients and plant community biomass in alpine swamp meadow in the headwaters region of the Yangtze River. Acta Agrestia Sinica 2020, 28, 483–491, (In Chinese with English Abstract). [Google Scholar]
- Bai, T.; Wang, P.; Ye, C.; Hu, S. Form of nitrogen input dominates N effects on root growth and soil aggregation: A meta-analysis. Soil Biol. Biochem. 2021, 157, 108251. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2010, 189, 1040–1050. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, And Global Change; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; An, S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Zhang, C. Rhizosphere soil microbial properties on abandoned croplands in the Loess Plateau, China during vegetation succession. Eur. J. Soil Biol. 2012, 50, 127–136. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: Implications for China. Adv. Agron. 2010, 107, 1–32. [Google Scholar]
- Bell, C.; Carrillo, Y.; Boot, C.M.; Rocca, J.D.; Pendall, E.; Wallenstein, M.D. Rhizosphere Stoichiometry: Are C:N:P ratios of plants, soils, and enzymes conserved at the plant species-level? New Phytol. 2014, 201, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Zeng, Q.; Lal, R.; Chen, Y.; An, S. Soil, leaf and root ecological stoichiometry of Caragana korshinskii on the Loess Plateau of China in relation to plantation age. PLoS ONE 2017, 12, e0168890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, D.R.; Gautam, R.K.; Yousuf, B.; Mishra, A.; Jha, B. Nutrients, microbial community structure and functional gene abundance of rhizosphere and bulk soils of halophytes. Appl. Soil Ecol. 2015, 91, 16–26. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Q.; Zhan, W.; Xu, Y.; Zhu, E.; Gao, Y.; Li, S.; Zheng, Q.; Zhu, D.; He, Y.; et al. The linkage between vegetation and soil nutrients and their variation under different grazing intensities in an alpine meadow on the eastern Qinghai-Tibetan Plateau. Ecol. Eng. 2018, 110, 128–136. [Google Scholar] [CrossRef]
- Lü, C.; Tian, H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res-Atmos. 2007, 112, 229–238. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, S.; Pang, Z.; Wang, C.; Kardol, P.; Zhou, X.; Rui, Y.; Lan, Z.; Wang, Y.; Xu, X. Grazing modifies inorganic and organic nitrogen uptake by coexisting plant species in alpine grassland. Biol. Fert. Soils 2016, 52, 211–221. [Google Scholar] [CrossRef]
- Liu, X.; Ju, X.; Zhang, Y.; He, C.; Kopsch, J.; Zhang, F. Nitrogen deposition in agroecosystems in the Beijing area. Agr. Ecosys. Environ. 2006, 113, 370–377. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Chistie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, P.; Wu, N.; Yi, S.; Chen, H. Biomass and nitrogen responses to grazing intensity in an alpine meadow on the eastern Tibetan Plateau. Pol. J. Ecol. 2007, 55, 469–479. [Google Scholar]
- Zhang, J.; Peng, C.; Xue, W.; Yang, Z.; Yang, B.; Li, P.; Zhu, Q.; Wang, M. Soil CH4 and CO2 dynamics and nitrogen transformations with incubation in mountain forest and meadow ecosystems. Catena 2018, 163, 24–32. [Google Scholar] [CrossRef]
- Lan, Z.; Bai, Y. Testing mechanisms of N-enrichment induced species loss in a semiarid Inner Mongolia grassland: Critical thresholds and implications for long-term ecosystem responses. Philos. T. R. Soc. B 2012, 367, 3125–3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China's soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Nosengo, N. Fertilized to death. Nature 2002, 425, 894–895. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Q.; Bai, E.; Lü, X.; Li, Q.; Xia, J.; Kardol, P.; Liang, W.; Wang, Z.; Han, X. Nitrogen deposition weakens plant-microbe interactions in grassland ecosystems. Global Change Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef]
- Nakaji, T.; Fukami, M.; Dokiya, Y.; Izuta, T. Effects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus densiflora seedlings. Trees 2001, 15, 453–461. [Google Scholar] [CrossRef]
- Yang, Z.; Xiong, W.; Xu, Y.; Jiang, L.; Zhu, E.; Zhan, W.; He, Y.; Zhu, D.; Zhu, Q.; Peng, C.; et al. Soil properties and species composition under different grazing intensity in an alpine meadow on the eastern Tibetan Plateau, China. Environ. Monit. Assess. 2016, 188, 678. [Google Scholar] [CrossRef]
- Yue, K.; Fornara, D.A.; Li, W.; Ni, X.; Peng, Y.; Liao, S.; Tan, S.; Wang, D.; Wu, F.; Yang, Y. Nitrogen addition affects plant biomass allocation but not allometric relationships among different organs across the globe. J. Plant Ecol. 2021, 14, 361–371. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, R.F.; Farquhar, G.D. Plants increase CO2 uptake by assimilating nitrogen via the photorespiratory pathway. Nat. Plants 2018, 4, 46–54. [Google Scholar] [CrossRef]
- Yu, Q.; Elser, J.J.; He, N.; Wu, H.; Chen, Q.; Zhang, G.; Han, X. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 2011, 166, 1–10. [Google Scholar] [CrossRef]
- Bragazza, L.; Tahvanainen, T.; Kutnar, L.; Rydin., H.; Limpens, J.; Hájek, M.; Grosvernier, P.; Hájek, T.; Hajkova, P.; Hansen, I.; et al. Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen deposition in Europe. New Phytol. 2004, 163, 609–616. [Google Scholar] [CrossRef]
- Haynes, R.J. Active ion uptake and maintenance of cation-anion balance: A critical examination of their role in regulating rhizosphere pH. Plant Soil 1990, 126, 247–264. [Google Scholar] [CrossRef]
- Weng, B.; Xie, X.; Yang, J.; Liu, J.; Lu, H.; Yan, C. Research on the nitrogen cycle in rhizosphere of Kandelia obovata under ammonium and nitrate addition. Mar. Pollut. Bull. 2013, 76, 227–240. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef]
- Deforest, J.L.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Atmospheric nitrate deposition and enhanced dissolved organic carbon leaching. Soil Sci. Soc. Am. J. 2005, 69, 1233–1237. [Google Scholar] [CrossRef]
- Craig, S.; Zhu, W. Impacts of deicing salt and nitrogen addition on soil nitrogen and carbon cycling in a roadside ecosystem. Water Air Soil Poll. 2018, 229, 187. [Google Scholar] [CrossRef]
- Miegroet, H.V.; Johnson, D.W.; Cole, D.W. Soil nitrification as affected by N fertility and changes in forest floor C/N ratio in four forest soils. Can. J. Forest Res. 1990, 20, 1013–1019. [Google Scholar] [CrossRef]
- Jiao, F.; Shi, X.; Han, F.; Yuan, Z. Increasing aridity, temperature and soil pH induce soil C-N-P imbalance in grasslands. Sci. Rep-UK 2016, 6, 19601. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, Z.; Zhang, K.; Cornelissen, J.H.C. C:N:P stoichiometry of Artemisia species and close relatives across northern China: Unraveling effects of climate, soil and taxonomy. J Ecol. 2015, 103, 1020–1031. [Google Scholar] [CrossRef]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhou, G.; Zhang, D.; Liu, S.; Chu, G.; Yan, J. N and P stoichiometry of plant and soil in lower subtropical forest successional series in southern China. Chin. J. Plant Ecol. 2010, 34, 64–71, (In Chinese with English Abstract). [Google Scholar]
- Yang, Z.; Luo, Z. Nitrogen and phosphorus distribution and relationship in soils and plants under different aged Chinese fir plantation. Forests 2021, 12, 1271. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbia-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Di Palo, F.; Fornara, D.A. Plant and soil nutrient stoichiometry along primary ecological successions: Is there any link? PLoS ONE 2017, 12, e0182569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Liu, G.; Li, P.; Xue, S. Ecological stoichiometry of plant-soil-enzyme interactions drives secondary plant succession in the abandoned grasslands of Loess Plateau, China. Catena 2021, 202, 105302. [Google Scholar] [CrossRef]
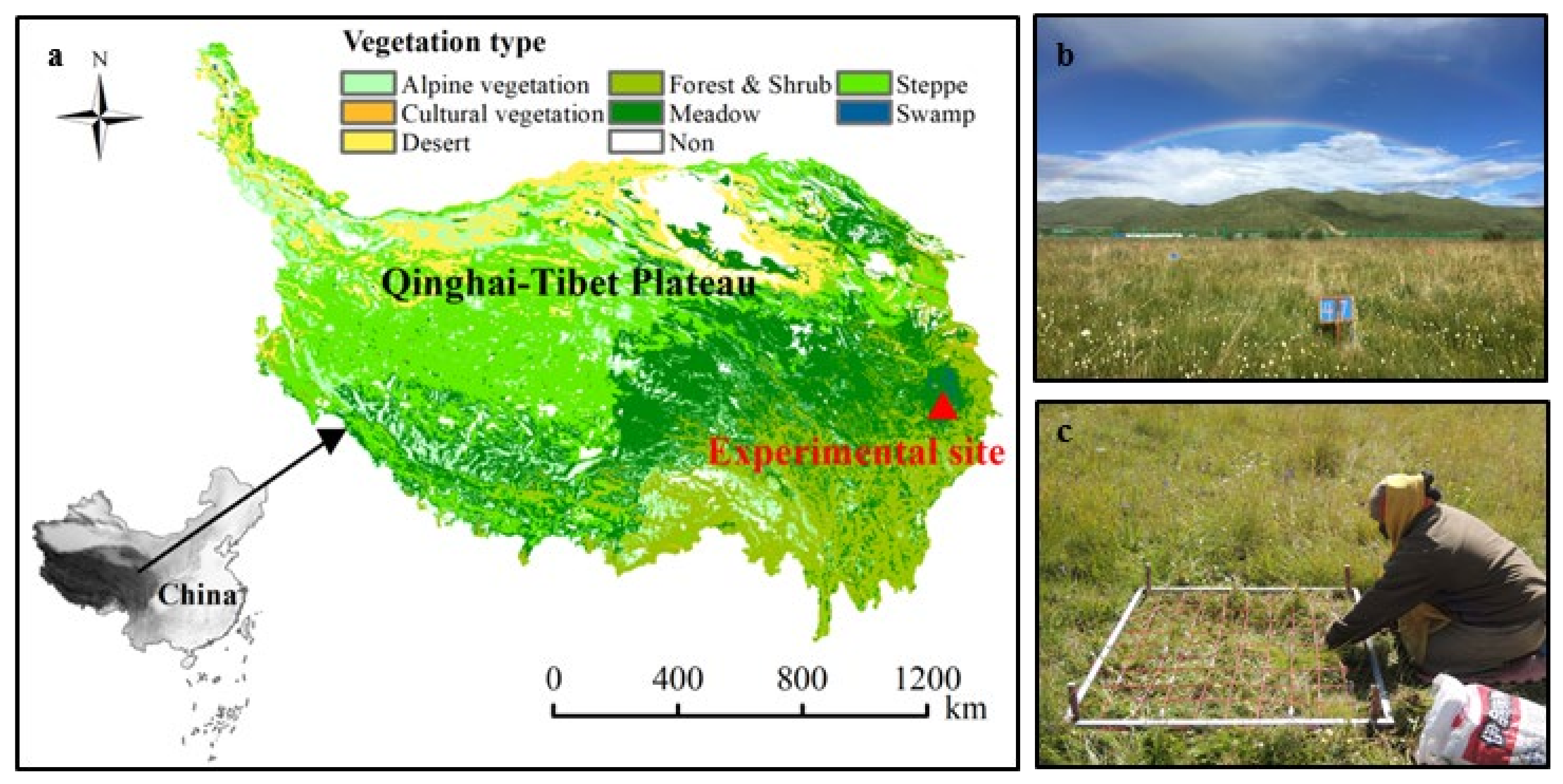



| Treatments | N Addition Rates (kg ha−1 y−1) | NH4Cl Used Amount (kg ha−1 y−1) |
|---|---|---|
| N0 | 0 | 0 |
| N2 | 20 | 76.43 |
| N4 | 40 | 152.86 |
| N8 | 80 | 305.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhan, W.; Jiang, L.; Chen, H. Effect of Short-Term Low-Nitrogen Addition on Carbon, Nitrogen and Phosphorus of Vegetation-Soil in Alpine Meadow. Int. J. Environ. Res. Public Health 2021, 18, 10998. https://doi.org/10.3390/ijerph182010998
Yang Z, Zhan W, Jiang L, Chen H. Effect of Short-Term Low-Nitrogen Addition on Carbon, Nitrogen and Phosphorus of Vegetation-Soil in Alpine Meadow. International Journal of Environmental Research and Public Health. 2021; 18(20):10998. https://doi.org/10.3390/ijerph182010998
Chicago/Turabian StyleYang, Zhen’an, Wei Zhan, Lin Jiang, and Huai Chen. 2021. "Effect of Short-Term Low-Nitrogen Addition on Carbon, Nitrogen and Phosphorus of Vegetation-Soil in Alpine Meadow" International Journal of Environmental Research and Public Health 18, no. 20: 10998. https://doi.org/10.3390/ijerph182010998
APA StyleYang, Z., Zhan, W., Jiang, L., & Chen, H. (2021). Effect of Short-Term Low-Nitrogen Addition on Carbon, Nitrogen and Phosphorus of Vegetation-Soil in Alpine Meadow. International Journal of Environmental Research and Public Health, 18(20), 10998. https://doi.org/10.3390/ijerph182010998





