Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
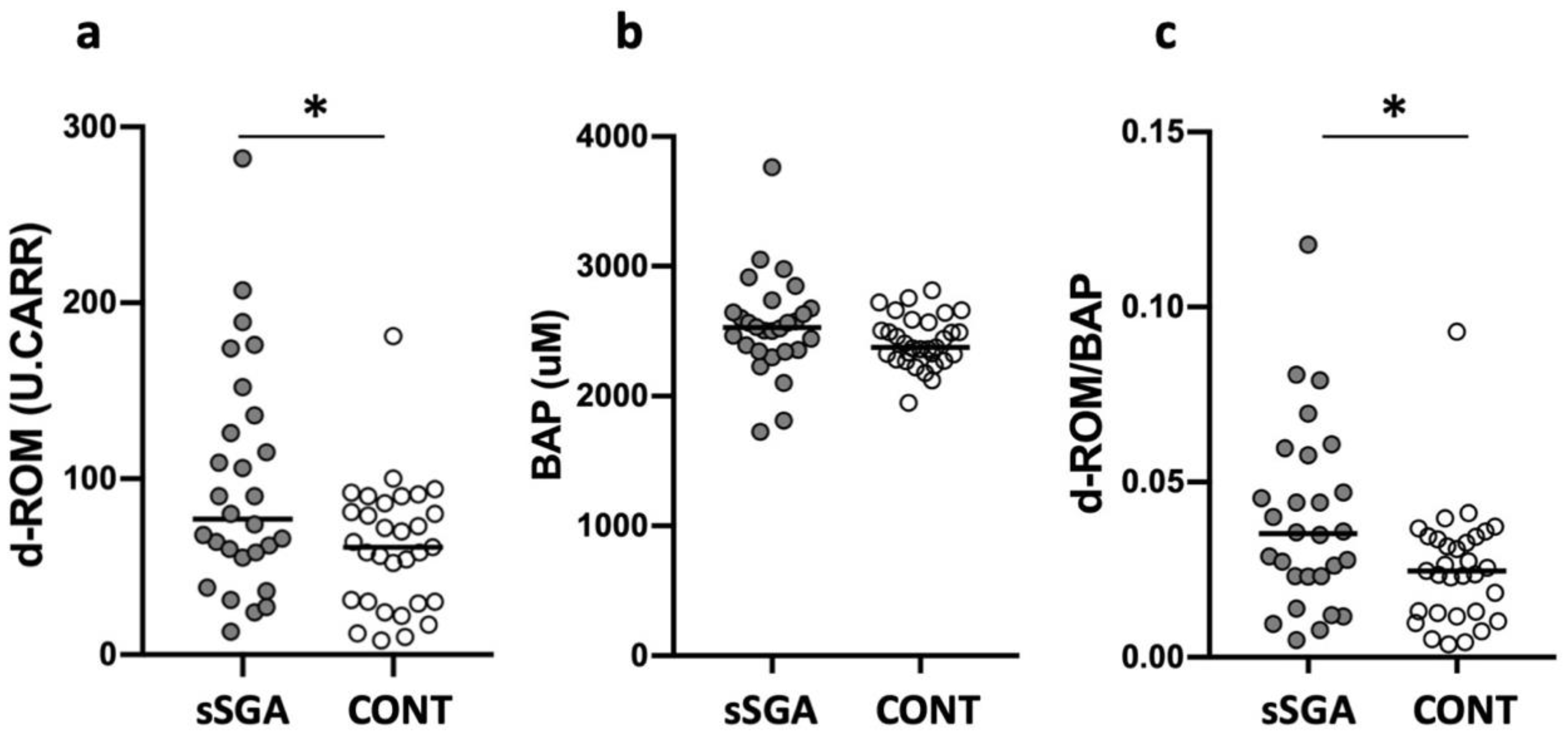
3.2. Oxidative Stress, Antioxidant Capacity, and Oxidant to Antioxidant Ratio in sSGA and Control Infants
3.3. Oxidative Stress, Antioxidant Capacity, and Oxidant to Antioxidant Ratio in Preterm and Term sSGA Infants
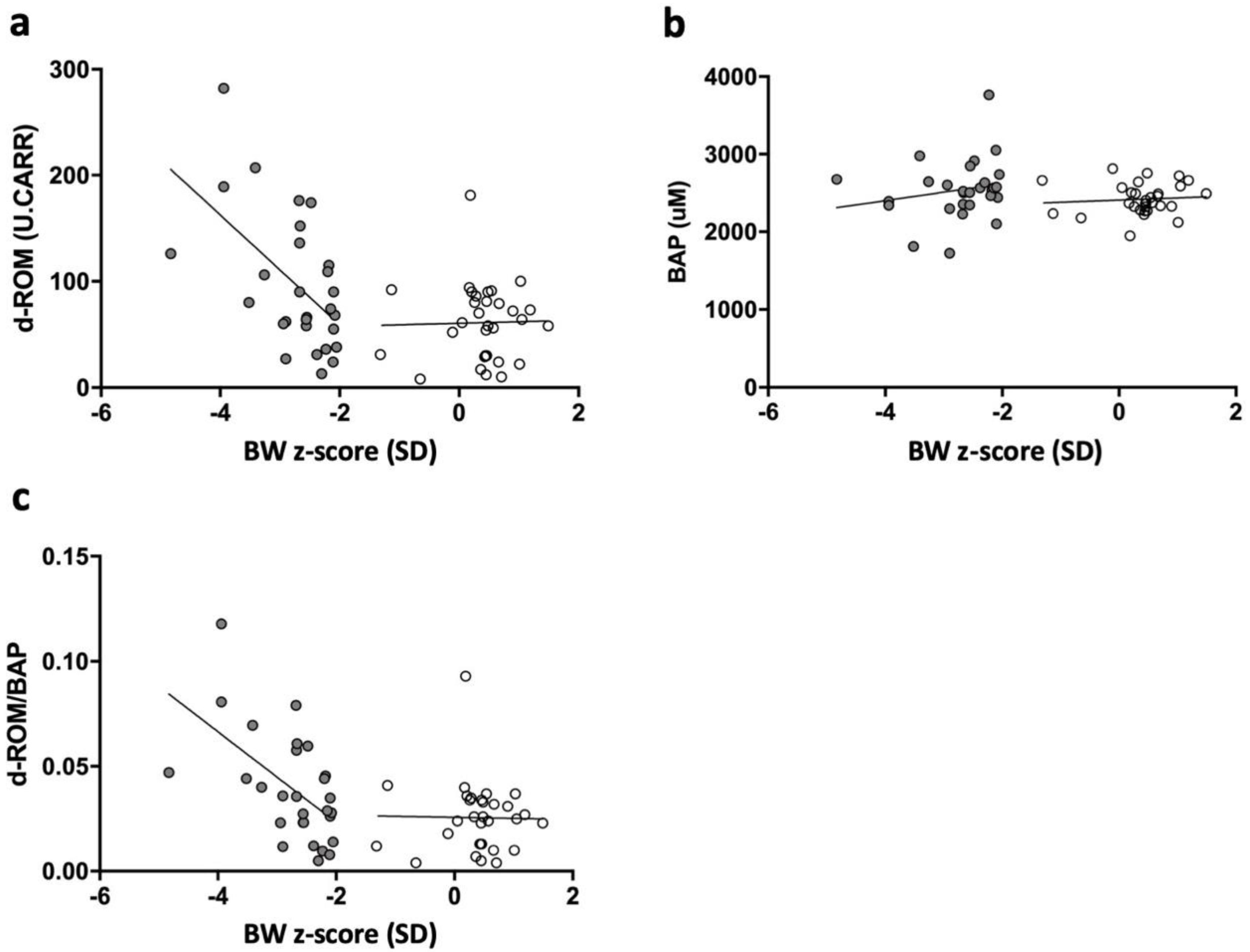
3.4. Correlation among Serum d-ROM, BAP, d-ROM/BAP Level, and BW z-Scores in sSGA and Control Infants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashina, M.; Fujioka, K.; Yoshimoto, S.; Ioroi, T.; Iijima, K. Incidence of hypospadias in severe small-for-gestational-age infants: A multicenter Asian population study. Pediatr. Neonatol. 2020, 61, 548–550. [Google Scholar] [CrossRef]
- Cohen, E.; Wong, F.Y.; Horne, R.S.; Yiallourou, S.R. Intrauterine growth restriction: Impact on cardiovascular development and function throughout infancy. Pediatr. Res. 2016, 79, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.A.; Chernausek, S.D.; Hokken-Koelega, A.C.; Czernichow, P.; for the International SGA Advisory Board. International Small for Gestational Age Advisory Board consensus development conference statement: Management of short children born small for gestational age, 24 April–1 October 2001. Pediatrics 2003, 111, 1253–1261. [Google Scholar] [CrossRef]
- Itabashi, K.; Miura, F.; Uehara, R.; Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 2014, 56, 702–708. [Google Scholar] [CrossRef]
- Fujioka, K.; Nishida, K.; Ashina, M.; Abe, S.; Fukushima, S.; Ikuta, T.; Ohyama, S.; Morioka, I.; Iijima, K. DNA methylation of the Rtl1 promoter in the placentas with fetal growth restriction. Pediatr. Neonatol. 2019, 60, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Mandai, T.; Iwatani, S.; Koda, T.; Nagasaka, M.; Fujita, K.; Kurokawa, D.; Yamana, K.; Nishida, K.; Taniguchi-Ikeda, M.; et al. Extremely preterm infants small for gestational age are at risk for motor impairment at 3 years corrected age. Brain Dev. 2016, 38, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Narang, M.; Banerjee, B.D.; Basu, S. Oxidative stress in term small for gestational age neonates born to undernourished mothers: A case control study. BMC Pediatr. 2004, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gveric-Ahmetasevic, S.; Sunjic, S.B.; Skala, H.; Andrisic, L.; Stroser, M.; Zarkovic, K.; Skrablin, S.; Tatzber, F.; Cipak, A.; Jaganjac, M.; et al. Oxidative stress in small-for-gestational age (SGA) term newborns and their mothers. Free Radic. Res. 2009, 43, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Faes, C.; Debevec, T.; Rytz, C.; Millet, G.; Pialoux, V. Preterm birth and oxidative stress: Effects of acute physical exercise and hypoxia physiological responses. Redox Biol. 2018, 17, 315–322. [Google Scholar] [CrossRef]
- Vento, M.; Moro, M.; Escrig, R.; Arruza, L.; Villar, G.; Izquierdo, I.; Roberts, L.J., 2nd; Arduini, A.; Escobar, J.J.; Sastre, J.; et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009, 124, e439–e449. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Y.; An, Y.; Jiao, W.; Xu, Y.; Han, Q.; Teng, X.; Teng, X. Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes. Theriogenology 2019, 131, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, J.; Sun, Q.; Xu, Y.; Teng, X. Oxidative stress and mitochondrial dysfunction involved in ammonia-induced nephrocyte necroptosis in chickens. Ecotoxicol. Environ. Saf. 2020, 203, 110974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ali Shah, S.W.; Zhou, Q.; Yin, X.; Teng, X. The contributions of miR-25-3p, oxidative stress, and heat shock protein in a complex mechanism of autophagy caused by pollutant cadmium in common carp (Cyprinus carpio L.) hepatopancreas. Environ. Pollut. 2021, 287, 117554. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Tanimura, K.; Morizane, M.; Fujioka, K.; Morioka, I.; Oohashi, M.; Minematsu, T.; Yamada, H. Clinical factors associated with congenital cytomegalovirus infection: A cohort study of pregnant women and newborns. Clin. Infect. Dis. 2020, 71, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Shibata, A.; Yokota, T.; Koda, T.; Nagasaka, M.; Yagi, M.; Takeshima, Y.; Yamada, H.; Iijima, K.; Morioka, I. Association of a vascular endothelial growth factor polymorphism with the development of bronchopulmonary dysplasia in Japanese premature newborns. Sci. Rep. 2014, 4, 4459. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rodriguez, M.A.; Mendoza-Nunez, V.M. Oxidative stress indexes for diagnosis of health or disease in humans. Oxid. Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Hussain, N.; Chaghtai, A.; Herndon, C.D.; Herson, V.C.; Rosenkrantz, T.S.; McKenna, P.H. Hypospadias and early gestation growth restriction in infants. Pediatrics 2002, 109, 473–478. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Lu, D.; Hammarstrom, L.; Cnattingius, S.; Fang, F. Small for gestational age and risk of childhood mortality: A Swedish population study. PLoS Med 2018, 15, e1002717. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Iwasaki, A.; Mori, T.; Kimura, C.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. PP043. Oxidative stress in the maternal body also affects the fetus in preeclamptic women with fetal growth restriction. Pregnancy Hypertens. 2013, 3, 82. [Google Scholar] [CrossRef]
- Hracsko, Z.; Orvos, H.; Novak, Z.; Pal, A.; Varga, I.S. Evaluation of oxidative stress markers in neonates with intra-uterine growth retardation. Redox Rep. 2008, 13, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.D.; Costantine, M.M. The role of statins in the prevention of preeclampsia. Am. J. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, G.; Perrone, S.; Longini, M.; Vezzosi, P.; Marzocchi, B.; Paffetti, P.; Bracci, R. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr. Res. 2002, 52, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Kamath, U.; Rao, G.; Kamath, S.U.; Rai, L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR). Indian J. Clin. Biochem. 2006, 21, 111–115. [Google Scholar] [CrossRef] [Green Version]

| sSGA n = 28 | CONT n = 31 | p Value | |
|---|---|---|---|
| Maternal data | |||
| Maternal age, years | 33 (24–40) | 34 (22–42) | 0.81 |
| Threatened preterm labor | 4 (14.3%) | 6 (19.4%) | 0.60 |
| Premature rupture of membrane | 2 (7.1%) | 6 (19.4%) | 0.17 |
| Hypertensive disorder of pregnancy | 6 (21.4%) | 6 (19.4%) | 0.84 |
| Smoking | 1 (3.6%) | 1 (3.2%) | 0.94 |
| Multiple pregnancy | 3 (10.7%) | 0 (0%) | 0.06 |
| Cesarean section | 17 (60.7%) | 17 (54.8%) | 0.65 |
| Neonatal data | |||
| GA, weeks | 36 (23–40) | 35 (23–40) | 0.88 |
| Extremely preterm infants (<28 weeks) | 4 (14.3%) | 5 (16.1%) | 0.84 |
| Preterm infants (28–36 weeks) | 12 (42.9%) | 11 (35.5%) | 0.56 |
| Term infants (≥37 weeks) | 12 (42.9%) | 15 (48.4%) | 0.65 |
| Male | 13 (46.4%) | 18 (58.1%) | 0.37 |
| BW, g | 1728 (284–2434) | 2560 (420–3516) | <0.01 |
| BW Z-score, SD | −2.6 (−4.8–−2.1) | 0.5 (−1.3–1.5) | <0.0001 |
| Height, cm | 40.4 (27.0–47.5) | 47.0 (27.0–51.2) | <0.01 |
| Head circumference, cm | 29.1 (20.2–40.4) | 32.0 (19.6–35.0) | 0.02 |
| Asymmetrical SGA * | 14 (50%) | - | - |
| Apgar score at 5 min | 9 (1–10) | 9 (5–10) | 0.07 |
| NRFS | 3 (10.7%) | 0 (0%) | 0.06 |
| Neonatal blood gas data | |||
| pH | 7.3 (7.2–7.6) | 7.3 (7.2–7.5) | 0.57 |
| Lactate | 3.6 (1.6–11.5) | 3.0 (0.9–6.7) | 0.10 |
| Base excess | −1.1 (−12.4–6.1) | 0.2 (−6–6.4) | 0.24 |
| HCO3 | 24.5 (12.6–29.6) | 25.3 (20.1–30.6) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashina, M.; Kido, T.; Kyono, Y.; Yoshida, A.; Suga, S.; Nakasone, R.; Abe, S.; Tanimura, K.; Nozu, K.; Fujioka, K. Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants. Int. J. Environ. Res. Public Health 2021, 18, 10726. https://doi.org/10.3390/ijerph182010726
Ashina M, Kido T, Kyono Y, Yoshida A, Suga S, Nakasone R, Abe S, Tanimura K, Nozu K, Fujioka K. Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants. International Journal of Environmental Research and Public Health. 2021; 18(20):10726. https://doi.org/10.3390/ijerph182010726
Chicago/Turabian StyleAshina, Mariko, Takumi Kido, Yuki Kyono, Asumi Yoshida, Shutaro Suga, Ruka Nakasone, Shinya Abe, Kenji Tanimura, Kandai Nozu, and Kazumichi Fujioka. 2021. "Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants" International Journal of Environmental Research and Public Health 18, no. 20: 10726. https://doi.org/10.3390/ijerph182010726
APA StyleAshina, M., Kido, T., Kyono, Y., Yoshida, A., Suga, S., Nakasone, R., Abe, S., Tanimura, K., Nozu, K., & Fujioka, K. (2021). Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants. International Journal of Environmental Research and Public Health, 18(20), 10726. https://doi.org/10.3390/ijerph182010726







