Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials
Abstract
:1. Introduction
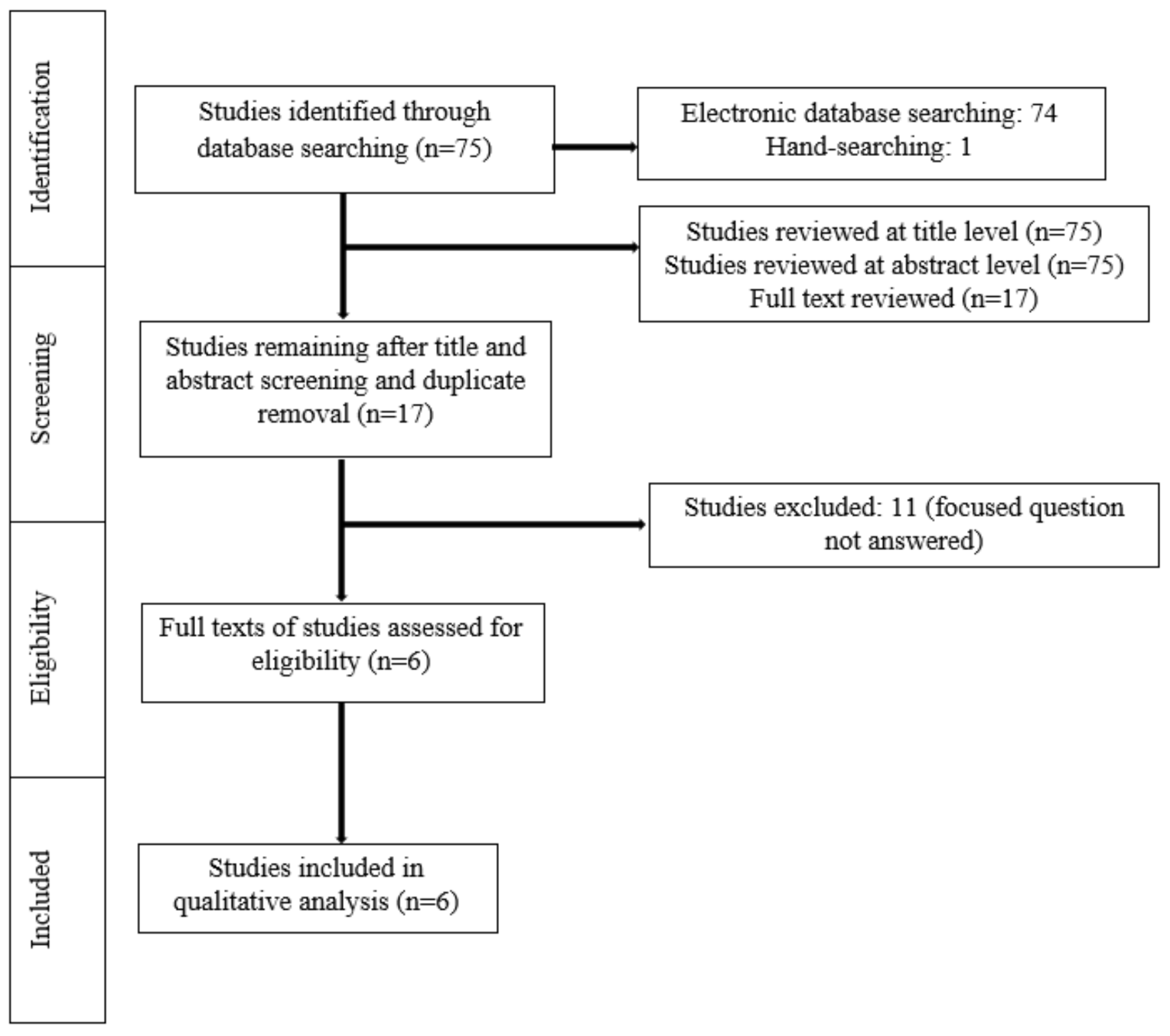
2. Materials and Methods
3. Results
3.1. Study Selection
3.2. General Characteristics of the Clinical Trials Assessed
3.3. Type, Concentration and Daily Frequency of Mouthwash Usage
3.4. Main Study Outcomes
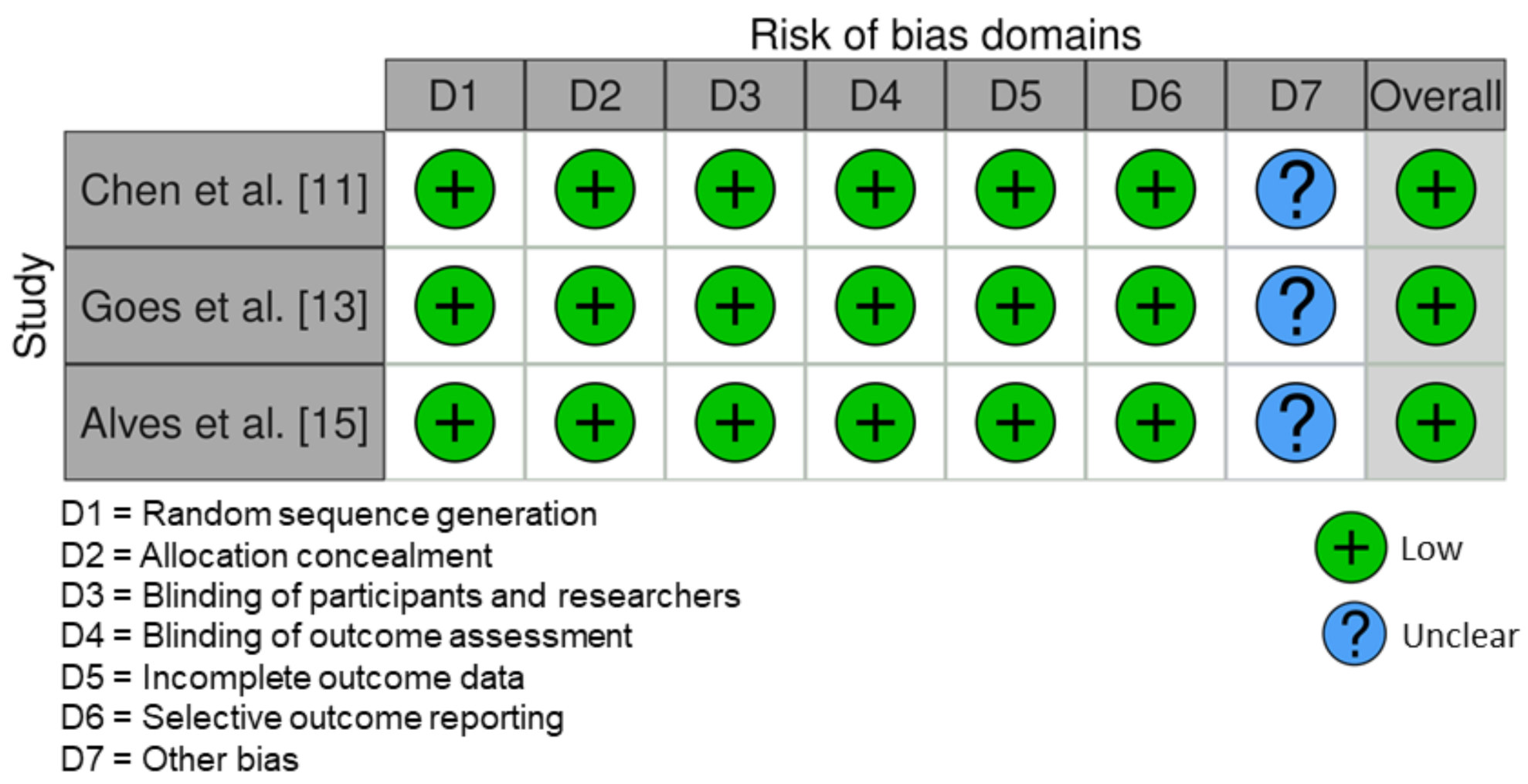
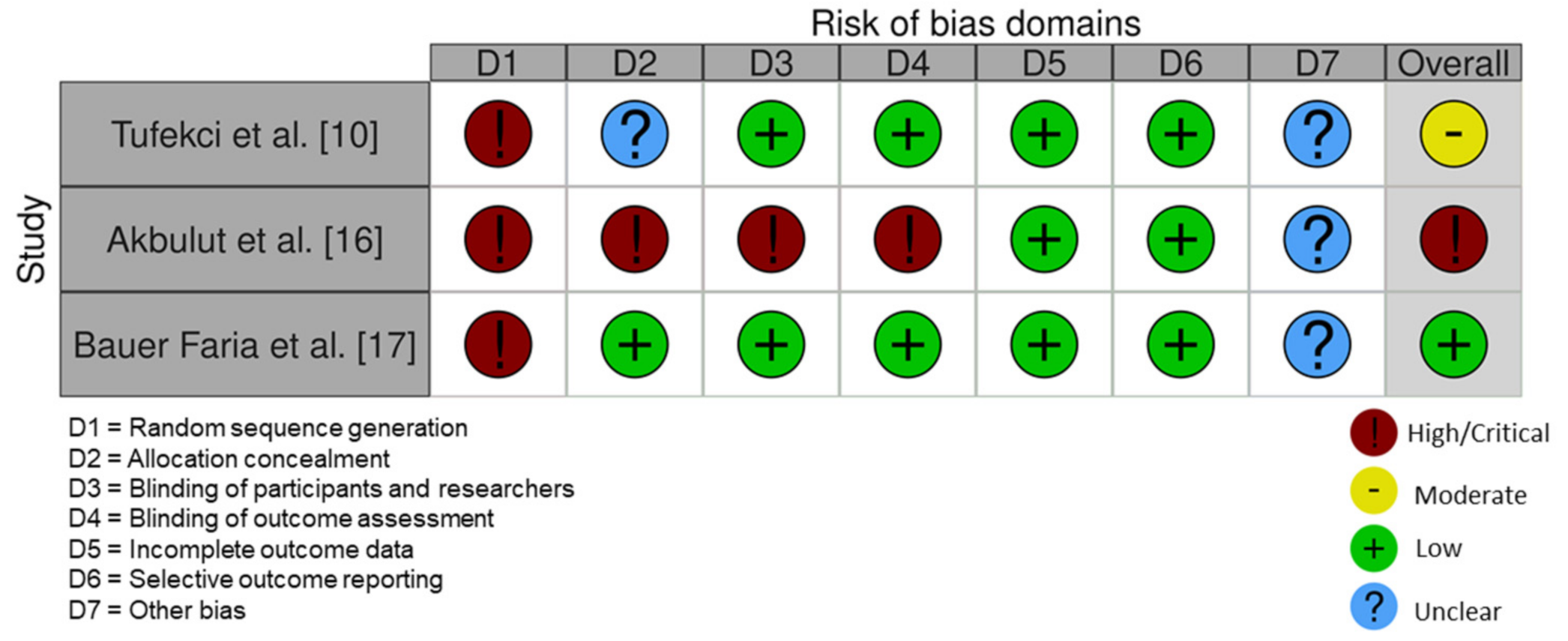
3.5. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dagli, N.; Dagli, R.J.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintans, J.S.; Brito, R.G.; Aquino, P.G.; França, P.H.; Siqueira-Lima, P.S.; Santana, A.E.; Ribeiro, E.A.; Salvador, M.J.; Araújo-Júnior, J.X.; Quintans-Júnior, L.J. Antinociceptive activity of Syzygium cumini leaves ethanol extract on orofacial nociception protocols in rodents. Pharm. Biol. 2014, 52, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Bonjardim, L.R.; Silva, A.M.; Oliveira, M.G.B.; Guimarães, A.G.; Antoniolli, A.R.; Santana, M.F.; Serafini, M.R.; Santos, R.C.; Araújo, A.A.S.; Estevam, C.S.; et al. Sida cordifolia Leaf Extract Reduces the Orofacial Nociceptive Response in Mice. Phytotherapy Res. 2011, 25, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Zabirunnisa, M.; Gadagi, J.S.; Gadde, P.; Koneru, J.; Myla, N.; Thatimatla, C. Dental patient anxiety: Possible deal with Lavender fragrance. J. Res. Pharm. Pract. 2014, 3, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Hasheminia, D.; Motamedi, M.R.K.; Ahmadabadi, F.K.; Hashemzehi, H.; Haghighat, A. Can Ambient Orange Fragrance Reduce Patient Anxiety During Surgical Removal of Impacted Mandibular Third Molars? J. Oral Maxillofac. Surg. 2014, 72, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.J.; Campbell, P.M.; Rees, T.D.; Buschang, P.H. A randomized controlled trial evaluating antioxidant–essential oil gel as a treatment for gingivitis in orthodontic patients. Angle Orthod. 2016, 86, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria, M.; Petermann, K.D.; Vedovello, S.A.S.; Degan, V.; Lucato, A.; Franzini, C.M. Antimicrobial effect of Melaleuca alternifolia dental gel in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, E.; Casagrande, Z.A.; Lindauer, S.J.; Fowler, C.E.; Williams, K.T. Effectiveness of an Essential Oil Mouthrinse in Improving Oral Health in Orthodontic Patients. Angle Orthod. 2008, 78, 294–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wong, R.W.K.; Seneviratne, C.J.; Hagg, U.; McGrath, C.P.J.; Samaranayake, L.P. The effects of natural compounds-containing mouthrinses on patients with fixed orthodontic appliance treatment: Clinical and microbiological outcomes. Int. J. Paediatr. Dent. 2012, 23, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kotsailidi, E.A.; Kalogirou, E.-M.; Michelogiannakis, D.; Vlachodimitropoulos, D.; Tosios, K.I. Hypersensitivity reaction of the gingiva to chlorhexidine: Case report and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Goes, P.; Dutra, C.S.; Lisboa, M.R.P.; Gondim, D.V.; Leitão, R.; Brito, G.A.C.; Rego, R.O. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J. Oral Sci. 2016, 58, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachojannis, C.; Chrubasik-Hausmann, S.; Hellwig, E.; Al-Ahmad, A. A Preliminary Investigation on the Antimicrobial Activity of Listerine®, Its Components, and of Mixtures Thereof. Phytother. Res. 2015, 29, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Zenóbio, E.; Alves, K.M.; Goursand, D.; Cruz, R.A. Effectiveness of Procedures for the Chemical-Mechanical Control of Dental Biofilm in Orthodontic Patients. J. Contemp. Dent. Pract. 2010, 11, 41–48. [Google Scholar] [CrossRef]
- Akbulut, Y. The effects of different antiseptic mouthwash on microbiota around orthodontic mini-screw. Niger. J. Clin. Pract. 2020, 23, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Bauer Faria, T.R.; Furletti-Goes, V.F.; Franzini, C.M.; de Aro, A.A.; de Andrade, T.A.M.; Sartoratto, A.; de Menezes, C.C. Anti-inflammatory and antimicrobial effects of Zingiber officinale mouthwash on patients with fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, T.W. The Importance of A Priori Sample Size Estimation in Strength and Conditioning Research. J. Strength Cond. Res. 2013, 27, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
| Authors | Type of Study | Participants | Gender | Age in Years (Range) | Clinical Indices | Duration of Follow-Up |
|---|---|---|---|---|---|---|
| Tufekci et al. (2008) [10] | Non-RCT | 47 | 20 M 27 F | 16.6 years * (10–64) | PI, BI and MGI | 90 and 180 days |
| Chen et al. (2013) [11] | RCT | 79 | NR | 17.7 ± 3.9 years | PI, BI and MGI | 90 and 180 days |
| Goes et al. (2016) [13] | RCT | 30 | 4 M 26 F | 28.8 ± 3.28 years (10–40) | VPI and GBI | 15 days |
| Alves et al. (2010) [15] | RCT | 30 | 10 M 20 F | 12–21 years | VPI and GI | 60 days |
| Akbulut ** (2020) [16] | Non-RCT | 38 (60 mini screws) | 18 M 20 F | NR | MPI and MGI | 21 days |
| Bauer Faria et al. (2021) [17] | Non-RCT | 31 | 14 M 17 F | 19.96 years (12–35) | GBI | 7 days |
| Authors (Year) | Test Group (n = Number of Patients) | Control Group (n = Number of Patients) | Power Analysis |
|---|---|---|---|
| Tufekci et al. (2008) [10] | EO-based MW (n = 24) | No MW (n = 23) | Yes |
| Chen et al. (2013) [11] | EO-based MW 1 (n = 28) EO-based MW 2 (n = 25) | No MW (n = 26) | Yes |
| Goes et al. (2016) [13] | EO-based MW (n = 10) | CHX (n = 10) Placebo MW (n = 10) | No |
| Alves et al. (2010) [15] | EO-based MW (n = 10) | Placebo MW (n = 10) No MW (n = 10) | No |
| Akbulut (2020) [16] | EO-based MW (n = NR) | CHX (n = NR) Povidone-iodine MW (n = NR) No MW (n = NR) | No |
| Bauer Faria et al. (2021) [17] | EO-based MW (n = 31) | CHX (n = 31) Placebo MW (n = 31) | Yes |
| Authors (Year) | Type of MW | Concentration of MW | Daily Frequency of Usage | |||
|---|---|---|---|---|---|---|
| Test-Group | Control-Group | Test-Group | Control-Group | Test-Group | Control-Group | |
| Tufekci et al. (2008) [10] | Listerine® | No MW | NR | NA | 2× daily | NA |
| Chen et al. (2013) [11] | Listerine® Fructus mume | No MW | NR 2.5% | NA | 2× daily 2× daily | NA |
| Goes et al. (2016) [13] | Matricaria chamomilla L. | CHX Placebo | 1% | 0.12% NR | 2× daily | 2× daily 2× daily |
| Alves et al. (2010) [15] | Listerine® | Placebo No MW | NR | NR NA | 2× daily | 2× daily NA |
| Akbulut (2020) [16] | Listerine® | CHX Povidone iodine No MW | NR | 0.12% 7.5% NA | NR | NR NR NA |
| Bauer Faria et al. (2021) [17] | Zingiber officinale | CHX Distilled water | 0.5% | 0.12% NA | NR | NR NA |
| Authors (Year) | Main Outcomes | Side-Effects/Complications | Conclusion |
|---|---|---|---|
| Tufekci et al. (2008) [10] | BI, MGI and PI were significantly higher in the group that did not use a mouthwash compared with the Listerine® group after 90 and 180 days. | N/R | Listerine® is effective in decreasing plaque accumulation and gingival bleeding in orthodontic patients. |
| Chen et al. (2013) [11] |
| No complications were mentioned by the patients. | Listerine® and FM mouthwashes lead to decreased bleeding of gingival tissue in patients undergoing fixed orthodontic treatment. |
| Goes et al. (2016) [13] |
|
|
|
| Alves et al. (2010) [15] |
| N/R | Listerine® is an effective adjunct to oral hygiene in patients undergoing orthodontic treatment. |
| Akbulut (2020) [16] |
| N/R | CHX, Listerine® and povidone-iodine are effective in promoting oral health in patients with orthodontic mini screws. |
| Bauer Faria et al. (2021) [17] | GBI was significantly higher in the CHX group compared with the ZO group after 7 days. | ZO and CHX have a low taste tolerance. | ZO reduces gingival bleeding and oral biofilm accumulation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotou, A.; Rossouw, P.E.; Michelogiannakis, D.; Javed, F. Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 10825. https://doi.org/10.3390/ijerph182010825
Panagiotou A, Rossouw PE, Michelogiannakis D, Javed F. Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials. International Journal of Environmental Research and Public Health. 2021; 18(20):10825. https://doi.org/10.3390/ijerph182010825
Chicago/Turabian StylePanagiotou, Aristeidis, P. Emile Rossouw, Dimitrios Michelogiannakis, and Fawad Javed. 2021. "Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials" International Journal of Environmental Research and Public Health 18, no. 20: 10825. https://doi.org/10.3390/ijerph182010825
APA StylePanagiotou, A., Rossouw, P. E., Michelogiannakis, D., & Javed, F. (2021). Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials. International Journal of Environmental Research and Public Health, 18(20), 10825. https://doi.org/10.3390/ijerph182010825








