Disturbed Lipid Metabolism in Diabetic Patients with Manifest Coronary Artery Disease Is Associated with Enhanced Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspect
2.2. Patient Selection and Tissue Harvesting
2.3. Blood Samples and Tissue Harvesting
2.4. Isolation of Cardiac Mitochondria and Mitochondrial Aldehyde Dehydrogenase 2 Activity
2.5. Western Blot Analysis
2.6. ELISA
2.7. CRP, Blood Lipids, and Lipoproteins
2.8. HPLC Assay for Dihydroethidium Oxidation Products
2.9. Statistical Analysis
3. Results
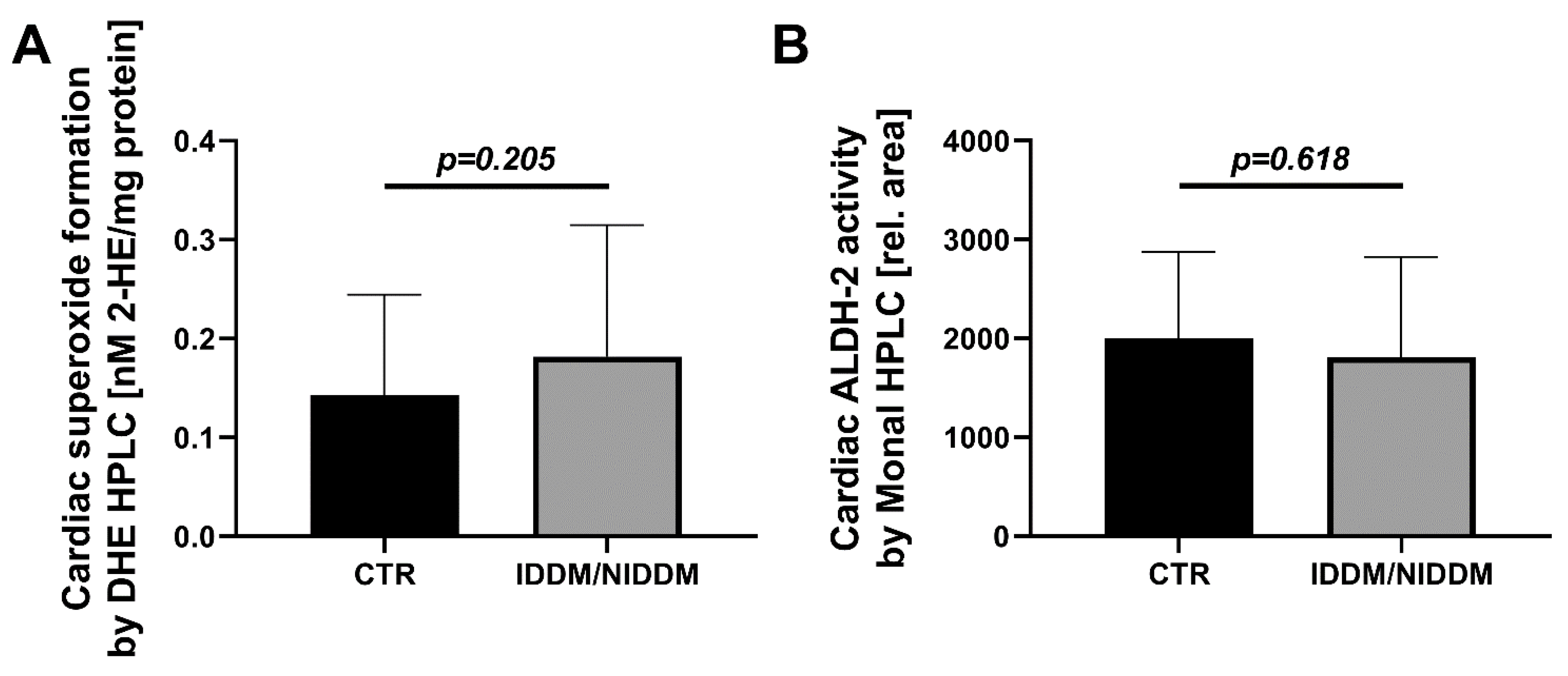
3.1. Myocardial Markers of Oxidative Stress
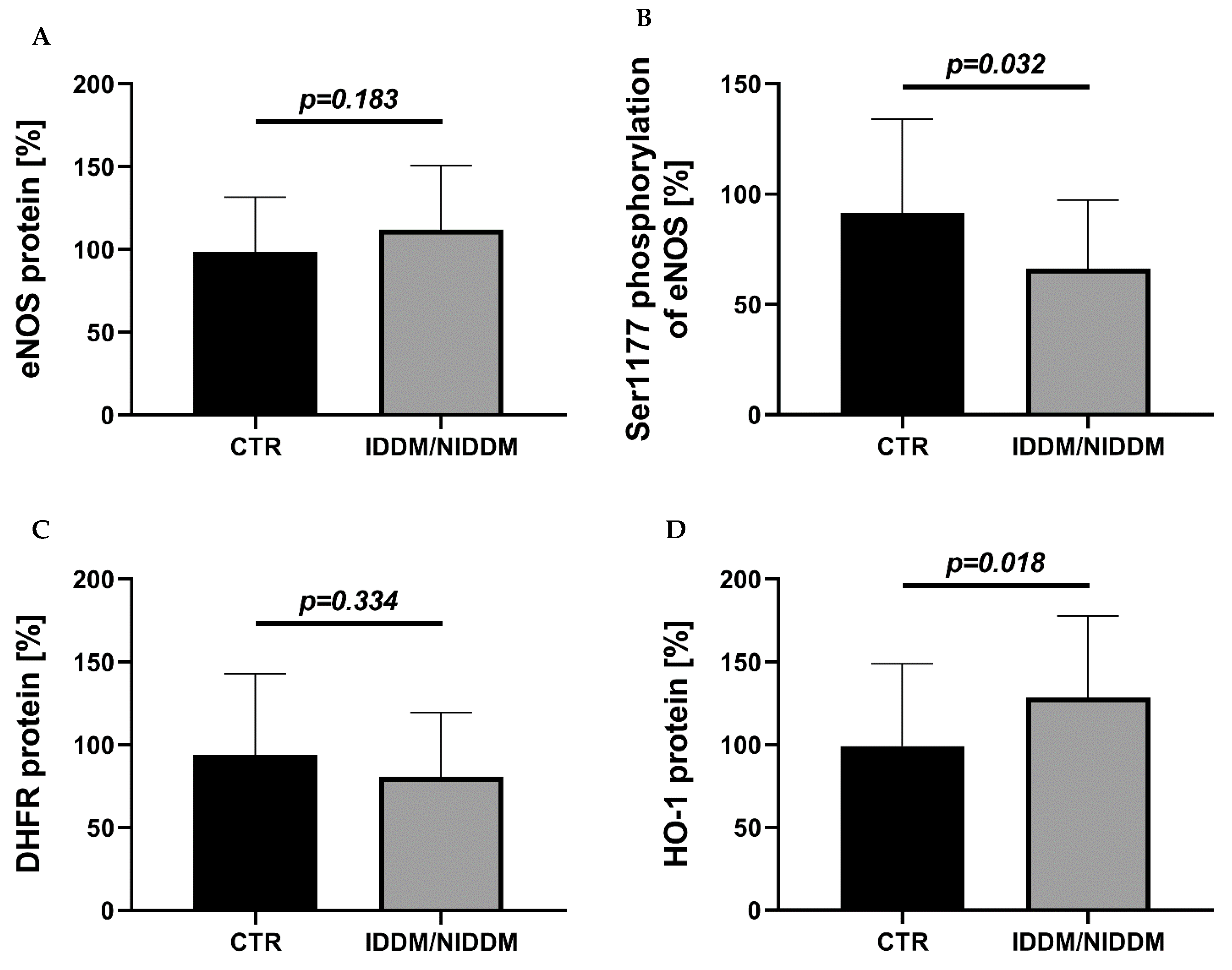
3.2. Myocardial Vasoactive Regulatory Proteins and Antioxidant Enzymes
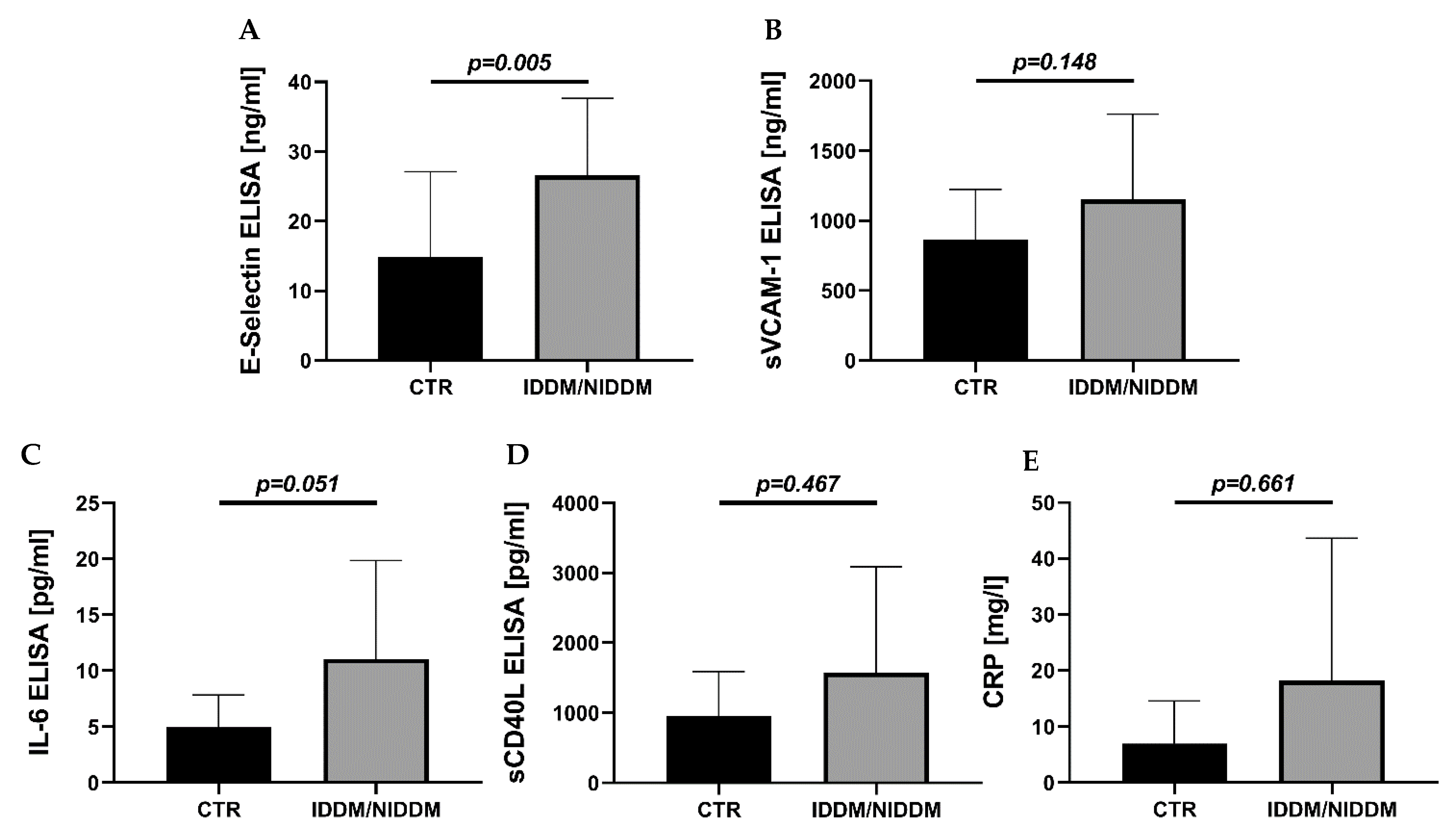
3.3. Serum Markers of Inflammation
3.4. Serum Markers of Lipid Metabolism
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, L.; Kang, Y.J. Cell Death and Diabetic Cardiomyopathy. Cardiovasc. Toxicol. 2003, 3, 219–228. [Google Scholar] [CrossRef]
- Watanabe, K.; Thandavarayan, R.A.; Harima, M.; Sari, F.R.; Gurusamy, N.; Veeraveedu, P.T.; Mito, S.; Arozal, W.; Sukumaran, V.; Laksmanan, A.P.; et al. Role of Differential Signaling Pathways and Oxidative Stress in Diabetic Cardiomyopathy. Curr. Cardiol. Rev. 2010, 6, 280–290. [Google Scholar] [CrossRef]
- Duerr, G.D.; Heinemann, J.C.; Arnoldi, V.; Feisst, A.; Kley, J.; Ghanem, A.; Welz, A.; Dewald, O. Cardiomyocyte specific peroxisome proliferator-activated receptor-α overexpression leads to irreversible damage in ischemic murine heart. Life Sci. 2014, 102, 88–97. [Google Scholar] [CrossRef]
- Parrinello, C.M.; Lutsey, P.L.; Ballantyne, C.M.; Folsom, A.R.; Pankow, J.; Selvin, E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am. Heart J. 2015, 170, 380–389.e4. [Google Scholar] [CrossRef]
- Ofstad, A.P.; Gullestad, L.; Orvik, E.; Aakhus, S.; Endresen, K.; Ueland, T.; Aukrust, P.; Fagerland, M.W.; I Birkeland, K.; Johansen, O.E. Interleukin-6 and activin A are independently associated with cardiovascular events and mortality in type 2 diabetes: The prospective Asker and Bærum Cardiovascular Diabetes (ABCD) cohort study. Cardiovasc. Diabetol. 2013, 12, 126. [Google Scholar] [CrossRef]
- Oelze, M.; Kroller-Schon, S.; Welschof, P.; Jansen, T.; Hausding, M.; Mikhed, Y.; Stamm, P.; Mader, M.; Zinssius, E.; Agdauletova, S.; et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by inter-fering with oxidative stress and glucotoxicity. PLoS ONE 2014, 9, e112394. [Google Scholar] [CrossRef] [PubMed]
- A Badreldin, A.M.; Muehle, A.; Misic, J.; Tvildiani, T.; Duerr, G.D.; Paulini-Heine, B.; Peivandi, A.A. Objective method to evaluate the competency of residents in cardiac surgery. Eur. J. Cardio Thoracic Surg. 2021, 59, 1059–1068. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Vujacic-Mirski, K.; Kalinovic, S.; Oelze, M.; Di Lisa, F.; Münzel, T. Regulation of Vascular Function and Inflammation via Cross Talk of Reactive Oxygen and Nitrogen Species from Mitochondria or NADPH Oxidase—Implications for Diabetes Progression. Int. J. Mol. Sci. 2020, 21, 3405. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Vujacic-Mirski, K.; Bayo Jimenez, M.T.; Kalinovic, S.; Kroller-Schon, S.; Munzel, T.; Daiber, A. SGLT2 inhibitors, diabetes and oxidative stress. In Diabetes—Oxidative Stress and Dietary Antioxidants, 2nd ed.; Preedy, V., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–12. [Google Scholar]
- Heitzer, T.; Finckh, B.; Albers, S.; Krohn, K.; Kohlschütter, A.; Meinertz, T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: Relation to parameters of oxidative stress. Free. Radic. Biol. Med. 2001, 31, 53–61. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Krohn, K.; Albers, S.; Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by in-creasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 2000, 43, 1435–1438. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Ramanathan, K.; Abel, J.G.; Park, J.E.; Fung, A.; Mathew, V.; Taylor, C.M.; Mancini, G.J.; Gao, M.; Ding, L.; Verma, S.; et al. Surgical Versus Percutaneous Coronary Revascularization in Patients with Diabetes and Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2017, 70, 2995–3006. [Google Scholar] [CrossRef]
- Stone, P.H.; E Muller, J.; Hartwell, T.; York, B.; Rutherford, J.D.; Parker, C.B.; Turi, Z.G.; Strauss, H.; Willerson, J.T.; Robertson, T.; et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: Contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. J. Am. Coll. Cardiol. 1989, 14, 49–57. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2013, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. eNOS Uncoupling in Cardiovascular Diseases—The Role of Oxidative Stress and Inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Euler, G.; Schulz, R. Vascular and cardiac oxidative stress and inflammation as targets for cardiopro-tection. Curr. Pharm. Des. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Howard, C.P.; Walter, V.; Everett, B.; Libby, P.; Hensen, J.; Thuren, T.; Group, C.P.I. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIb randomized, placebo-controlled trial. Circulation 2012, 126, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Jansen, T.; Wenzel, P.; Daiber, A.; Münzel, T. Nitric Oxide, Tetrahydrobiopterin, Oxidative Stress, and Endothelial Dysfunction in Hypertension. Antioxid. Redox Signal. 2008, 10, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.; Crabtree, M.J. Tetrahydrobiopterin in Cardiovascular Health and Disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Deshane, J.; Jozkowicz, A.; Agarwal, A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: Focus on angiogenesis. Circulation 2008, 117, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Junge, J.M.; Drummond, G.S. Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome. Trends Pharmacol. Sci. 2016, 37, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M. Mechanisms of Cell Protection by Heme Oxygenase. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D’Orazio, N.; Torella, M.; Gazzolo, D.; Volti, G.L. Potential Therapeutic Effects of Natural Heme Oxygenase-1 Inducers in Cardiovascular Diseases. Antioxid. Redox Signal. 2013, 18, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.; Kastelein, J.J.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.; Butterworth, A.; et al. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef]
- Xu, Y.; He, Z.; King, G.L. Introduction of hyperglycemia and dyslipidemia in the pathogenesis of diabetic vascular com-plications. Curr. Diabetes Rep. 2005, 5, 1–7. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Gramlich, Y.; Daiber, A.; Buschmann, K.; Oelze, M.; Vahl, C.-F.; Münzel, T.; Hink, U. Oxidative Stress in Cardiac Tissue of Patients Undergoing Coronary Artery Bypass Graft Surgery: The Effects of Overweight and Obesity. Oxidative Med. Cell. Longev. 2018, 2018, 6598326. [Google Scholar] [CrossRef]
- Denk, K.; Albers, J.; Kayhan, N.; Ister, D.; Bonz, A.; Werner, C.; Münzel, T.; Vahl, C.-F. Evidence for a negative inotropic effect of obesity in human myocardium? Eur. J. Cardio-Thoracic Surg. 2009, 36, 300–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Kröller-Schön, S.; Daiber, A.; Steven, S.; Oelze, M.; Frenis, K.; Kalinovic, S.; Heimann, A.; Schmidt, F.P.; Pinto, A.; Kvandova, M.; et al. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur. Heart J. 2018, 39, 3528–3539. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Dib, M.; Hausding, M.; Kashani, F.; Oelze, M.; Kröller-Schön, S.; Hanf, A.; Daub, S.; Roohani, S.; Gramlich, Y.; et al. CD40L controls obesity-associated vascular inflammation, oxidative stress, and endothelial dysfunction in high fat diet-treated and db/db mice. Cardiovasc. Res. 2017, 114, 312–323. [Google Scholar] [CrossRef]
- Giddings, J.C. Field-flow fractionation: Analysis of macromolecular, colloidal, and particulate materials. Science 1993, 260, 1456–1465. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and charac-terization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 2005, 102, 5727–5732. [Google Scholar] [CrossRef]
- Wenzel, P.; Mollnau, H.; Oelze, M.; Schulz, E.; Wickramanayake, J.M.; Muller, J.; Schuhmacher, S.; Hortmann, M.; Baldus, S.; Gori, T.; et al. First evidence for a crosstalk between mitochondrial and NADPH oxi-dase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid. Redox Signal. 2008, 10, 1435–1447. [Google Scholar] [CrossRef]
- Kroller-Schon, S.; Steven, S.; Kossmann, S.; Scholz, A.; Daub, S.; Oelze, M.; Xia, N.; Hausding, M.; Mikhed, Y.; Zinssius, E.; et al. Molecular mecha-nisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014, 20, 247–266. [Google Scholar] [CrossRef]
- Verhulst, M.J.L.; Loos, B.G.; Gerdes, V.E.A.; Teeuw, W.J. Evaluating All Potential Oral Complications of Diabetes Mellitus. Front. Endocrinol. 2019, 10, 56. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Huang, Q.; Ren, Q.; Chen, S.; Zhang, A.; Zhao, L.; Zhen, Q.; Peng, Y. Impaired beta cell function in Chinese newly diagnosed type 2 diabetes mellitus with hyperlipidemia. J. Diabetes Res. 2014, 2014, 493039. [Google Scholar] [CrossRef]
- Hao, W.; Friedman, A. The LDL-HDL Profile Determines the Risk of Atherosclerosis: A Mathematical Model. PLoS ONE 2014, 9, e90497. [Google Scholar] [CrossRef]
- Martinovic, I.; Abegunewardene, N.; Seul, M.; Vosseler, M.; Horstick, G.; Buerke, M.; Darius, H.; Lindemann, S. Elevated Monocyte Chemoattractant Protein-1 Serum Levels in Patients at Risk for Coronary Artery Disease. Circ. J. 2005, 69, 1484–1489. [Google Scholar] [CrossRef]
- Gall, T.; Balla, G.; Balla, J. Heme, Heme Oxygenase, and Endoplasmic Reticulum Stress-A New Insight into the Pathophysiology of Vascular Diseases. Int. J. Mol. Sci. 2019, 20, 3675. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Zhang, H.; Hill, M.A.; Zhang, C.; Park, Y. Interaction of IL-6 and TNF-alpha contributes to endothelial dys-function in type 2 diabetic mouse hearts. PLoS ONE 2017, 12, e0187189. [Google Scholar]
- Einbinder, Y.; Ohana, M.; Benchetrit, S.; Zehavi, T.; Nacasch, N.; Bernheim, J.; Zitman-Gal, T. Glucagon-like peptide-1 and vitamin D: Anti-inflammatory response in diabetic kidney disease in db/db mice and in cultured endothelial cells. Diabetes/Metabolism Res. Rev. 2016, 32, 805–815. [Google Scholar] [CrossRef]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef] [PubMed]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.; Vita, J.A. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Wong, W.T.; Wang, N.; Lu, Y.; Cheang, W.S.; Liu, J.; Liu, L.; Liu, Y.; Lee, S.S.; Chen, Z.Y.; et al. PPARdelta activation protects endothelial function in diabetic mice. Diabetes 2012, 61, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Tiefenbacher, C.P.; Bleeke, T.; Vahl, C.; Amann, K.; Vogt, A.; Kübler, W. Endothelial Dysfunction of Coronary Resistance Arteries Is Improved by Tetrahydrobiopterin in Atherosclerosis. Circulation 2000, 102, 2172–2179. [Google Scholar] [CrossRef]
- Yamamoto, E.; Nakamura, T.; Kataoka, K.; Tokutomi, Y.; Dong, Y.-F.; Fukuda, M.; Nako, H.; Yasuda, O.; Ogawa, H.; Kim-Mitsuyama, S. Nifedipine prevents vascular endothelial dysfunction in a mouse model of obesity and type 2 diabetes, by improving eNOS dysfunction and dephosphorylation. Biochem. Biophys. Res. Commun. 2010, 403, 258–263. [Google Scholar] [CrossRef]
- Wang, C.; Chao, Y.; Xu, W.; Liang, M.; Deng, S.; Zhang, D.; Huang, K. CTRP13 Preserves Endothelial Function by Targeting GTP Cyclohydrolase 1 in Diabetes. Diabetes 2019, 69, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. C-Reactive Protein Levels and Outcomes after Statin Therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Habeos, I.G.; Ziros, P.G.; Chartoumpekis, D.; Psyrogiannis, A.; Kyriazopoulou, V.; Papavassiliou, A.G. Simvastatin activates Keap1/Nrf2 signaling in rat liver. J. Mol. Med. 2008, 86, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Zakkar, M.; Karu, K.; Lidington, E.A.; Hamdulay, S.S.; Boyle, J.J.; Zloh, M.; Bauer, A.; Haskard, D.O.; Evans, P.C.; et al. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium ex-posed to laminar shear stress and impaired by disturbed flow. J. Biol. Chem. 2009, 284, 18882–18892. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Münzel, T.; Daiber, A. Exploiting the Pleiotropic Antioxidant Effects of Established Drugs in Cardiovascular Disease. Int. J. Mol. Sci. 2015, 16, 18185–18223. [Google Scholar] [CrossRef]
- Takemoto, M.; Liao, J.K. Pleiotropic Effects of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Cannon, C.P. Pathological Changes in Acute Coronary Syndromes: The Role of Statin Therapy in the Modulation of Inflammation, Endothelial Function and Coagulation. J. Thromb. Thrombolysis 2004, 18, 89–101. [Google Scholar] [CrossRef]
- Patel, T.N.; Shishehbor, M.H.; Bhatt, D.L. A review of high-dose statin therapy: Targeting cholesterol and inflammation in atherosclerosis. Eur. Heart J. 2007, 28, 664–672. [Google Scholar] [CrossRef]
- Adam, O.; Laufs, U. Rac1-mediated effects of HMG-CoA reductase inhibitors (statins) in cardiovascular disease. Antioxid. Redox Signal. 2014, 20, 1238–1250. [Google Scholar] [CrossRef]
- Wenzel, P.; Daiber, A.; Oelze, M.; Brandt, M.; Closs, E.; Xu, J.; Thum, T.; Bauersachs, J.; Ertl, G.; Zou, M.H.; et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozoto-cin-induced diabetes mellitus. Atherosclerosis 2008, 198, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, M.; Channon, K.M.; Antoniades, C. Statins as regulators of redox state in the vascular endothelium: Beyond lipid lowering. Antioxid. Redox Signal. 2014, 20, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, B.; Simard, T.; Ramirez, F.D.; Pourdjabbar, A.; Raizman, J.E.; Maze, R.; Wilson, K.R.; Hawken, S.; O’Brien, E.R. The effect of statins on circulating endothelial progenitor cells in humans: A systematic review. J. Cardiovasc. Pharmacol. 2013, 62, 491–496. [Google Scholar] [CrossRef]

| CTR | IDDM/NIDDM | p-Value | |
|---|---|---|---|
| Age (years) | 65.15 ± 9.08 | 66.90 ± 9.83 | 0.5782 |
| Female (%) | 61.54% | 61.11% | 0.9754 |
| Height (cm) | 1.694 ± 0.081 | 1.681 ± 0.104 | 0.5856 |
| Body weight (kg) | 80.62 ± 13.5 | 79.56 ± 12.6 | 0.7794 |
| BMI (ratio) | 27.99 ± 3.62 | 28.43 ± 5.60 | 0.7234 |
| Waist circumference (cm) | 102.4 ± 8.69 | 102.6 ± 9.53 | 0.9257 |
| Hip circumference (cm) | 100.5 ± 6.49 | 104.9 ± 8.59 | 0.1357 |
| Waist/Hip ratio | 1.018 ± 0.077 | 0.986 ± 0.096 | 0.3452 |
| Oral anti-diabetics (%) | 0.00% | 88.89% | <0.0001 |
| Insulin dependence (%) | 0.00% | 16.67% | 0.0088 |
| ACE inhibitors (%) | 35.90% | 50.00% | 0.3131 |
| AT1 antagonists (%) | 12.82% | 22.22% | 0.3656 |
| Beta-blocker (%) | 58.97% | 55.56% | 0.8080 |
| Calcium-channel blocker/%) | 20.51% | 16.67% | 0.7323 |
| Spironolactone (%) | 2.56% | 0.00% | 0.4931 |
| Statins (%) | 64.10% | 50.00% | 0.3131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buschmann, K.; Gramlich, Y.; Chaban, R.; Oelze, M.; Hink, U.; Münzel, T.; Treede, H.; Daiber, A.; Duerr, G.D. Disturbed Lipid Metabolism in Diabetic Patients with Manifest Coronary Artery Disease Is Associated with Enhanced Inflammation. Int. J. Environ. Res. Public Health 2021, 18, 10892. https://doi.org/10.3390/ijerph182010892
Buschmann K, Gramlich Y, Chaban R, Oelze M, Hink U, Münzel T, Treede H, Daiber A, Duerr GD. Disturbed Lipid Metabolism in Diabetic Patients with Manifest Coronary Artery Disease Is Associated with Enhanced Inflammation. International Journal of Environmental Research and Public Health. 2021; 18(20):10892. https://doi.org/10.3390/ijerph182010892
Chicago/Turabian StyleBuschmann, Katja, Yves Gramlich, Ryan Chaban, Matthias Oelze, Ulrich Hink, Thomas Münzel, Hendrik Treede, Andreas Daiber, and Georg Daniel Duerr. 2021. "Disturbed Lipid Metabolism in Diabetic Patients with Manifest Coronary Artery Disease Is Associated with Enhanced Inflammation" International Journal of Environmental Research and Public Health 18, no. 20: 10892. https://doi.org/10.3390/ijerph182010892
APA StyleBuschmann, K., Gramlich, Y., Chaban, R., Oelze, M., Hink, U., Münzel, T., Treede, H., Daiber, A., & Duerr, G. D. (2021). Disturbed Lipid Metabolism in Diabetic Patients with Manifest Coronary Artery Disease Is Associated with Enhanced Inflammation. International Journal of Environmental Research and Public Health, 18(20), 10892. https://doi.org/10.3390/ijerph182010892








