The Interplay between Housing Environmental Attributes and Design Exposures and Psychoneuroimmunology Profile—An Exploratory Review and Analysis Paper in the Cancer Survivors’ Mental Health Morbidity Context
Abstract
1. Introduction
2. The Complexity of Mental Health and Comorbidities in Cancer Survivorship
3. COVID-19 Disruptive Changes Adding Hardship to Psychological Stressors
3.1. Substantial Changes of Ambient and Household Environmental Quality during COVID-19 Lockdown
3.2. Buffering Effect of Housing Conditions on Resident’s Mental Health during COVID-19 Lockdown
| Residents’ Location [Reference] | Home Environmental Attributes | Mental Health Outcomes |
|---|---|---|
| Portugal, Spain [18,19]; Italy [18,155]; France, Germany, United Kingdom, New Zealand, United States, Mexico [18]; Bulgaria [148]; Scotland [150]; Sweden [157]; Japan [149] |
|
|
| Portugal [18,19]; Italy [18,155]; France, Germany, Spain, United Kingdom, New Zealand, United States, Mexico [18]; Bulgaria [148]; Japan [149] |
|
|
| Spain [19,152]; Chile, Colombia, Brazil, Argentina, Mexico, United States, United Kingdom, France, Italy, Germany, Greece [152] |
|
|
| Italy [152,155]; Spain [22,152]; Chile, Colombia, Brazil, Argentina, Mexico, United States, United Kingdom, France, Germany, Greece [152] |
|
|
| Bulgaria [153] |
|
|
3.3. Housing Design and Constructive Characteristics Impact on Environmental Conditions
4. The Interconnectedness between Housing, Public Health and Primary Healthcare
4.1. New Models of Primary Care-Based Comprehensive Cancer Survivorship Care
4.2. Housing as an Essential Component for Sustainable Primary Care and Public Health Integration
5. The Role of Psychoneuroimmunology, Inflammation and Built Environment on Recovery following Post-Cancer Treatment
5.1. PNI Contributions in the Development and Neuroprogression of Mental Disorders
5.2. Dual Role of Multi-Level PNI Biomarkers—Clinical Information and Built Environment Exposure
5.3. Evidence of Intervention Trials and Therapeutic Approaches through Housing Design and Environmental Factors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, T.; Jia, X.; Shi, H.; Niu, J.; Yin, X.; Xie, J.; Wang, X. Prevalence of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Duddu, V.; Anyim, N.; Neelam, J.; Lewis, S. Pandemics and pre-existing mental illness: A systematic review and meta-analysis. Brain Behav. Immun. Health 2021, 10, 100177. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef]
- Brown, A.; Flint, S.W.; Kalea, A.Z.; O’Kane, M.; Williams, S.; Batterham, R.L. Negative impact of the first COVID-19 lockdown upon health-related behaviours and psychological wellbeing in people living with severe and complex obesity in the UK. EClinicalMedicine 2021, 34, 100796. [Google Scholar] [CrossRef] [PubMed]
- Medline, A.; Hayes, L.; Valdez, K.; Hayashi, A.; Vahedi, F.; Capell, W.; Sonnenberg, J.; Glick, Z.; Klausner, J.D. Evaluating the impact of stay-at-home orders on the time to reach the peak burden of Covid-19 cases and deaths: Does timing matter? BMC Public Health 2020, 20, 1750. [Google Scholar] [CrossRef] [PubMed]
- Tatapudi, H.; Das, R.; Das, T.K. Impact assessment of full and partial stay-at-home orders, face mask usage, and contact tracing: An agent-based simulation study of COVID-19 for an urban region. Glob. Epidemiol. 2020, 2, 100036. [Google Scholar] [CrossRef] [PubMed]
- Marroquín, B.; Vine, V.; Morgan, R. Mental health during the COVID-19 pandemic: Effects of stay-at-home policies, social distancing behavior, and social resources. Psychiatry Res. 2020, 293, 113419. [Google Scholar] [CrossRef]
- Jacobson, N.C.; Lekkas, D.; Price, G.; Heinz, M.V.; Song, M.; James O’Malley, A.; Barr, P.J. Flattening the mental health curve: COVID-19 stay-at-home orders are associated with alterations in mental health search behavior in the United States. JMIR Ment. Health 2020, 7, e19347. [Google Scholar] [CrossRef]
- Bartoszek, A.; Walkowiak, D.; Bartoszek, A.; Kardas, G. Mental well-being (Depression, loneliness, insomnia, daily life fatigue) during COVID-19 related home-confinement—A study from Poland. Int. J. Environ. Res. Public Health 2020, 17, 7417. [Google Scholar] [CrossRef]
- Ammar, A.; Mueller, P.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Brach, M.; Schmicker, M.; Bentlage, E.; et al. Psychological consequences of COVID-19 home confinement: The ECLB-COVID19 multicenter study. PLoS ONE 2020, 15, e0240204. [Google Scholar] [CrossRef]
- Tull, M.T.; Edmonds, K.A.; Scamaldo, K.M.; Richmond, J.R.; Rose, J.P.; Gratz, K.L. Psychological Outcomes Associated with Stay-at-Home Orders and the Perceived Impact of COVID-19 on Daily Life. Psychiatry Res. 2020, 289, 113098. [Google Scholar] [CrossRef]
- Yarrington, J.S.; Lasser, J.; Garcia, D.; Vargas, J.H.; Couto, D.D.; Marafon, T.; Craske, M.G.; Niles, A.N. Impact of the COVID-19 Pandemic on Mental Health among 157,213 Americans. J. Affect. Disord. 2021, 286, 64–70. [Google Scholar] [CrossRef]
- Liu, Y.; Mattke, S. Association between state stay-at-home orders and risk reduction behaviors and mental distress amid the COVID-19 pandemic. Prev. Med. 2020, 141, 106299. [Google Scholar] [CrossRef]
- Havnen, A.; Anyan, F.; Hjemdal, O.; Solem, S.; Riksfjord, M.G.; Hagen, K. Resilience moderates negative outcome from stress during the COVID-19 pandemic: A moderatedmediation approach. Int. J. Environ. Res. Public Health 2020, 17, 6461. [Google Scholar] [CrossRef]
- Röhr, S.; Reininghaus, U.; Riedel-Heller, S. Mental and social health in the German old age population largely unaltered during COVID-19 lockdown: Results of a representative survey. BMC Geriatr. 2020, 20, 489. [Google Scholar] [CrossRef]
- Prati, G.; Mancini, A.D. The psychological impact of COVID-19 pandemic lockdowns: A review and meta-analysis of longitudinal studies and natural experiments. Psychol. Med. 2021, 51, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.; Den Daas, C.; Johnston, M.; Murchie, P.; Thompson, C.W.; Dixon, D. Are rurality, area deprivation, access to outside space, and green space associated with mental health during the covid-19 pandemic? A cross sectional study (charis-e). Int. J. Environ. Res. Public Health 2021, 18, 3869. [Google Scholar] [CrossRef]
- Pouso, S.; Borja, Á.; Fleming, L.E.; Gómez-Baggethun, E.; White, M.P.; Uyarra, M.C. Contact with blue-green spaces during the COVID-19 pandemic lockdown beneficial for mental health. Sci. Total Environ. 2021, 756, 143984. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Triguero-Mas, M.; Jardim Santos, C.; Gómez-Nieto, A.; Cole, H.; Anguelovski, I.; Silva, F.M.; Baró, F. Exposure to nature and mental health outcomes during COVID-19 lockdown. A comparison between Portugal and Spain. Environ. Int. 2021, 154, 106664. [Google Scholar] [CrossRef]
- Poortinga, W.; Bird, N.; Hallingberg, B.; Phillips, R.; Williams, D. The role of perceived public and private green space in subjective health and wellbeing during and after the first peak of the COVID-19 outbreak. Landsc. Urban Plan. 2021, 211, 104092. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y. Which Risk Factors Matter More for Psychological Distress during the COVID-19 Pandemic? An Application Approach of Gradient Boosting Decision Trees. Int. J. Environ. Res. Public Health 2021, 18, 5879. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Ruiz-Jaramillo, J.; Cuerdo-vilches, T.; Joyanes-Diaz, M.D.; Montiel-Vega, L.; Cano-martos, V.; Navas-Martín, M.Á. Natural Lighting in Historic Houses during Times of Pandemic. The Case of Housing in the Mediterranean Climate. Int. J. Environ. Res. Public Health 2021, 18, 7264. [Google Scholar] [CrossRef]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Riera, R.; Bagattini, Â.M.; Pacheco, R.L.; Pachito, D.V.; Roitberg, F.; Ilbawi, A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Jammu, A.S.; Chasen, M.R.; Lofters, A.K.; Bhargava, R. Systematic rapid living review of the impact of the COVID-19 pandemic on cancer survivors: Update to August 27, 2020. Support. Care Cancer 2021, 29, 2841–2850. [Google Scholar] [CrossRef]
- Namin, S.; Zhou, Y.; Neuner, J.; Beyer, K. Neighborhood Characteristics and Cancer Survivorship: An Overview of the Current Literature on Neighborhood Landscapes and Cancer Care. Int. J. Environ. Res. Public Health 2021, 18, 7192. [Google Scholar] [CrossRef]
- Wray, A.J.D.; Minaker, L.M. Is cancer prevention influenced by the built environment? A multidisciplinary scoping review. Cancer 2019, 125, 3299–3311. [Google Scholar] [CrossRef]
- Namin, S.; Zhou, Y.; Neuner, J.; Beyer, K. The role of residential history in cancer research: A scoping review. Soc. Sci. Med. 2021, 270, 113657. [Google Scholar] [CrossRef]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 2021. [Google Scholar] [CrossRef]
- Parpa, E.; Tsilika, E.; Gennimata, V.; Mystakidou, K. Elderly cancer patients’ psychopathology: A systematic review aging and mental health. Arch. Gerontol. Geriatr. 2015, 60, 9–15. [Google Scholar] [CrossRef]
- Huang, W.K.; Juang, Y.Y.; Chung, C.C.; Chang, S.H.; Chang, J.W.C.; Lin, Y.C.; Wang, H.M.; Chang, H.K.; Chen, J.S.; Tsai, C.S.; et al. Timing and risk of mood disorders requiring psychotropics in long-term survivors of adult cancers: A nationwide cohort study. J. Affect. Disord. 2018, 236, 80–87. [Google Scholar] [CrossRef]
- Ji, X.; Cummings, J.R.; Gilleland Marchak, J.; Han, X.; Mertens, A.C. Mental Health Among Nonelderly Adult Cancer Survivors: A National Estimate. Cancer 2020, 126, 3768–3776. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Ferguson, D.W.; Gill, J.; Paul, J.; Symonds, P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 721–732. [Google Scholar] [CrossRef]
- Carreira, H.; Williams, R.; Müller, M.; Harewood, R.; Stanway, S.; Bhaskaran, K. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J. Natl. Cancer Inst. 2018, 110, djy177. [Google Scholar] [CrossRef] [PubMed]
- Osazuwa-Peters, N.; Simpson, M.C.; Zhao, L.; Boakye, E.A.; Olomukoro, S.I.; Deshields, T.; Loux, T.M.; Varvares, M.A.; Schootman, M. Suicide Risk Among Cancer Survivors: Head and Neck Versus Other Cancers. Cancer 2018, 124, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Recklitis, C.J.; Zhou, E.S.; Zwemer, E.K.; Hu, J.C.; Kantoff, P.W. Suicidal Ideation in Prostate Cancer Survivors: Understanding the Role of Physical and Psychological Health Outcomes. Cancer 2014, 120, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Shi, H.Y.; Yu, H.R.; Liu, X.M.; Jin, X.H.; Yan-Qian; Fu, X.L.; Song, Y.P.; Cai, J.Y.; Chen, H.L. Incidence of suicide death in patients with cancer: A systematic review and meta-analysis. J. Affect. Disord. 2020, 276, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, H.J.G.; Gielissen, M.F.M.; Verhagen, C.; Knoop, H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: A systematic review. Clin. Psychol. Rev. 2018, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leysen, L.; Lahousse, A.; Nijs, J.; Adriaenssens, N.; Mairesse, O.; Ivakhnov, S.; Bilterys, T.; Van Looveren, E.; Pas, R.; Beckwée, D. Prevalence and risk factors of sleep disturbances in breast cancer survivors: Systematic review and meta-analyses. Support. Care Cancer 2019, 27, 4401–4433. [Google Scholar] [CrossRef]
- Bamonti, P.M.; Moye, J.; Naik, A.D. Pain is associated with continuing depression in cancer survivors. Psychol. Health Med. 2018, 23, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.; Lu, L.; Drummond, F.J.; Gavin, A.; Sharp, L. Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Support. Care Cancer 2020, 28, 4813–4824. [Google Scholar] [CrossRef] [PubMed]
- Reynolds-Cowie, P.; Fleming, L. Living with persistent insomnia after cancer: A qualitative analysis of impact and management. Br. J. Health Psychol. 2021, 26, 33–49. [Google Scholar] [CrossRef]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Renzi, C.; Rubin, G.P.; Lyratzopoulos, G. The prevalence of chronic conditions in patients diagnosed with one of 29 common and rarer cancers: A cross-sectional study using primary care data. Cancer Epidemiol. 2020, 69, 101845. [Google Scholar] [CrossRef]
- Leach, C.R.; Weaver, K.E.; Aziz, N.M.; Alfano, C.M.; Bellizzi, K.M.; Kent, E.E.; Forsythe, L.P.; Rowland, J.H. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. J. Cancer Surviv. 2015, 9, 239–251. [Google Scholar] [CrossRef]
- Kenzik, K.M.; Kent, E.E.; Martin, M.Y.; Bhatia, S.; Pisu, M. Chronic condition clusters and functional impairment in older cancer survivors: A population-based study. J. Cancer Surviv. 2016, 10, 1096–1103. [Google Scholar] [CrossRef]
- Siembida, E.J.; Smith, A.W.; Potosky, A.L.; Graves, K.D.; Jensen, R.E. Examination of individual and multiple comorbid conditions and health-related quality of life in older cancer survivors. Qual. Life Res. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Götze, H.; Taubenheim, S.; Dietz, A.; Lordick, F.; Mehnert, A. Comorbid conditions and health-related quality of life in long-term cancer survivors—associations with demographic and medical characteristics. J. Cancer Surviv. 2018, 12, 712–720. [Google Scholar] [CrossRef]
- Keats, M.R.; Cui, Y.; DeClercq, V.; Grandy, S.A.; Sweeney, E.; Dummer, T.J.B. Burden of multimorbidity and polypharmacy among cancer survivors: A population-based nested case–control study. Support. Care Cancer 2021, 29, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.F.; Schiltz, N.K.; Stange, K.C.; Given, C.W.; Owusu, C.; Berger, N.A.; Koroukian, S.M. Complex multimorbidity and health outcomes in older adult cancer survivors. Fam. Med. Community Health 2017, 5, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Holmes, H.M.; Nguyen, H.T.; Nayak, P.; Oh, J.H.; Escalante, C.P.; Elting, L.S. Chronic conditions and health status in older cancer survivors. Eur. J. Intern. Med. 2014, 25, 374–378. [Google Scholar] [CrossRef]
- Jansana, A.; Poblador-Plou, B.; Gimeno-Miguel, A.; Lanzuela, M.; Prados-Torres, A.; Domingo, L.; Comas, M.; Sanz-Cuesta, T.; del Cura-Gonzalez, I.; Ibañez, B.; et al. Multimorbidity clusters among long-term breast cancer survivors in Spain: Results of the SURBCAN Study. Int. J. Cancer 2021, 149, 1755–1767. [Google Scholar] [CrossRef]
- Diederichs, C.; Berger, K.; Bartels, D.B. The measurement of multiple chronic diseases—A systematic review on existing multimorbidity indices. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66 A, 301–311. [Google Scholar] [CrossRef]
- Xu, X.; Mishra, G.D.; Jones, M. Evidence on multimorbidity from definition to intervention: An overview of systematic reviews. Ageing Res. Rev. 2017, 37, 53–68. [Google Scholar] [CrossRef]
- Lee, E.S.; Koh, H.L.; Ho, E.Q.Y.; Teo, S.H.; Wong, F.Y.; Ryan, B.L.; Fortin, M.; Stewart, M. Systematic review on the instruments used for measuring the association of the level of multimorbidity and clinically important outcomes. BMJ Open 2021, 11, e041219. [Google Scholar] [CrossRef]
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health 2019, 29, 182–189. [Google Scholar] [CrossRef]
- Le Reste, J.Y.; Nabbe, P.; Manceau, B.; Lygidakis, C.; Doerr, C.; Lingner, H.; Czachowski, S.; Munoz, M.; Argyriadou, S.; Claveria, A.; et al. The European General Practice Research Network Presents a Comprehensive Definition of Multimorbidity in Family Medicine and Long Term Care, Following a Systematic Review of Relevant Literature. J. Am. Med. Dir. Assoc. 2013, 14, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, T.G.; Bebe, A.; Køster-Rasmussen, R.; Jarbøl, D.E.; Guassora, A.D.; Waldorff, F.B.; Reventlow, S.; Olivarius, N. de F. The role of diseases, risk factors and symptoms in the definition of multimorbidity—A systematic review. Scand. J. Prim. Health Care 2016, 34, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, T.K.; Andersen, E.A.W.; Winther, J.F.; Bidstrup, P.E.; Borre, M.; Møller, H.; Larsen, S.B.; Johansen, C.; Dalton, S.O. Long-term Somatic Disease Risk in Adult Danish Cancer Survivors. JAMA Oncol. 2019, 5, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.F.; Fu, M.R.; Merlin, J.S.; Paice, J.A.; Bernacki, R.; Lee, C.; Wood, L.J. Exploring Factors Associated with Long-Term Opioid Therapy in Cancer Survivors: An Integrative Review. J. Pain Symptom Manag. 2020, 61, 395–415. [Google Scholar] [CrossRef]
- Murphy, C.C.; Fullington, H.M.; Alvarez, C.A.; Betts, A.C.; Lee, S.J.C.; Haggstrom, D.A.; Halm, E.A. Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer 2018, 124, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.A.; Soman, A.; Lunsford, N.B.; Leadbetter, S.; Rodriguez, J.L. Use of medications for treating anxiety and depression in cancer survivors in the United States. J. Clin. Oncol. 2017, 35, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, C.; Forsythe, L.; Lerro, C.; Soni, A. Mental health services utilization and expenditures associated with cancer survivorship in the United States. J. Cancer Surviv. 2015, 9, 50–58. [Google Scholar] [CrossRef][Green Version]
- Costa, A.R.; Alves, L.; Lunet, N. Healthcare services and medication use among cancer survivors and their partners: A cross-sectional analysis of 16 European countries. J. Cancer Surviv. 2020, 14, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Treanor, C.; Donnelly, M. An international review of the patterns and determinants of health service utilisation by adult cancer survivors. BMC Health Serv. Res. 2012, 12, 316. [Google Scholar] [CrossRef]
- Palladino, R.; Lee, J.T.; Ashworth, M.; Triassi, M.; Millett, C. Associations between multimorbidity, healthcare utilisation and health status: Evidence from 16 European countries. Age Ageing 2016, 45, 431–435. [Google Scholar] [CrossRef]
- Glynn, L.G.; Valderas, J.M.; Healy, P.; Burke, E.; Newell, J.; Gillespie, P.; Murphy, A.W. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam. Pract. 2011, 28, 516–523. [Google Scholar] [CrossRef]
- Bähler, C.; Huber, C.A.; Brüngger, B.; Reich, O. Multimorbidity, health care utilization and costs in an elderly community-dwelling population: A claims data based observational study. BMC Health Serv. Res. 2015, 15, 23. [Google Scholar] [CrossRef]
- Van Oostrom, S.H.; Picavet, H.S.J.; De Bruin, S.R.; Stirbu, I.; Korevaar, J.C.; Schellevis, F.G.; Baan, C.A. Multimorbidity of chronic diseases and health care utilization in general practice. BMC Fam. Pract. 2014, 15, 61. [Google Scholar] [CrossRef]
- Violán, C.; Foguet-boreu, Q.; Roso-llorach, A.; Rodriguez-blanco, T.; Pons-vigués, M.; Pujol-ribera, E.; Muñoz-pérez, M.Á.; Valderas, J.M. Burden of multimorbidity, socioeconomic status and use of health services across stages of life in urban areas: A cross-sectional study. BMC Public Health 2014, 14, 530. [Google Scholar] [CrossRef]
- Soley-Bori, M.; Ashworth, M.; Bisquera, A.; Dodhia, H.; Lynch, R.; Wang, Y.; Fox-Rushby, J. Impact of multimorbidity on healthcare costs and utilisation: A systematic review of the UK literature. Br. J. Gen. Pract. 2021, 71, e39–e46. [Google Scholar] [CrossRef]
- Kim, J.; Keshavjee, S.; Atun, R. Trends, patterns and health consequences of multimorbidity among South Korea adults: Analysis of nationally representative survey data 2007–2016. J. Glob. Health 2020, 10, 020426. [Google Scholar] [CrossRef]
- Hopman, P.; Heins, M.J.; Korevaar, J.C.; Rijken, M.; Schellevis, F.G. Health care utilization of patients with multiple chronic diseases in the Netherlands: Differences and underlying factors. Eur. J. Intern. Med. 2016, 35, 44–50. [Google Scholar] [CrossRef]
- Déruaz-Luyet, A.; N’Goran, A.A.; Senn, N.; Bodenmann, P.; Pasquier, J.; Widmer, D.; Tandjung, R.; Rosemann, T.; Frey, P.; Streit, S.; et al. Multimorbidity and patterns of chronic conditions in a primary care population in Switzerland: A cross-sectional study. BMJ Open 2017, 7, e013664. [Google Scholar] [CrossRef] [PubMed]
- Cassell, A.; Edwards, D.; Harshfield, A.; Rhodes, K.; Brimicombe, J.; Payne, R.; Griffin, S. The epidemiology of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 2018, 68, e245–e251. [Google Scholar] [CrossRef] [PubMed]
- Violan, C.; Foguet-Boreu, Q.; Flores-Mateo, G.; Salisbury, C.; Blom, J.; Freitag, M.; Glynn, L.; Muth, C.; Valderas, J.M. Prevalence, Determinants and Patterns of Multimorbidity in Primary Care: A Systematic Review of Observational Studies. PLoS ONE 2014, 9, e102149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Edwards, D.; Mant, J.; Payne, R.A.; Kiddle, S. Characteristics, service use and mortality of clusters of multimorbid patients in England: A population-based study. BMC Med. 2020, 18, 78. [Google Scholar] [CrossRef]
- Huang, I.C.; Hudson, M.M.; Robison, L.L.; Krull, K.R. Differential impact of symptom prevalence and chronic conditions on quality of life in cancer survivors and non-cancer individuals: A population study. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1124–1132. [Google Scholar] [CrossRef]
- Kenzik, K.; Richman, J.; Kent, E.E.; Pisu, M.; Bhatia, S. Impact of precancer multimorbidity on survival and functional outcomes after cancer in old patients. J. Clin. Oncol. 2016, 34, 291. [Google Scholar] [CrossRef]
- Luque-Fernandez, M.A.; Gonçalves, K.; Salamanca-Fernández, E.; Redondo-Sanchez, D.; Lee, S.F.; Rodríguez-Barranco, M.; Carmona-García, M.C.; Marcos-Gragera, R.; Sánchez, M.J. Multimorbidity and short-term overall mortality among colorectal cancer patients in Spain: A population-based cohort study. Eur. J. Cancer 2020, 129, 4–14. [Google Scholar] [CrossRef]
- Gilbertson-White, S.; Srivastava, S.; Li, Y.; Laures, E.; Saeidzadeh, S.; Yeung, C.; Chae, S. Multimorbidity and Cancer: Using Electronic Health Record (EHR) Data to Cluster Patients in Multimorbidity Phenotypes (GP735). J. Pain Symptom Manag. 2020, 60, 266. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Roso-Llorach, A.; Fernández, S.; Guisado-Clavero, M.; Violán, C.; Onder, G.; Fratiglioni, L.; Calderón-Larrañaga, A.; Marengoni, A. Twelve-year clinical trajectories of multimorbidity in a population of older adults. Nat. Commun. 2020, 11, 3223. [Google Scholar] [CrossRef]
- Molassiotis, A.; Yates, P.; Li, Q.; So, W.K.W.; Pongthavornkamol, K.; Pittayapan, P.; Komatsu, H.; Thandar, M.; Yi, M.; Titus Chacko, S.; et al. Mapping unmet supportive care needs, quality-of-life perceptions and current symptoms in cancer survivors across the Asia-Pacific region: Results from the International STEP Study. Ann. Oncol. 2017, 28, 2552–2558. [Google Scholar] [CrossRef]
- Shakeel, S.; Tung, J.; Rahal, R.; Finley, C. Evaluation of Factors Associated with Unmet Needs in Adult Cancer Survivors in Canada. JAMA Netw. Open 2020, 3, e200506. [Google Scholar] [CrossRef]
- Moreno, P.I.; Ramirez, A.G.; San Miguel-Majors, S.L.; Castillo, L.; Fox, R.S.; Gallion, K.J.; Munoz, E.; Estabrook, R.; Perez, A.; Lad, T.; et al. Unmet supportive care needs in Hispanic/Latino cancer survivors: Prevalence and associations with patient-provider communication, satisfaction with cancer care, and symptom burden. Support. Care Cancer 2019, 27, 1383–1394. [Google Scholar] [CrossRef]
- Swash, B.; Bramwell, R.; Hulbert-Williams, N.J. Unmet psychosocial supportive care needs and psychological distress in haematological cancer survivors: The moderating role of psychological flexibility. J. Context. Behav. Sci. 2017, 6, 187–194. [Google Scholar] [CrossRef]
- Hoekstra, R.A.; Heins, M.J.; Korevaar, J.C. Health care needs of cancer survivors in general practice: A systematic review. BMC Fam. Pract. 2014, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Katayama, K.; Yoshioka, T.; Narimatsu, H. Impact of individual background on the unmet needs of cancer survivors and caregivers—A mixed-methods analysis. BMC Cancer 2020, 20, 263. [Google Scholar] [CrossRef] [PubMed]
- Recklitis, C.J.; Syrjala, K.L. Provision of integrated psychosocial services for cancer survivors post-treatment. Lancet Oncol. 2017, 18, e39–e50. [Google Scholar] [CrossRef]
- La Cour, K.; Cutchin, M.P. Developing community based rehabilitation for cancer survivors: Organizing for coordination and coherence in practice. BMC Health Serv. Res. 2013, 13, 339. [Google Scholar] [CrossRef]
- Chen, X.; Gong, X.; Shi, C.; Sun, L.; Tang, Z.; Yuan, Z.; Wang, J.; Yu, J. Multi-focused psychosocial residential rehabilitation interventions improve quality of life among cancer survivors: A community-based controlled trial. J. Transl. Med. 2018, 16, 250. [Google Scholar] [CrossRef]
- Smith, S.M.; Wallace, E.; O’Dowd, T.; Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst. Rev. 2021, CD006560. [Google Scholar] [CrossRef]
- Islam, J.Y.; Vidot, D.C.; Camacho-Rivera, M. Evaluating Mental Health–Related Symptoms Among Cancer Survivors During the COVID-19 Pandemic: An Analysis of the COVID Impact Survey. JCO Oncol. Pract. 2021, OP.20.00752. [Google Scholar] [CrossRef]
- Han, J.; Zhou, F.; Zhang, L.; Su, Y.; Mao, L. Psychological symptoms of cancer survivors during the COVID-19 outbreak: A longitudinal study. Psychooncology 2021, 30, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Mazariego, C.; Li, Z.; Canfell, K.; Miller, A.; Koczwara, B.; Shaw, J.; Taylor, N. Psychosocial impact of COVID-19 on cancer patients, survivors, and carers in Australia: A real-time assessment of cancer support services. Support. Care Cancer 2021, 1–11. [Google Scholar] [CrossRef]
- Leach, C.R.; Kirkland, E.G.; Masters, M.; Sloan, K.; Rees-Punia, E.; Patel, A.V.; Watson, L. Cancer survivor worries about treatment disruption and detrimental health outcomes due to the COVID-19 pandemic. J. Psychosoc. Oncol. 2021, 29, 5463–5473. [Google Scholar] [CrossRef]
- Amaniera, I.; Bach, C.; Vachani, C.; Hampshire, M.; Arnold-Korzeniowski, K.; Healy, M.; Rodriguez, A.; Misher, C.; Kendrick, L.; Metz, J.M.; et al. Psychosocial impact of the COVID-19 pandemic on cancer patients, survivors and caregivers. J. Psychosoc. Oncol. 2021, 39, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.E.; Kempski, K.A.; Galantino, M.L.A. Effect of disrupted rehabilitation services on distress and quality of life in breast cancer survivors during the COVID-19 pandemic. Rehabil. Oncol. 2020, 38, 153–158. [Google Scholar] [CrossRef]
- Carreira, H.; Strongman, H.; Peppa, M.; McDonald, H.I.; dos-Santos-Silva, I.; Stanway, S.; Smeeth, L.; Bhaskaran, K. Prevalence of COVID-19-related risk factors and risk of severe influenza outcomes in cancer survivors: A matched cohort study using linked English electronic health records data. EClinicalMedicine 2020, 29–30, 100656. [Google Scholar] [CrossRef]
- Walker, J.L.; Grint, D.J.; Strongman, H.; Eggo, R.M.; Peppa, M.; Minassian, C.; Mansfield, K.E.; Rentsch, C.T.; Douglas, I.J.; Mathur, R.; et al. UK prevalence of underlying conditions which increase the risk of severe COVID-19 disease: A point prevalence study using electronic health records. BMC Public Health 2021, 21, 484. [Google Scholar] [CrossRef]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Tisminetzky, M.; Delude, C.; Hebert, T.; Carr, C.; Goldberg, R.J.; Gurwitz, J.H. Age, Multiple Chronic Conditions, and COVID-19: A Literature Review. J. Gerontol. A. Biol. Sci. Med. Sci. 2020, glaa320. [Google Scholar] [CrossRef]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2021, 11, 571416. [Google Scholar] [CrossRef]
- Nidadavolu, L.S.; Walston, J.D. Underlying Vulnerabilities to the Cytokine Storm and Adverse COVID-19 Outcomes in the Aging Immune System. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e13–e18. [Google Scholar] [CrossRef]
- Derosa, L.; Melenotte, C.; Griscelli, F.; Gachot, B.; Marabelle, A.; Kroemer, G.; Zitvogel, L. The immuno-oncological challenge of COVID-19. Nat. Cancer 2020, 1, 946–964. [Google Scholar] [CrossRef]
- Danhieux, K.; Buffel, V.; Pairon, A.; Benkheil, A.; Remmen, R.; Wouters, E.; van Olmen, J. The impact of COVID-19 on chronic care according to providers: A qualitative study among primary care practices in Belgium. BMC Fam. Pract. 2020, 21, 255. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Gillies, C.L.; Zaccardi, F.; Coles, B.; Davies, M.J.; Seidu, S.; Khunti, K. Impact of COVID-19 on routine care for chronic diseases: A global survey of views from healthcare professionals. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 965–967. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pulse Survey on Continuity of Essential Health Services during the COVID-19 Pandemic. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1 (accessed on 15 April 2021).
- Mansfield, K.E.; Mathur, R.; Tazare, J.; Henderson, A.D.; Mulick, A.R.; Carreira, H.; Matthews, A.A.; Bidulka, P.; Gayle, A.; Forbes, H.; et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: A population-based study. Lancet Digit. Health 2021, 3, e217–e230. [Google Scholar] [CrossRef]
- Chan, A.; Ashbury, F.; Fitch, M.I.; Koczwara, B.; Chan, R.J. Cancer survivorship care during COVID-19—Perspectives and recommendations from the MASCC survivorship study group. Support. Care Cancer 2020, 28, 3485–3488. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Steeg, S.; Webb, R.T.; Kapur, N.; Chew-Graham, C.A.; Abel, K.M.; Hope, H.; Pierce, M.; Ashcroft, D.M. Effects of the COVID-19 pandemic on primary care-recorded mental illness and self-harm episodes in the UK: A population-based cohort study. Lancet Public Health 2021, 6, e124–e135. [Google Scholar] [CrossRef]
- Pierce, M.; Hope, H.; Ford, T.; Hatch, S.; Hotopf, M.; John, A.; Kontopantelis, E.; Webb, R.; Wessely, S.; McManus, S.; et al. Mental health before and during the COVID-19 pandemic: A longitudinal probability sample survey of the UK population. Lancet Psychiatry 2020, 7, 883–892. [Google Scholar] [CrossRef]
- O’Connor, R.C.; Wetherall, K.; Cleare, S.; McClelland, H.; Melson, A.J.; Niedzwiedz, C.L.; O’Carroll, R.E.; O’Connor, D.B.; Platt, S.; Scowcroft, E.; et al. Mental health and well-being during the COVID-19 pandemic: Longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. Br. J. Psychiatry 2021, 218, 326–333. [Google Scholar] [CrossRef]
- Kaul, S.; Avila, J.C.; Mutambudzi, M.; Russell, H.; Kirchhoff, A.C.; Schwartz, C.L. Mental distress and health care use among survivors of adolescent and young adult cancer: A cross-sectional analysis of the National Health Interview Survey. Cancer 2017, 123, 869–878. [Google Scholar] [CrossRef]
- Akbari, P.; Yazdanfar, S.-A.; Hosseini, S.-B.; Norouzian-Maleki, S. Housing and mental health during outbreak of COVID-19. J. Build. Eng. 2021, 43, 102919. [Google Scholar] [CrossRef]
- Guzman, V.; Garrido-Cumbrera, M.; Braçe, O.; Hewlett, D.; Foley, R. Associations of the natural and built environment with mental health and wellbeing during COVID-19: Irish perspectives from the GreenCOVID study. Lancet Glob. Health 2021, 9, S20. [Google Scholar] [CrossRef]
- Amerio, A.; Brambilla, A.; Morganti, A.; Aguglia, A.; Bianchi, D.; Santi, F.; Costantini, L.; Odone, A.; Costanza, A.; Signorelli, C.; et al. COVID-19 Lockdown: Housing Built Environment’s Effects on Mental Health. Int. J. Environ. Res. Public Health 2020, 17, 5973. [Google Scholar] [CrossRef]
- National Housing Federation. Housing Issues during Lockdown: Health, Space and Overcrowding; National Housing Federation: London, UK, 2020. [Google Scholar]
- Groot, J.; Keller, A.C.; Joensen, A.; Nguyen, T.L.; Nybo Andersen, A.M.; Strandberg-Larsen, K. Impact of housing conditions on changes in youth’s mental health following the initial national COVID-19 lockdown: A cohort study. medRxiv Prepr. Serv. Health Sci. 2020. [Google Scholar] [CrossRef]
- Zarrabi, M.; Yazdanfar, S.-A.; Hosseini, S.-B. COVID-19 and healthy home preferences: The case of apartment residents in Tehran. J. Build. Eng. 2021, 35, 102021. [Google Scholar] [CrossRef]
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. USA 2020, 117, 18984–18990. [Google Scholar] [CrossRef]
- He, C.; Hong, S.; Zhang, L.; Mu, H.; Xin, A.; Zhou, Y.; Liu, J.; Liu, N.; Su, Y.; Tian, Y.; et al. Global, continental, and national variation in PM2.5, O3, and NO2 concentrations during the early 2020 COVID-19 lockdown. Atmos. Pollut. Res. 2021, 12, 136–145. [Google Scholar] [CrossRef]
- Chossière, G.P.; Xu, H.; Dixit, Y.; Isaacs, S.; Eastham, S.D.; Allroggen, F.; Speth, R.L.; Barrett, S.R.H. Air pollution impacts of COVID-19–related containment measures. Sci. Adv. 2021, 7, eabe1178. [Google Scholar] [CrossRef] [PubMed]
- Briz-Redón, Á.; Belenguer-Sapiña, C.; Serrano-Aroca, Á. Changes in air pollution during COVID-19 lockdown in Spain: A multi-city study. J. Environ. Sci. (China) 2021, 101, 16–26. [Google Scholar] [CrossRef]
- Sicard, P.; De Marco, A.; Agathokleous, E.; Feng, Z.; Xu, X.; Paoletti, E.; Rodriguez, J.J.D.; Calatayud, V. Amplified ozone pollution in cities during the COVID-19 lockdown. Sci. Total Environ. 2020, 735, 139542. [Google Scholar] [CrossRef] [PubMed]
- Velders, G.J.M.; Willers, S.M.; Wesseling, J.; van den Elshout, S.; van der Swaluw, E.; Mooibroek, D.; van Ratingen, S. Improvements in air quality in the Netherlands during the corona lockdown based on observations and model simulations. Atmos. Environ. 2021, 247, 118158. [Google Scholar] [CrossRef]
- Jephcote, C.; Hansell, A.L.; Adams, K.; Gulliver, J. Changes in air quality during COVID-19 ‘lockdown’ in the United Kingdom. Environ. Pollut. 2021, 272, 116011. [Google Scholar] [CrossRef] [PubMed]
- Higham, J.E.; Ramírez, C.A.; Green, M.A.; Morse, A.P. UK COVID-19 lockdown: 100 days of air pollution reduction? Air Qual. Atmos. Health 2021, 14, 325–332. [Google Scholar] [CrossRef]
- Fan, L.; Fu, S.; Wang, X.; Fu, Q.; Jia, H.; Xu, H.; Qin, G.; Hu, X.; Cheng, J. Spatiotemporal variations of ambient air pollutants and meteorological influences over typical urban agglomerations in China during the COVID-19 lockdown. J. Environ. Sci. 2021, 106, 26–38. [Google Scholar] [CrossRef]
- Nigam, R.; Pandya, K.; Luis, A.J.; Sengupta, R.; Kotha, M. Positive effects of COVID-19 lockdown on air quality of industrial cities (Ankleshwar and Vapi) of Western India. Sci. Rep. 2021, 11, 4285. [Google Scholar] [CrossRef]
- Roy, S.; Saha, M.; Dhar, B.; Pandit, S.; Nasrin, R. Geospatial analysis of COVID-19 lockdown effects on air quality in the South and Southeast Asian region. Sci. Total Environ. 2021, 756, 144009. [Google Scholar] [CrossRef]
- Mashayekhi, R.; Pavlovic, R.; Racine, J.; Moran, M.D.; Manseau, P.M.; Duhamel, A.; Katal, A.; Miville, J.; Niemi, D.; Peng, S.J.; et al. Isolating the impact of COVID-19 lockdown measures on urban air quality in Canada. Air Qual. Atmos. Health 2021, 18, 1–22. [Google Scholar] [CrossRef]
- Liu, Q.; Harris, J.T.; Chiu, L.S.; Sun, D.; Houser, P.R.; Yu, M.; Duffy, D.Q.; Little, M.M.; Yang, C. Spatiotemporal impacts of COVID-19 on air pollution in California, USA. Sci. Total Environ. 2021, 750, 141592. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.A.; Chien, L.C.; Li, Y.; Lin, G. Nonuniform impacts of COVID-19 lockdown on air quality over the United States. Sci. Total Environ. 2020, 745, 141105. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Paniagua, I.Y.; Valdez, S.I.; Almanza, V.; Rivera-Cárdenas, C.; Grutter, M.; Stremme, W.; García-Reynoso, A.; Ruiz-Suárez, L.G. Impact of the COVID-19 Lockdown on Air Quality and Resulting Public Health Benefits in the Mexico City Metropolitan Area. Front. Public Health 2021, 9, 642630. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, G. Indoor Air Pollution was Nonnegligible during COVID-19 Lockdown. Aerosol Air Qual. Res. 2020, 20, 1851–1855. [Google Scholar] [CrossRef]
- Mousavi, A.; Wu, J. Indoor-Generated PM2.5 During COVID-19 Shutdowns Across California: Application of the PurpleAir Indoor-Outdoor Low-Cost Sensor Network. Environ. Sci. Technol. 2021, 55, 5648–5656. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Casari, L.; Demaria, G.; Russo, M. Indoor Air Quality in Domestic Environments during Periods Close to Italian COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 4060. [Google Scholar] [CrossRef]
- Wang, F.; Du, W.; Lv, S.; Ding, Z.; Wang, G. Spatial and Temporal Distributions and Sources of Anthropogenic NMVOCs in the Atmosphere of China: A Review. Adv. Atmos. Sci. 2021, 38, 1085–1100. [Google Scholar] [CrossRef]
- Domínguez-Amarillo, S.; Fernández-Agüera, J.; Cesteros-García, S.; González-Lezcano, R.A. Bad air can also kill: Residential indoor air quality and pollutant exposure risk during the COVID-19 crisis. Int. J. Environ. Res. Public Health 2020, 17, 7183. [Google Scholar] [CrossRef]
- Tahmasebi, F.; Cooper, E.; Godoy-Shimizu, D.; Stamp, S. Is home working? CIBSE J. 2020, 24–25. Available online: http://portfolio.cpl.co.uk/CIBSE/202012/24/ (accessed on 18 April 2021).
- Zheng, G.; Filippelli, G.M.; Salamova, A. Increased Indoor Exposure to Commonly Used Disinfectants during the COVID-19 Pandemic. Environ. Sci. Technol. Lett. 2020, 7, 760–765. [Google Scholar] [CrossRef]
- Braithwaite, I.; Zhang, S.; Kirkbride, J.B.; Osborn, D.P.J.; Hayes, J.F. Air Pollution (Particulate matter) Exposure and Associations with Depression, Anxiety, Bipolar, Psychosis and Suicide Risk: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2019, 127, 126002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, W.; Gu, X.; Deng, F.; Wang, X.; Lin, H.; Guo, X.; Wu, S. Association between particulate matter air pollution and risk of depression and suicide: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 9029–9049. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.C.; Ezzati, M.; Marshall, J.D.; Robinson, A.L.; Burnett, R.T.; Pope, C.A. Fine Particulate Matter Air Pollution and Mortality Risk Among US Cancer Patients and Survivors. JNCI Cancer Spectr. 2021, 5, pkab001. [Google Scholar] [CrossRef]
- Edwards, L.; Rutter, G.; Iverson, L.; Wilson, L.; Chadha, T.S.; Wilkinson, P.; Milojevic, A. Personal exposure monitoring of PM2.5 among US diplomats in Kathmandu during the COVID-19 lockdown, March to June 2020. Sci. Total Environ. 2021, 772, 144836. [Google Scholar] [CrossRef] [PubMed]
- Dzhambov, A.M.; Lercher, P.; Browning, M.H.E.M.; Stoyanov, D.; Petrova, N.; Novakov, S.; Dimitrova, D.D. Does greenery experienced indoors and outdoors provide an escape and support mental health during the COVID-19 quarantine? Environ. Res. 2020, 196, 110420. [Google Scholar] [CrossRef] [PubMed]
- Soga, M.; Evans, M.J.; Tsuchiya, K.; Fukano, Y. A room with a green view: The importance of nearby nature for mental health during the COVID-19 pandemic. Ecol. Appl. 2021, 31, e02248. [Google Scholar] [CrossRef]
- Corley, J.; Okely, J.A.; Taylor, A.M.; Page, D.; Welstead, M.; Skarabela, B.; Redmond, P.; Cox, S.R.; Russ, T.C. Home garden use during COVID-19: Associations with physical and mental wellbeing in older adults. J. Environ. Psychol. 2021, 73, 101545. [Google Scholar] [CrossRef]
- Theodorou, A.; Panno, A.; Carrus, G.; Carbone, G.A.; Massullo, C.; Imperatori, C. Stay home, stay safe, stay green: The role of gardening activities on mental health during the Covid-19 home confinement. Urban For. Urban Green. 2021, 61, 127091. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Kaltsidi, M.P.; Nektarios, P.A.; Markakis, G.; Loges, V.; Perini, K.; Fernández-Cañero, R. Particularities of having plants at home during the confinement due to the COVID-19 pandemic. Urban For. Urban Green. 2021, 59, 126919. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Lercher, P.; Stoyanov, D.; Petrova, N.; Novakov, S.; Dimitrova, D.D. University students’ self-rated health in relation to perceived acoustic environment during the covid-19 home quarantine. Int. J. Environ. Res. Public Health 2021, 18, 2538. [Google Scholar] [CrossRef]
- Lenzi, S.; Sádaba, J.; Lindborg, P.M. Soundscape in Times of Change: Case Study of a City Neighbourhood During the COVID-19 Lockdown. Front. Psychol. 2021, 12, 570741. [Google Scholar] [CrossRef]
- Spano, G.; D’Este, M.; Giannico, V.; Elia, M.; Cassibba, R.; Lafortezza, R.; Sanesi, G. Association between indoor-outdoor green features and psychological health during the COVID-19 lockdown in Italy: A cross-sectional nationwide study. Urban For. Urban Green. 2021, 62, 127156. [Google Scholar] [CrossRef]
- Olszewska-Guizzo, A.; Fogel, A.; Escoffier, N.; Ho, R. Effects of COVID-19-related stay-at-home order on neuropsychophysiological response to urban spaces: Beneficial role of exposure to nature? J. Environ. Psychol. 2021, 75, 101590. [Google Scholar] [CrossRef]
- Lõhmus, M.; Stenfors, C.U.D.; Lind, T.; Lauber, A.; Georgelis, A. Mental Health, Greenness, and Nature Related Behaviors in the Adult Population of Stockholm County during COVID-19-Related Restrictions. Int. J. Environ. Res. Public Health 2021, 18, 3303. [Google Scholar] [CrossRef]
- Robinson, J.M.; Brindley, P.; Cameron, R.; MacCarthy, D.; Jorgensen, A. Nature’s Role in Supporting Health during the COVID-19 Pandemic: A Geospatial and Socioecological Study. Int. J. Environ. Res. Public Health 2021, 18, 2227. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Solà, D.; Martínez-Suquía, C.; Alsina-Pagès, R.M.; Bergadà, P. The Soundscape of the COVID-19 Lockdown: Barcelona Noise Monitoring Network Case Study. Int. J. Environ. Res. Public Health 2021, 18, 5799. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.; Pavón, I.; de Arcas, G. Changes in noise levels in the city of Madrid during COVID-19 lockdown in 2020. J. Acoust. Soc. Am. 2020, 148, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Hornberg, J.; Haselhoff, T.; Lawrence, B.T.; Fischer, J.L.; Ahmed, S.; Gruehn, D.; Moebus, S. Impact of the COVID-19 Lockdown Measures on Noise Levels in Urban Areas—A Pre/during Comparison of Long-Term Sound Pressure Measurements in the Ruhr Area, Germany. Int. J. Environ. Res. Public Health 2021, 18, 4653. [Google Scholar] [CrossRef]
- Aletta, F.; Oberman, T.; Mitchell, A.; Tong, H.; Kang, J. Assessing the changing urban sound environment during the COVID-19 lockdown period using short-term acoustic measurements. Noise Mapp. 2020, 7, 123–134. [Google Scholar] [CrossRef]
- Rumpler, R.; Venkataraman, S.; Göransson, P. An observation of the impact of CoViD-19 recommendation measures monitored through urban noise levels in central Stockholm, Sweden. Sustain. Cities Soc. 2020, 63, 102469. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Aletta, F.; Mitchell, A.; Oberman, T.; Kang, J. Increases in noise complaints during the COVID-19 lockdown in Spring 2020: A case study in Greater London, UK. Sci. Total Environ. 2021, 785, 147213. [Google Scholar] [CrossRef]
- Lee, P.J.; Jeong, J.H. Attitudes towards outdoor and neighbour noise during the COVID-19 lockdown: A case study in London. Sustain. Cities Soc. 2021, 67, 102768. [Google Scholar] [CrossRef]
- Brown, M.; O’Connor, D.; Murphy, C.; McClean, M.; McMeekin, A.; Prue, G. Impact of COVID-19 on an established physical activity and behaviour change support programme for cancer survivors: An exploratory survey of the Macmillan Move More service for Northern Ireland. Support. Care Cancer 2021, 29, 6135–6143. [Google Scholar] [CrossRef]
- Thomson, C.A.; Overholser, L.S.; Hébert, J.R.; Risendal, B.C.; Morrato, E.H.; Wheeler, S.B. Addressing Cancer Survivorship Care Under COVID-19: Perspectives from the Cancer Prevention and Control Research Network. Am. J. Prev. Med. 2021, 60, 732–736. [Google Scholar] [CrossRef]
- Nekhlyudov, L.; Duijts, S.; Hudson, S.V.; Jones, J.M.; Keogh, J.; Love, B.; Lustberg, M.; Smith, K.C.; Tevaarwerk, A.; Yu, X.; et al. Addressing the needs of cancer survivors during the COVID-19 pandemic. J. Cancer Surviv. 2020, 14, 601–606. [Google Scholar] [CrossRef]
- Tinson, A.; Clair, A. Better Housing Is Crucial for Our Health and the COVID-19 Recovery. The Health Foundation, 2020; pp. 1–25. Available online: https://www.health.org.uk/publications/long-reads/better-housing-is-crucial-for-our-health-and-the-covid-19-recovery (accessed on 18 April 2021).
- Nwanaji-Enwerem, J.C.; Allen, J.G.; Beamer, P.I. Another invisible enemy indoors: COVID-19, human health, the home, and United States indoor air policy. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 773–775. [Google Scholar] [CrossRef]
- Nekhlyudov, L.; Mollica, M.A.; Jacobsen, P.B.; Mayer, D.K.; Shulman, L.N.; Geiger, A.M. Developing a Quality of Cancer Survivorship Care Framework: Implications for Clinical Care, Research, and Policy. J. Natl. Cancer Inst. 2019, 111, 1120–1130. [Google Scholar] [CrossRef]
- Halpern, M.T.; Viswanathan, M.; Evans, T.S.; Birken, S.A.; Basch, E.; Mayer, D.K. Models of Cancer Survivorship Care: Overview and Summary of Current Evidence. J. Oncol. Pract. 2015, 11, e19–e27. [Google Scholar] [CrossRef]
- Choi, Y.; Radhakrishnan, A.; Mahabare, D.; Patole, S.; Dy, S.M.; Pollack, C.E.; Berger, Z.D.; Peairs, K.S. The Johns Hopkins Primary Care for Cancer Survivor Clinic: Lessons learned in our first 4 years. J. Cancer Surviv. 2020, 14, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Nekhlyudov, L.; O’malley, D.M.; Hudson, S.V. Integrating primary care providers in the care of cancer survivors: Gaps in evidence and future opportunities. Lancet Oncol. 2017, 18, e30–e38. [Google Scholar] [CrossRef]
- Rubin, G.; Berendsen, A.; Crawford, S.M.; Dommett, R.; Earle, C.; Emery, J.; Fahey, T.; Grassi, L.; Grunfeld, E.; Gupta, S.; et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015, 16, 1231–1272. [Google Scholar] [CrossRef]
- Clinical Oncology Society of Australia Model of Survivorship Care. Critical Components of Cancer Survivorship Care in Australia. Position Statement; Clinical Oncology Society of Australia Model of Survivorship Care: Sydney, NSW, Australia, 2016; Available online: https://www.cosa.org.au/media/332340/cosa-model-of-survivorship-care-full-version-final-20161107.pdf (accessed on 27 July 2021).
- Coughlan, A. Evaluation of the Victorian Cancer Survivorship Program Phase II Deliverable 1: VCSP II Evaluation; Australian Cancer Survivorship Centre: Ashburton, New Zealand, 2019. [Google Scholar]
- Vardy, J.L.; Chan, R.J.; Koczwara, B.; Lisy, K.; Cohn, R.J.; Joske, D.; Dhillon, H.M.; Jefford, M. Clinical Oncology Society of Australia position statement on cancer survivorship care. Aust. J. Gen. Pract. 2019, 48, 833–836. [Google Scholar] [CrossRef]
- National Health Service. Achieving World-Class Cancer Outcomes: A Strategy for England 2015–2020. 2017. Progress Report 2016-17. Available online: https://www.england.nhs.uk/wp-content/uploads/2017/10/national-cancer-transformation-programme-2016-17-progress.pdf (accessed on 27 July 2021).
- Rubinstein, E.B.; Miller, W.L.; Hudson, S.V.; Howard, J.; O’Malley, D.; Tsui, J.; Lee, H.S.; Bator, A.; Crabtree, B.F. Cancer Survivorship Care in Advanced Primary Care Practices: A Qualitative Study of Challenges and Opportunities. JAMA Intern. Med. 2017, 177, 1726–1732. [Google Scholar] [CrossRef]
- De Wit, N.; Groome, P.; Helsper, C.; McBride, M.; Watson, E.; Wind, J. Increased survival means increasing roles for primary care after cancer diagnosis. Br. J. Gen. Pract. 2017, 67, 349. [Google Scholar] [CrossRef]
- Lisy, K.; Kent, J.; Piper, A.; Jefford, M. Facilitators and barriers to shared primary and specialist cancer care: A systematic review. Support. Care Cancer 2021, 29, 85–96. [Google Scholar] [CrossRef]
- Rechel, B. How to Enhance the Integration of Primary Care and public Health? Approaches, Facilitating Factors and Policy Options. Copenhagen, Denmark, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553739/ (accessed on 27 July 2021).
- Shahzad, M.; Upshur, R.; Donnelly, P.; Bharmal, A.; Wei, X.; Feng, P.; Brown, A.D. A population-based approach to integrated healthcare delivery: A scoping review of clinical care and public health collaboration. BMC Public Health 2019, 19, 708. [Google Scholar] [CrossRef] [PubMed]
- Gosling, R.; Davies, S.M.; Hussey, J.A. How integrating primary care and public health can improve population health outcomes: A view from Liverpool, UK. Public Health Res. Pract. 2016, 26, e2611605. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.S.; Wheeler, B.W.; White, M.P.; Economou, T.; Osborne, N.J. Research note: Urban street tree density and antidepressant prescription rates—A cross-sectional study in London, UK. Landsc. Urban Plan. 2015, 136, 174–179. [Google Scholar] [CrossRef]
- Grigoroglou, C.; Munford, L.; Webb, R.T.; Kapur, N.; Ashcroft, D.M.; Kontopantelis, E. Prevalence of mental illness in primary care and its association with deprivation and social fragmentation at the small-area level in England. Psychol. Med. 2019, 50, 293–302. [Google Scholar] [CrossRef] [PubMed]
- WHO; Regional Office for Europe. From Alma-Ata to Astana: Primary Health Care—Reflecting on the Past, Transforming for the Future. 2018. Available online: https://www.who.int/docs/default-source/primary-health-care-conference/phc-regional-report-europe.pdf?sfvrsn=cf2badeb_2 (accessed on 27 July 2021).
- WHO; Universal Health Coverage—Life Course Team. Declaration of Astana. Global Conference on Primary Health Care; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/docs/default-source/primary-health/declaration/gcphc-declaration.pdf (accessed on 27 July 2021).
- Leong, M.Q.; Lim, C.W.; Lai, Y.F. Comparison of Hospital-at-Home models: A systematic review of reviews. BMJ Open 2021, 11, e043285. [Google Scholar] [CrossRef]
- Buck, D.; Gregory, S. Housing and Health: Opportunities for Sustainability and Transformation Partnerships. 2018. Available online: https://www.kingsfund.org.uk/sites/default/files/2018-03/Housing_and_health_final.pdf (accessed on 28 July 2021).
- Public Health England. Improving Health and Care through the Home: A National Memorandum of Understanding. PHE Publications, 2018. Available online: https://www.gov.uk/government/publications/improving-health-and-care-through-the-home-mou (accessed on 28 July 2021).
- Krieger, J.; Higgins, D.L. Housing and Health: Time Again for Public Health Action. Am. J. Public Health 2002, 92, 758–768. [Google Scholar] [CrossRef]
- Shaw, M. Housing and Public Health. Annu. Rev. Public Health 2004, 25, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Braubach, M. Key challenges of housing and health from WHO perspective. Int. J. Public Health 2011, 56, 579–580. [Google Scholar] [CrossRef]
- Palacios, J.; Eichholtz, P.; Kok, N.; Aydin, E. The impact of housing conditions on health outcomes. Real Estate Econ. 2020, 1–29. [Google Scholar] [CrossRef]
- Fyfe, C.; Telfar-Barnard, L.; Howden-Chapman, P.; Douwes, J. Association between home insulation and hospital admission rates: Retrospective cohort study using linked data from a national intervention programme. BMJ 2020, 371, m4571. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.; Burns, P.; Jones, A.; Winrow, E.; Edwards, R.T. Costs and outcomes of improving population health through better social housing: A cohort study and economic analysis. Int. J. Public Health 2017, 62, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.; Coxon, J. Boiler on Prescription Trial. Closing Report. 2016. Available online: https://www.gentoogroup.com/media/1061811/boiler-on-prescription-closing-report.pdf (accessed on 28 July 2021).
- Yeo, N.L.; Elliott, L.R.; Bethel, A.; White, M.P.; Dean, S.G.; Garside, R. Indoor nature interventions for health and wellbeing of older adults in residential settings: A systematic review. Gerontologist 2020, 60, e184–e199. [Google Scholar] [CrossRef] [PubMed]
- Braçe, O.; Garrido-Cumbrera, M.; Foley, R.; Correa-Fernández, J.; Suárez-Cáceres, G.; Lafortezza, R. Is a view of green spaces from home associated with a lower risk of anxiety and depression? Int. J. Environ. Res. Public Health 2020, 17, 7014. [Google Scholar] [CrossRef]
- Jo, H.; Song, C.; Miyazaki, Y. Physiological Benefits of Viewing Nature: A Systematic Review of Indoor Experiments. Int. J. Environ. Res. Public Health 2019, 16, 4739. [Google Scholar] [CrossRef]
- Osibona, O.; Solomon, B.D.; Fecht, D. Lighting in the Home and Health: A systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 609. [Google Scholar] [CrossRef]
- Janus, S.I.M.; Kosters, J.; Van Den Bosch, K.A.; Andringa, T.C.; Andringa, T.C.; Zuidema, S.U.; Luijendijk, H.J. Sounds in nursing homes and their effect on health in dementia: A systematic review. Int. Psychogeriatr. 2020, 33, 627–644. [Google Scholar] [CrossRef]
- Lee, K.K.; Bing, R.; Kiang, J.; Bashir, S.; Spath, N.; Stelzle, D.; Mortimer, K.; Bularga, A.; Doudesis, D.; Joshi, S.S.; et al. Adverse health effects associated with household air pollution: A systematic review, meta-analysis, and burden estimation study. Lancet Glob. Health 2020, 8, e1427–e1434. [Google Scholar] [CrossRef]
- National Institute for Health and Care. Indoor Air Quality at Home. [2] Evidence Review for Exposure to Pollutants and Health Outcomes. 2020. Available online: https://www.nice.org.uk/guidance/ng149/evidence/2-exposure-to-pollutants-and-health-outcomes-pdf-7020943887 (accessed on 18 April 2021).
- Thomson, H.; Thomas, S.; Sellstrom, E.; Petticrew, M. Housing improvements for health and associated socio-economic outcomes. Cochrane Database Syst. Rev. 2013, 12, CD008657. [Google Scholar] [CrossRef] [PubMed]
- Curl, A.; Kearns, A.; Mason, P.; Egan, M.; Tannahill, C.; Ellaway, A. Physical and mental health outcomes following housing improvements: Evidence from the GoWell study. J. Epidemiol. Community Health 2015, 69, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.W.; Yeager, R.; Conklin, D.J.; DeJarnett, N.; Keith, R.J.; DeFilippis, A.P.; Rai, S.N.; Bhatnagar, A. Residential proximity to greenness mitigates the hemodynamic effects of ambient air pollution. Am. J. Physiol. Hear. Circ. Physiol. 2021, 320, H1102–H1111. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gong, G.; He, X.; Shi, X.; Mo, L. Control of exhaled SARS-CoV-2-laden aerosols in the interpersonal breathing microenvironment in a ventilated room with limited space air stability. J. Environ. Sci. 2021, 108, 175–187. [Google Scholar] [CrossRef]
- Wiktorczyk-kapischke, N.; Grudlewska-buda, K.; Wa, E.; Kwieci, J.; Radtke, L.; Gospodarek-komkowska, E.; Skowron, K. Science of the Total Environment SARS-CoV-2 in the environment—Non-droplet spreading routes. Sci. Total Environ. 2021, 770, 145260. [Google Scholar] [CrossRef]
- Rahimi, N.R.; Fouladi-Fard, R.; Aali, R.; Shahryari, A.; Rezaali, M.; Ghafouri, Y.; Ghalhari, M.R.; Ghalhari, M.A.; Farzinnia, B.; Gea, O.C.; et al. Bidirectional association between COVID-19 and the environment: A systematic review. Environ. Res. 2021, 194, 110692. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kumari, N.; Karmakar, S.; Behera, M.; Siddiqui, A.J.; Rajput, V.D.; Minkina, T.; Bauddh, K.; Kumar, N. Current understanding of the influence of environmental factors on SARS-CoV-2 transmission, persistence, and infectivity. Environ. Sci. Pollut. Res. 2021, 28, 6267–6288. [Google Scholar] [CrossRef]
- Green McDonald, P.; O’Connell, M.; Lutgendorf, S.K. Psychoneuroimmunology and cancer: A decade of discovery, paradigm shifts, and methodological innovations. Brain Behav. Immun. 2013, 30, S1–S9. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.E.; Ganz, P.A.; Haque, R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav. Immun. 2013, 30, S58–S67. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Lamkin, D.M. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 2013, 30, S48–S57. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.L.; Rosenstiel, P. Systems Medicine in Chronic Inflammatory Diseases. Immunity 2018, 48, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Microbes Immunity and Behavior: Psychoneuroimmunology Meets the Microbiome. Neuropsychopharmacology 2017, 42, 178–192. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 2014, 54, 281–288. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-Resident Macrophage Enhancer Landscapes Are Shaped by the Local Microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Teeling, J.L.; Felton, L.M.; Deacon, R.M.J.; Cunningham, C.; Rawlins, J.N.P.; Perry, V.H. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav. Immun. 2007, 21, 836–850. [Google Scholar] [CrossRef]
- Braig, D.; Nero, T.L.; Koch, H.G.; Kaiser, B.; Wang, X.; Thiele, J.R.; Morton, C.J.; Zeller, J.; Kiefer, J.; Potempa, L.A.; et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat. Commun. 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef]
- Sander, L.E.; Sackett, S.D.; Dierssen, U.; Beraza, N.; Linke, R.P.; Müller, M.; Blander, J.M.; Tacke, F.; Trautwein, C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 2010, 207, 1453–1464. [Google Scholar] [CrossRef]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- McKenzie, A.N.J.; Spits, H.; Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 2014, 41, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Kaissi, S.; Homaidan, F.R.; Naim, H.Y.; El-Sabban, M.E. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Nowarski, R.; Jackson, R.; Flavell, R.A. The Stromal Intervention: Regulation of Immunity and Inflammation at the Epithelial-Mesenchymal Barrier. Cell 2017, 168, 362–375. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Brücher, B.L.D.M.; Jamall, I.S. Epistemology of the origin of cancer: A new paradigm. BMC Cancer 2014, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Shurin, G.V.; Zlotnikov, S.B.; Bunimovich, Y.L. The Neuroimmune Axis in the Tumor Microenvironment. J. Immunol. 2020, 204, 280–285. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.C.; Molofsky, A.V. The immune system and psychiatric disease: A basic science perspective. Clin. Exp. Immunol. 2019, 197, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, V.; Vernon, A.C.; Turkheimer, F.; Dazzan, P.; Pariante, C.M. Brain microglia in psychiatric disorders. Lancet Psychiatry 2017, 4, 563–572. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Miller, A.H.; Haroon, E.; Felger, J.C. The Immunology of Behavior-Exploring the Role of the Immune System in Brain Health and Illness. Neuropsychopharmacology 2017, 42, 1–4. [Google Scholar] [CrossRef]
- Baumeister, D.; Russell, A.; Pariante, C.M.; Mondelli, V. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social and lifestyle factors. Soc. Psychiatry Psychiatr. Epidemiol. 2014, 49, 841–849. [Google Scholar] [CrossRef]
- Travert, A.S.; Annerstedt, K.; Daivadanam, M. Built Environment and Health Behaviors: Deconstructing the Black Box of Interactions—A Review of Reviews. Int. J. Environ. Res. Public Health 2019, 16, 1454. [Google Scholar] [CrossRef]
- Kärmeniemi, M.; Lankila, T.; Ikäheimo, T.; Koivumaa-Honkanen, H.; Korpelainen, R. The Built Environment as a Determinant of Physical Activity: A Systematic Review of Longitudinal Studies and Natural Experiments. Ann. Behav. Med. 2018, 52, 239–251. [Google Scholar] [CrossRef]
- Smith, M.; Hosking, J.; Woodward, A.; Witten, K.; MacMillan, A.; Field, A.; Baas, P.; Mackie, H. Systematic literature review of built environment effects on physical activity and active transport—An update and new findings on health equity. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Bivoltsis, A.; Cervigni, E.; Trapp, G.; Knuiman, M.; Hooper, P.; Ambrosini, G.L. Food environments and dietary intakes among adults: Does the type of spatial exposure measurement matter? A systematic review. Int. J. Health Geogr. 2018, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Sulicka-Grodzicka, J.; Surdacki, A.; Seweryn, M.; Mikołajczyk, T.; Rewiuk, K.; Guzik, T.; Grodzicki, T. Low-grade chronic inflammation and immune alterations in childhood and adolescent cancer survivors: A contribution to accelerated aging? Cancer Med. 2021, 10, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Scuric, Z.; Carroll, J.E.; Bower, J.E.; Ramos-Perlberg, S.; Petersen, L.; Esquivel, S.; Hogan, M.; Chapman, A.M.; Irwin, M.R.; Breen, E.C.; et al. Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer 2017, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Léveillé, P.; Samoilenko, M.; Franco, A.; England, J.; Malaquin, N.; Tu, V.; Cardin, G.B.; Drouin, S.; Rodier, F.; et al. Biomarkers of cardiometabolic complications in survivors of childhood acute lymphoblastic leukemia. Sci. Rep. 2020, 10, 21507. [Google Scholar] [CrossRef]
- Tromp, J.; Boerman, L.M.; Sama, I.E.; Maass, S.W.M.C.; Maduro, J.H.; Hummel, Y.M.; Berger, M.Y.; de Bock, G.H.; Gietema, J.A.; Berendsen, A.J.; et al. Long-term survivors of early breast cancer treated with chemotherapy are characterized by a pro-inflammatory biomarker profile compared to matched controls. Eur. J. Heart Fail. 2020, 22, 1239–1246. [Google Scholar] [CrossRef]
- Van Der Willik, K.D.; Koppelmans, V.; Hauptmann, M.; Compter, A.; Ikram, M.A.; Schagen, S.B. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study. Breast Cancer Res. 2018, 20, 135. [Google Scholar] [CrossRef]
- Maurer, T.; Jaskulski, S.; Behrens, S.; Jung, A.Y.; Obi, N.; Johnson, T.; Becher, H.; Chang-Claude, J. Tired of feeling tired—The role of circulating inflammatory biomarkers and long-term cancer related fatigue in breast cancer survivors. Breast 2021, 56, 103–109. [Google Scholar] [CrossRef]
- Collado-Hidalgo, A.; Bower, J.E.; Ganz, P.A.; Cole, S.W.; Irwin, M.R. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006, 12, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Miller, A.H.; Felger, J.; Mister, D.; Liu, T.; Torres, M.A. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol. Med. 2017, 47, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Russ, E.; Srinivasan, M.; Eidelman, O.; Eklund, M.; Hueman, M.; Pollard, H.B.; Hu, H.; Shriver, C.D.; Srivastava, M. Proteomic Analysis of Inflammatory Biomarkers Associated with Breast Cancer Recurrence. Mil. Med. 2020, 185, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Lim, J.; McSorley, S.T.; Horgan, P.G.; McMillan, D.C. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci. Rep. 2017, 7, 16717. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Laird, B.J.A.; Horgan, P.G.; McMillan, D.C. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2018, 132, 130–137. [Google Scholar] [CrossRef]
- Lurie, D.I. An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J. Exp. Neurosci. 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Almeida, P.G.C.; Nani, J.V.; Oses, J.P.; Brietzke, E.; Hayashi, M.A.F. Neuroinflammation and glial cell activation in mental disorders. Brain Behav. Immun. Health 2020, 2, 100034. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef]
- Chan, M.K.; Cooper, J.D.; Bot, M.; Steiner, J.; Penninx, B.W.J.H.; Bahn, S. Identification of an Immune-Neuroendocrine Biomarker Panel for Detection of Depression: A Joint Effects Statistical Approach. Neuroendocrinology 2016, 103, 693–710. [Google Scholar] [CrossRef]
- Haenisch, F.; Cooper, J.D.; Reif, A.; Kittel-Schneider, S.; Steiner, J.; Leweke, F.M.; Rothermundt, M.; van Beveren, N.J.M.; Crespo-Facorro, B.; Niebuhr, D.W.; et al. Towards a blood-based diagnostic panel for bipolar disorder. Brain Behav. Immun. 2016, 52, 49–57. [Google Scholar] [CrossRef]
- Chan, M.K.; Krebs, M.O.; Cox, D.; Guest, P.C.; Yolken, R.H.; Rahmoune, H.; Rothermundt, M.; Steiner, J.; Leweke, F.M.; Van Beveren, N.J.M.; et al. Development of a blood-based molecular biomarker test for identification of schizophrenia before disease onset. Transl. Psychiatry 2015, 5, e601. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Karmakar, C.; Tamouza, R.; Tran, T.; Yearwood, J.; Hamdani, N.; Laouamri, H.; Richard, J.R.; Yolken, R.; Berk, M.; et al. Precision psychiatry with immunological and cognitive biomarkers: A multi-domain prediction for the diagnosis of bipolar disorder or schizophrenia using machine learning. Transl. Psychiatry 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Shelton, R.C.; Kinrys, G.; Henry, M.E.; Bakow, B.R.; Lipkin, S.H.; Pi, B.; Thurmond, L.; Bilello, J.A. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: A Pilot and Replication Study. Mol. Psychiatry 2013, 18, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.R.; Graham, C.; D’Amato, A.; Gentry-Maharaj, A.; Ryan, A.; Kalsi, J.K.; Ainley, C.; Whetton, A.D.; Menon, U.; Jacobs, I.; et al. A combined biomarker panel shows improved sensitivity for the early detection of ovarian cancer allowing the identification of the most aggressive type II tumours. Br. J. Cancer 2017, 117, 666–674. [Google Scholar] [CrossRef]
- Guida, F.; Sun, N.; Bantis, L.E.; Muller, D.C.; Li, P.; Taguchi, A.; Dhillon, D.; Kundnani, D.L.; Patel, N.J.; Yan, Q.; et al. Assessment of Lung Cancer Risk on the Basis of a Biomarker Panel of Circulating Proteins. JAMA Oncol. 2018, 4, e182078. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Basil, C.F.; Zhao, Y.; Zavaglia, K.; Jin, P.; Panelli, M.C.; Voiculescu, S.; Mandruzzato, S.; Lee, H.M.; Seliger, B.; Freedman, R.S.; et al. Common cancer biomarkers. Cancer Res. 2006, 66, 2953–2961. [Google Scholar] [CrossRef]
- Yan, Q. Translational implications of inflammatory biomarkers and cytokine networks in psychoneuroimmunology. Methods Mol. Biol. 2012, 934, 105–120. [Google Scholar]
- Ghezzi, P.; Floridi, L.; Boraschi, D.; Cuadrado, A.; Manda, G.; Levic, S.; D’Acquisto, F.; Hamilton, A.; Athersuch, T.J.; Selley, L. Oxidative Stress and Inflammation Induced by Environmental and Psychological Stressors: A Biomarker Perspective. Antioxid. Redox Signal. 2018, 28, 852–872. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; García, R.; et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adult. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Bakolis, I.; Hammoud, R.; Stewart, R.; Beevers, S.; Dajnak, D.; MacCrimmon, S.; Broadbent, M.; Pritchard, M.; Shiode, N.; Fecht, D.; et al. Mental health consequences of urban air pollution: Prospective population-based longitudinal survey. Soc. Psychiatry Psychiatr. Epidemiol. 2020, 56, 1–13. [Google Scholar] [CrossRef]
- Hooper, L.G.; Kaufman, J.D. Ambient air pollution and clinical implications for susceptible populations. Ann. Am. Thorac. Soc. 2018, 15, S64–S68. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.G.W.; Spruit, M.A.; Groenen, M.; Gaffron, S.; Van Empel, V.P.M.; Bruijnzeel, P.L.B.; Rutten, E.P.A.; Op’t Roodt, J.; Wouters, E.F.M.; Franssen, F.M.E. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 728–735. [Google Scholar] [CrossRef]
- Herder, C.; Maalmi, H.; Strassburger, K.; Zaharia, O.P.; Ratter, J.M.; Karusheva, Y.; Elhadad, M.A.; Báodis, K.; Bongaerts, B.W.C.; Rathmann, W.; et al. Differences in biomarkers of inflammation between novel subgroups of recent-onset diabetes. Diabetes 2021, 70, 1198–1208. [Google Scholar] [CrossRef]
- Happo, M.S.; Sippula, O.; Jalava, P.I.; Rintala, H.; Leskinen, A.; Komppula, M.; Kuuspalo, K.; Mikkonen, S.; Lehtinen, K.; Jokiniemi, J.; et al. Role of microbial and chemical composition in toxicological properties of indoor and outdoor air particulate matter. Part. Fibre Toxicol. 2014, 11, 60. [Google Scholar] [CrossRef]
- Hoisington, A.J.; Brenner, L.A.; Kinney, K.A.; Postolache, T.T.; Lowry, C.A. The microbiome of the built environment and mental health. Microbiome 2015, 3, 60. [Google Scholar] [CrossRef]
- Thomas, S.J.; Larkin, T. Cognitive Distortions in Relation to Plasma Cortisol and Oxytocin Levels in Major Depressive Disorder. Front. Psychiatry 2020, 10, 971. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Bus, B.A.A.; Spinhoven, P.; Penninx, B.W.J.H.; Kenis, G.; Prickaerts, J.; Voshaar, R.C.O.; Elzinga, B.M. Serum levels of brain-derived neurotrophic factor in major depressive disorder: State-trait issues, clinical features and pharmacological treatment. Mol. Psychiatry 2011, 16, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Bos, I.; Jacobs, L.; Nawrot, T.S.; de Geus, B.; Torfs, R.; Int Panis, L.; Degraeuwe, B.; Meeusen, R. No exercise-induced increase in serum BDNF after cycling near a major traffic road. Neurosci. Lett. 2011, 500, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Tähkämö, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Nowozin, C.; Wahnschaffe, A.; Rodenbeck, A.; de Zeeuw, J.; Haedel, S.; Kozakov, R.; Schoepp, H.; Muench, M.; Kunz, D. Applying Melanopic Lux to Measure Biological Light Effects on Melatonin Suppression and Subjective Sleepiness. Curr. Alzheimer Res. 2017, 14, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Melhuish Beaupre, L.M.; Brown, G.M.; Gonçalves, V.F.; Kennedy, J.L. Melatonin’s neuroprotective role in mitochondria and its potential as a biomarker in aging, cognition and psychiatric disorders. Transl. Psychiatry 2021, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.C.L.; Ng, T.K.S.; Le Lee, J.; Lim, P.Y.; Chua, S.K.J.; Tan, C.; Chua, M.; Tan, J.; Lee, S.; Sia, A.; et al. Horticultural Therapy Reduces Biomarkers of Immunosenescence and Inflammaging in Community Dwelling Older Adults: A Feasibility Pilot Randomized Controlled Trial. Gerontologist 2021, 76, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Durvasula, S.; Kok, C.; Sambrook, P.N.; Cumming, R.G.; Lord, S.R.; March, L.M.; Mason, R.S.; Seibel, M.J.; Simpson, J.M.; Cameron, I.D. Sunlight and health: Attitudes of older people living in intermediate care facilities in southern Australia. Arch. Gerontol. Geriatr. 2010, 51, e94–e99. [Google Scholar] [CrossRef] [PubMed]
- Durvasula, S.; Gies, P.; Mason, R.S.; Chen, J.S.; Henderson, S.; Seibel, M.J.; Sambrook, P.N.; March, L.M.; Lord, S.R.; Kok, C.; et al. Vitamin D response of older people in residential aged care to sunlight-derived ultraviolet radiation. Arch. Osteoporos. 2014, 9, 197. [Google Scholar] [CrossRef]
- Jankowska, M.M.; Natarajan, L.; Godbole, S.; Meseck, K.; Sears, D.D.; Patterson, R.E.; Kerr, J. Kernel Density Estimation as a Measure of Environmental Exposure Related to Insulin Resistance in Breast Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1078–1084. [Google Scholar] [CrossRef]
- Watson, K.T.; Simard, J.F.; Henderson, V.W.; Nutkiewicz, L.; Lamers, F.; Rasgon, N.; Penninx, B. Association of Insulin Resistance with Depression Severity and Remission Status: Defining a Metabolic Endophenotype of Depression. JAMA Psychiatry 2021, 78, 439–441. [Google Scholar] [CrossRef]
- Pan, K.; Chlebowski, R.T.; Mortimer, J.E.; Gunther, M.J.; Rohan, T.; Vitolins, M.Z.; Adams-Campbell, L.L.; Ho, G.Y.F.; Cheng, T.Y.D.; Nelson, R.A. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer 2020, 126, 3638–3647. [Google Scholar] [CrossRef]
- Subnis, U.B.; Starkweather, A.R.; McCain, N.L.; Brown, R.F. Psychosocial Therapies for Patients with Cancer: A Current Review of Interventions Using Psychoneuroimmunology-Based Outcome Measures. Integr. Cancer Ther. 2014, 13, 85–104. [Google Scholar] [CrossRef]
- Meneses-Echavez, J.F.; Correa-Bautista, J.E.; González-Jiménez, E.; Río-Valle, J.S.; Elkins, M.R.; Lobelo, F.; Ramírez-Velez, R. The effect of exercise training on mediators of inflammation in breast cancer survivors: A systematic review with meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Lee, J.; Suh, S.H.; Ligibel, J.; Courneya, K.S.; Jeon, J.Y. Effects of Exercise on Insulin, IGF Axis, Adipocytokines, and Inflammatory Markers in Breast Cancer Survivors: A systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2017, 26, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, N.; Stoner, L.; Farajivafa, V.; Hanson, E.D. Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain Behav. Immun. 2019, 81, 92–104. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, B.; Paxton, R.J.; Yang, W.; Wang, X.; Jiao, Y.; Yu, C.; Chen, X. The effects of exercise on insulin, glucose, IGF-axis and CRP in cancer survivors: Meta-analysis and meta-regression of randomised controlled trials. Eur. J. Cancer Care (Engl.) 2020, 29, e13186. [Google Scholar] [CrossRef]
- Chrysikou, E. Architecture for psychiatric environments and therapeutic spaces; IOS Press: Amsterdam, The Netherlands, 2014; ISBN 9781614994602. [Google Scholar]
- Karottki, D.G.; Spilak, M.; Frederiksen, M.; Gunnarsen, L.; Brauner, E.V.; Kolarik, B.; Andersen, Z.J.; Sigsgaard, T.; Barregard, L.; Strandberg, B.; et al. An indoor air filtration study in homes of elderly: Cardiovascular and respiratory effects of exposure to particulate matter. Environ. Health 2013, 12, 116. [Google Scholar] [CrossRef]
- Padró-Martínez, L.T.; Owusu, E.; Reisner, E.; Zamore, W.; Simon, M.C.; Mwamburi, M.; Brown, C.A.; Chung, M.; Brugge, D.; Durant, J.L. A randomized cross-over air filtration intervention trial for reducing cardiovascular health risks in residents of public housing near a highway. Int. J. Environ. Res. Public Health 2015, 12, 7814–7838. [Google Scholar] [CrossRef]
- Shao, D.; Du, Y.; Liu, S.; Brunekreef, B.; Meliefste, K.; Zhao, Q.; Chen, J.; Song, X.; Wang, M.; Wang, J.; et al. Cardiorespiratory responses of air filtration: A randomized crossover intervention trial in seniors living in Beijing: Beijing Indoor Air Purifier StudY, BIAPSY. Sci. Total Environ. 2017, 603–604, 541–549. [Google Scholar] [CrossRef]
- Chuang, H.C.; Ho, K.F.; Lin, L.Y.; Chang, T.Y.; Hong, G.B.; Ma, C.M.; Liu, I.J.; Chuang, K.J. Long-term indoor air conditioner filtration and cardiovascular health: A randomized crossover intervention study. Environ. Int. 2017, 106, 91–96. [Google Scholar] [CrossRef]
- Allen, R.W.; Carlsten, C.; Karlen, B.; Leckie, S.; Van Eeden, S.; Vedal, S.; Wong, I.; Brauer, M. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am. J. Respir. Crit. Care Med. 2011, 183, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, M.; Brauer, M.; Karlen, B.; Carlsten, C.; Van Eeden, S.; Allen, R.W. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: A HEPA filter intervention study. Occup. Environ. Med. 2015, 72, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Wahnschaffe, A.; Haedel, S.; Rodenbeck, A.; Stoll, C.; Rudolph, H.; Kozakov, R.; Schoepp, H.; Kunz, D. Out of the Lab and into the Bathroom: Evening Short-Term Exposure to Conventional Light Suppresses Melatonin and Increases Alertness Perception. Int. J. Mol. Sci. 2013, 14, 2573–2589. [Google Scholar] [CrossRef]
- Sander, B.; Markvart, J.; Kessel, L.; Argyraki, A.; Johnsen, K. Can sleep quality and wellbeing be improved by changing the indoor lighting in the homes of healthy, elderly citizens? Chronobiol. Int. 2015, 32, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Pedrinolla, A.; Tamburin, S.; Brasioli, A.; Sollima, A.; Fonte, C.; Muti, E.; Smania, N.; Schena, F.; Venturelli, M. An Indoor Therapeutic Garden for Behavioral Symptoms in Alzheimer’s Disease: A Randomized Controlled Trial. J. Alzheimer’s Dis. 2019, 71, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Takahashi, K.; Mase, A.; Kotani, M.; Ami, K.; Maeda, M.; Shirahama, T.; Lee, S.; Matsuzaki, H.; Kumagai-Takei, N.; et al. Enhancement of NK Cell Cytotoxicity Induced by Long-Term Living in Negatively Charged-Particle Dominant Indoor Air-Conditions. PLoS ONE 2015, 10, e0132373. [Google Scholar] [CrossRef]
- Dumbuya, J.S.; Chen, L.; Shu, S.Y.; Ma, L.; Luo, W.; Li, F.; Wu, J.Y.; Wang, B. G-CSF attenuates neuroinflammation and neuronal apoptosis via the mTOR/p70SK6 signaling pathway in neonatal Hypoxia-Ischemia rat model. Brain Res. 2020, 1739, 146817. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef]
- Perez, V.; Alexander, D.D.; Bailey, W.H. Air ions and mood outcomes: A review and meta-analysis. BMC Psychiatry 2013, 13, 29. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Yao, L.; Ding, N.; Jiang, L.; Wu, Y. Bright light therapy in the treatment of patients with bipolar disorder: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232798. [Google Scholar] [CrossRef]
- Johnson, J.A.; Garland, S.N.; Carlson, L.E.; Savard, J.; Simpson, J.S.A.; Ancoli-Israel, S.; Campbell, T.S. Bright light therapy improves cancer-related fatigue in cancer survivors: A randomized controlled trial. J. Cancer Surviv. 2018, 12, 206–215. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Zheng, X.; Du, X. Therapeutic strategies of melatonin in cancer patients: A systematic review and meta-analysis. OncoTargets Ther. 2018, 11, 7895–7908. [Google Scholar] [CrossRef]
- Cheng, D.C.Y.; Ganner, J.L.; Gordon, C.J.; Phillips, C.L.; Grunstein, R.R.; Comas, M. The efficacy of combined bright light and melatonin therapies on sleep and circadian outcomes: A systematic review. Sleep Med. Rev. 2021, 58, 101491. [Google Scholar] [CrossRef] [PubMed]
- Kalajian, T.A.; Aldoukhi, A.; Veronikis, A.J.; Persons, K.; Holick, M.F. Ultraviolet B Light Emitting Diodes (LEDs) Are More Efficient and Effective in Producing Vitamin D3 in Human Skin Compared to Natural Sunlight. Sci. Rep. 2017, 7, 11489. [Google Scholar] [CrossRef]
- Karkeni, E.; Morin, S.O.; Tayeh, B.B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.S.; Yilmaz, M.K.; Falkmer, U.G.; Poulsen, L.; Bøgsted, M.; Christensen, H.S.; Bojesen, S.E.; Jensen, B.V.; Chen, I.M.; Johansen, A.Z.; et al. Pre-treatment serum vitamin D deficiency is associated with increased inflammatory biomarkers and short overall survival in patients with pancreatic cancer. Eur. J. Cancer 2021, 144, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-Analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Lisi, E.; Bizzarri, M. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J. Pineal Res. 2011, 50, 150–158. [Google Scholar] [CrossRef]
- Vitti-Ruela, B.V.; Dokkedal-Silva, V.; Hachul, H.; Tufik, S.; Andersen, M.L. Melatonin and vitamin D: Complementary therapeutic strategies for breast cancer. Support. Care Cancer 2021, 29, 3433–3434. [Google Scholar] [CrossRef]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef]
- Burkett, J.P.; Miller, G.W. Using the exposome to understand environmental contributors to psychiatric disorders. Neuropsychopharmacology 2021, 46, 263–264. [Google Scholar] [CrossRef]
- Parajuli, D.R.; Kuot, A.; Hamiduzzaman, M.; Gladman, J.; Isaac, V. Person-centered, non-pharmacological intervention in reducing psychotropic medications use among residents with dementia in Australian rural aged care homes. BMC Psychiatry 2021, 21, 36. [Google Scholar] [CrossRef]
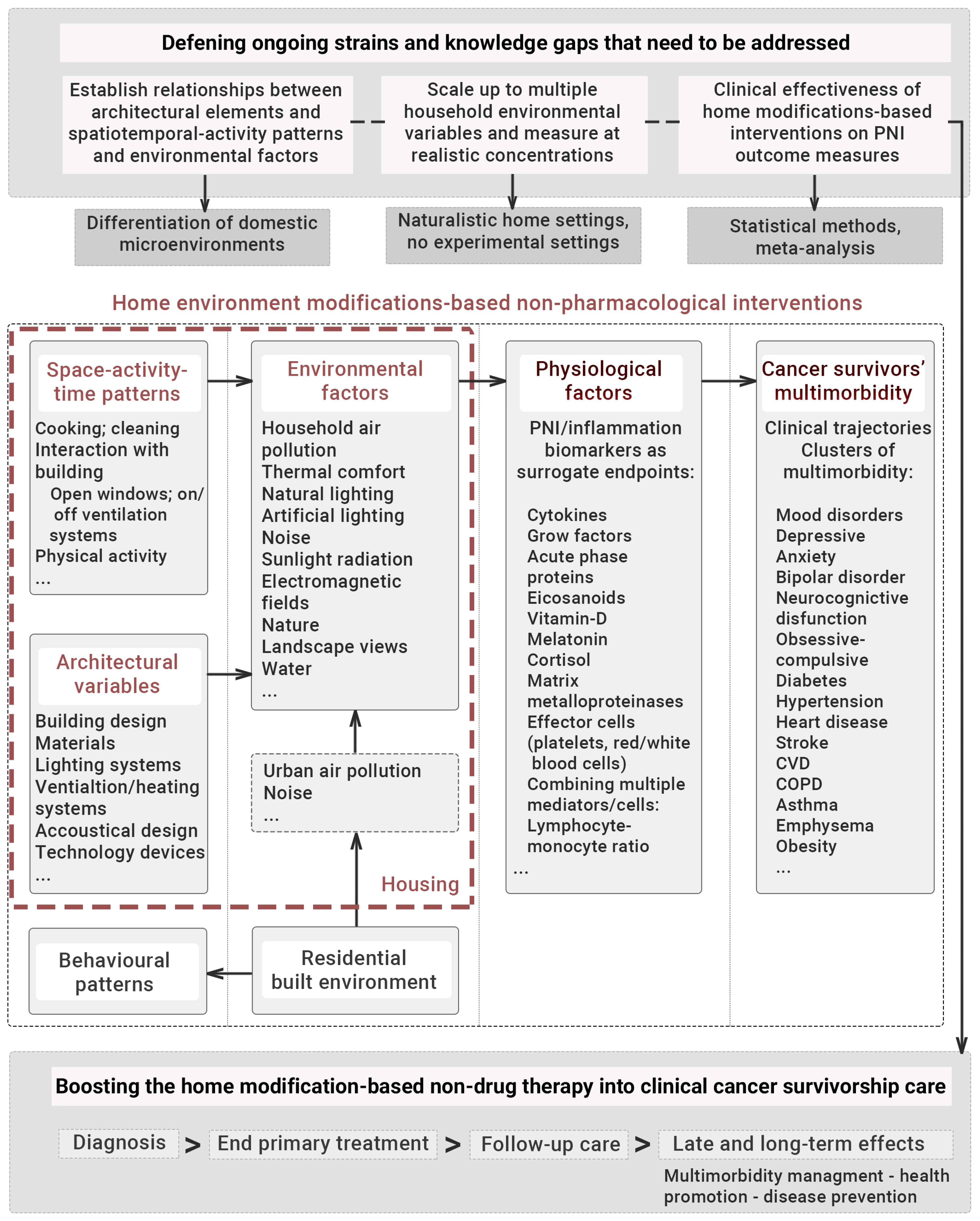
| First Author (Date) | Study Location | Study Design (Period) | Environmental Factor | Intervention | PNI Biomarkers 1 | Key Findings |
|---|---|---|---|---|---|---|
| Air quality-based interventions | ||||||
| Karottki (2013) [300] | Copenhagen, DK | Randomised, double-blind, crossover intervention (November 2010–June 2011) | PM2.5; PNC; PAH | Recirculated particle-filtered or sham-filtered indoor air (HEPA filter)/14-day period for each exposure. Time home median = 83% | Haemoglobin, leukocyte counts, lymphocytes, monocytes, CRP, HDL, LDL | Reductions in the median concentration of: PM2.5, from 8 to 4 μg/m3; PNC, from 7669 to 5352 particles/cm3; PAH, from 0.5 to 0.25 ng/m3. |
| No statistically significant effects on inflammatory biomarkers. | ||||||
| Padró-Martínez (2015) [301] | Somerville, MA, USA | Randomised, double-blind, crossover trial (February 2011–November 2012) | PNC | Recirculated particle-filtered or sham-filtered indoor air (HEPA filter)/21-day period for each exposure. Time home median = 81% | hs-CRP, fibrinogen, TNF-RII, IL-6 | Reductions in the median concentration of PNC, from 6820 to 4850 particles/cm3. |
| IL-6 concentrations were significantly higher following HEPA filtration (0.668 pg/mL; CI = 0.465–0.959). | ||||||
| Shao (2017) [302] | Beijing, CN | Randomised, double-blind, crossover trial (December 2013–March 2014) | PM2.5; BC | Active-mode and sham-mode air filtration (HEPA filter)/14-day period for each exposure. Time home median = 95% | CRP, fibrinogen, IL-6, IL-8 | Reductions in the median concentration of: PM2.5, from 60 to 24 μg/m3; BC, from 3.9 to 1.8 μg/m3. |
| Significant reductions in IL-8, from 120.30 to 47.65 pg/mL. | ||||||
| Chuang (2017) [303] | Taipei, CN | Randomised, double-blind, crossover trial (January 2013–December 2014) | PM ≤ 2.5 μm; total VOCs | Active-filtered indoor air and placebo air conditioner filter/365-day period for each exposure | hs-CRP, fibrinogen | Reductions in the median concentration of: PM2.5, from 21.4 to 12.8 μg/m3; VOCs, from 1.2 to 0.43 ppm. |
| Significant reduction in hs-CRP, from 0.36 to 0.18 mg/dL. | ||||||
| Allen (2011) [304] | Smithers, British Columbia, CA | Randomised, crossover trial (November 2008–December 2009) | PM2.5 mass concentration; LG, a marker of WS PM | Active-mode and sham-mode air filtration (HEPA filter)/7-day period for each exposure. Time home median = 77% | CRP, IL-6 | Reductions in the median concentration of: PM2.5, from 11.2 to 4.6 μg/m3; LG, from 127 to 33 ng/m3. |
| Decreased concentrations of CRP (32%) and IL-6 following active-mode air filtration. | ||||||
| Kajbafza-deh (2015) [305] | Vancouver, British Columbia, CA | Randomised, single-blind, cross-over intervention (December 2011–August 2012) | PM2.5 mass concentration; LG, a marker of TRAP PM | HEPA filtration and placebo filtration/7-day period for each exposure. Time home median = 74% | CRP, IL-6 | Reductions in PM2.5, from 7.1 to 4.3 μg/m3 following HEPA filtration. |
| Increase in CRP levels with increasing indoor PM2.5 concentrations. | ||||||
| Nishimura (2015) [309] | Japan | Non-randomised, non-blinded trial (21 months) | NCPDIAC; negatively charged- particles | NCPDIAC and indoor conditions with device generating NCP OFF/3-month period for each exposure | Measures of 29 PNI markers 2 | EGF, G-CSF and Eotaxin concentrations increased during NCPDIAC trials. |
| First Author (Date) | Study Location | Study Design (Period) | Environmental Factor | Intervention | PNI Biomarkers 1 | Key Findings |
|---|---|---|---|---|---|---|
| Artificial lighting-based interventions | ||||||
| Wahn-schaffe (2013) [306] | Berlin, Germany | Randomised, crossover design (February 2007) | Artificial lighting; LAN exposure | Exposure to polychromatic lights via everyday domestic lamps of different types in two intensities (130 and 500 lx at the cornea) and spectral distributions in the evening/6-day period of exposure | Melatonin | Melatonin levels decreased from 44 ± 26 at baseline to 23 ± 9 (bathroom daylight white), and 22 ± 26 (hall daylight white). Exposure to lighting conditions in the evening reduced melatonin levels both during and after the intervention, even the light with the lowest blue proportion. Yellow light showed no effect on melatonin concentrations. |
| Sander (2015) [307] | Albertslund, Copenhagen, DK | Randomised cross-over design (October 2013–November 2013) | Artificial lighting; day-time light | Exposure to two light epochs: blue-enriched light and blue-suppressed light conditions from 8 am to 1 pm/21-day period for each exposure. After 1 pm, 140 lx blue-depressed light in both epochs; 6 pm-bedtime, 100 lx blue-suppressed light | Melatonin | No significant difference between blue-enriched and blue-suppressed light for saliva melatonin concentrations. |
| Nature-based interventions | ||||||
| Pedrinolla (2019) [308] | Mantua, Italy | Single-blind randomized controlled trial (June 2015–2016) | Indoor natural environment | Exposure to indoor therapeutic garden (experimental group) or standard care environment (control group)/sessions of 2 h, 5 times/week for 6-month period | Cortisol | Significant reductions in cortisol levels, from −6.4 to −2.1 Nmol/L. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Garcia, E.; Chrysikou, E.; Kalea, A.Z. The Interplay between Housing Environmental Attributes and Design Exposures and Psychoneuroimmunology Profile—An Exploratory Review and Analysis Paper in the Cancer Survivors’ Mental Health Morbidity Context. Int. J. Environ. Res. Public Health 2021, 18, 10891. https://doi.org/10.3390/ijerph182010891
Hernandez-Garcia E, Chrysikou E, Kalea AZ. The Interplay between Housing Environmental Attributes and Design Exposures and Psychoneuroimmunology Profile—An Exploratory Review and Analysis Paper in the Cancer Survivors’ Mental Health Morbidity Context. International Journal of Environmental Research and Public Health. 2021; 18(20):10891. https://doi.org/10.3390/ijerph182010891
Chicago/Turabian StyleHernandez-Garcia, Eva, Evangelia Chrysikou, and Anastasia Z. Kalea. 2021. "The Interplay between Housing Environmental Attributes and Design Exposures and Psychoneuroimmunology Profile—An Exploratory Review and Analysis Paper in the Cancer Survivors’ Mental Health Morbidity Context" International Journal of Environmental Research and Public Health 18, no. 20: 10891. https://doi.org/10.3390/ijerph182010891
APA StyleHernandez-Garcia, E., Chrysikou, E., & Kalea, A. Z. (2021). The Interplay between Housing Environmental Attributes and Design Exposures and Psychoneuroimmunology Profile—An Exploratory Review and Analysis Paper in the Cancer Survivors’ Mental Health Morbidity Context. International Journal of Environmental Research and Public Health, 18(20), 10891. https://doi.org/10.3390/ijerph182010891







