Urinary Proteomics of Simulated Firefighting Tasks and Its Relation to Fitness Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethical Approval
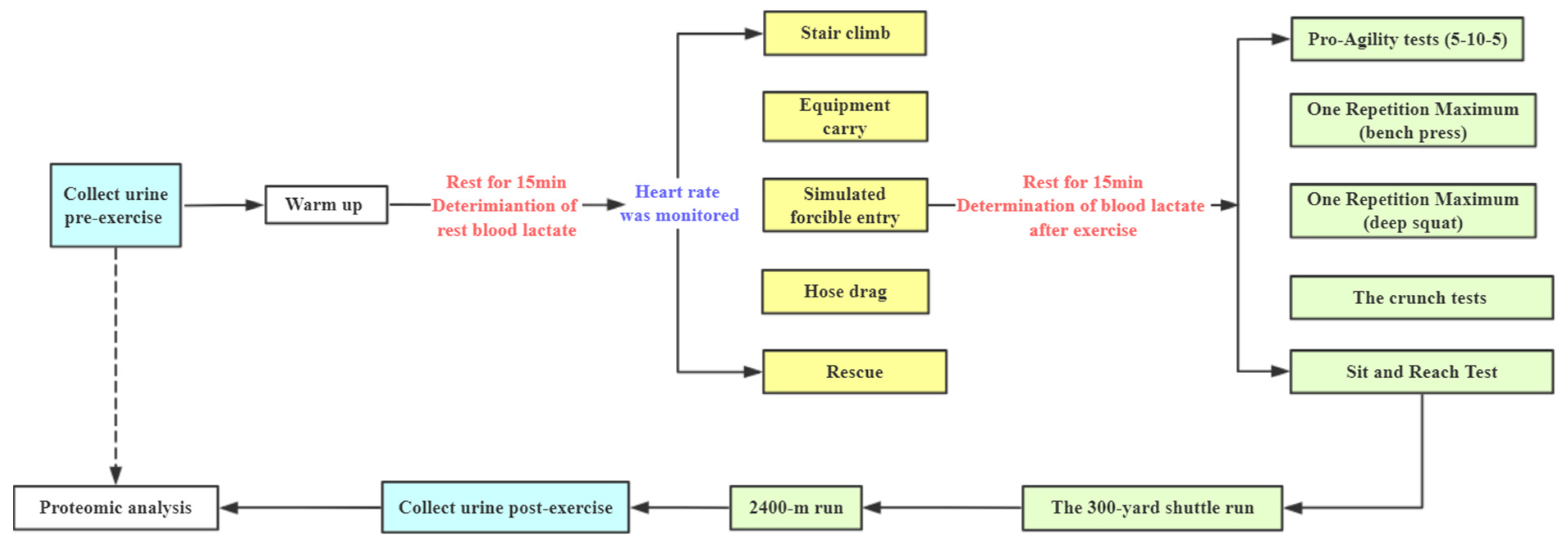
2.3. Simulated Firefighting Tasks
2.4. Performance Tests
2.4.1. Pro-Agility (5–10–5)
2.4.2. One Repetition Maximum
2.4.3. The Crunch Tests
2.4.4. The Sit-and-Reach Test
2.4.5. The 300-Yard Shuttle Run
2.4.6. 2400 Run
2.5. The Combined Simulated Firefighting Tasks Procedures
2.6. Urine Proteomics
2.6.1. Sample Pretreatment
2.6.2. LC–MS/MS Analysis
2.7. Statistical Analysis
3. Results
3.1. The Results of Physical Performance Tests and Physiological Responses of the Combined Simulated Firefighting Tasks
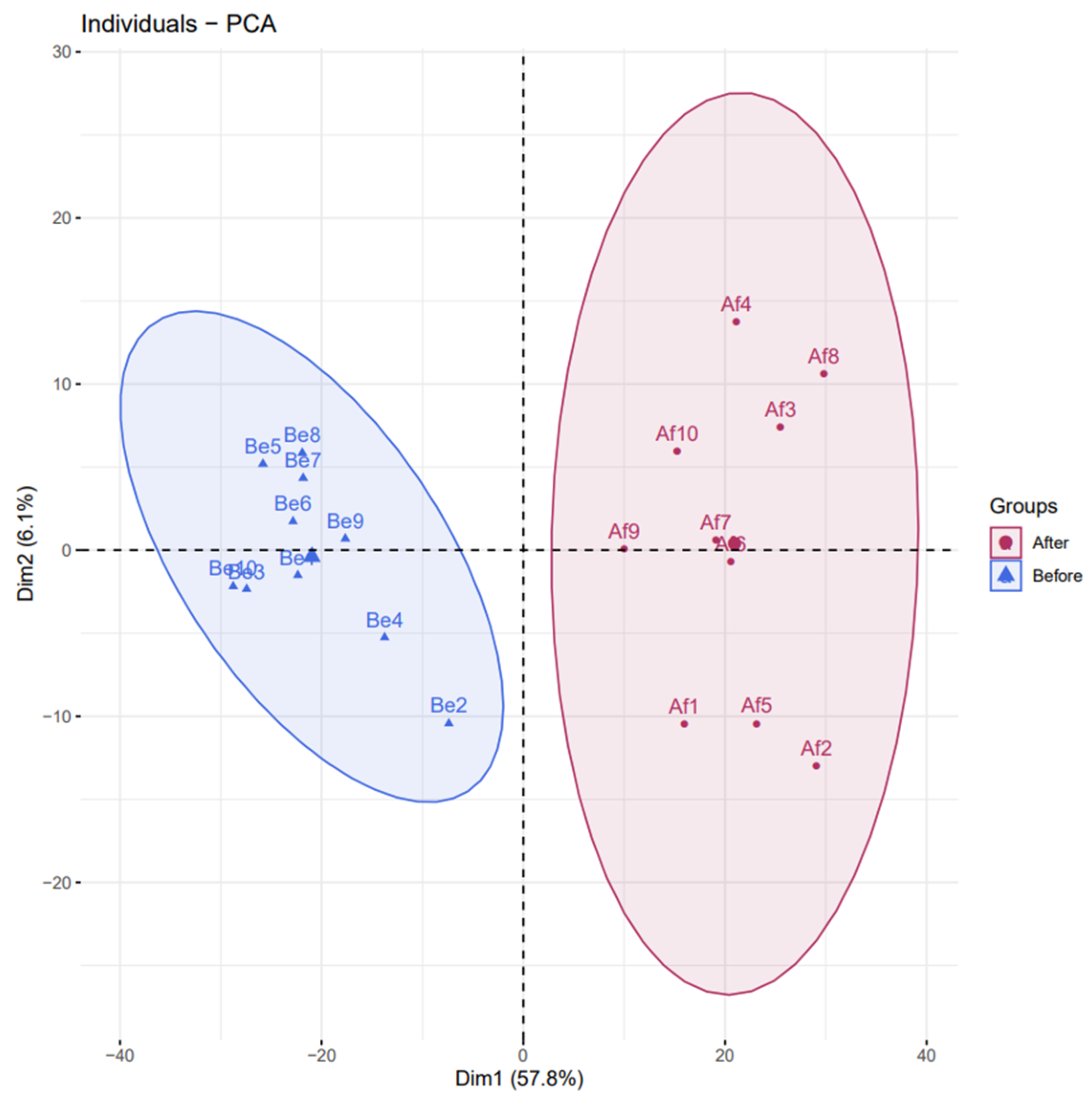
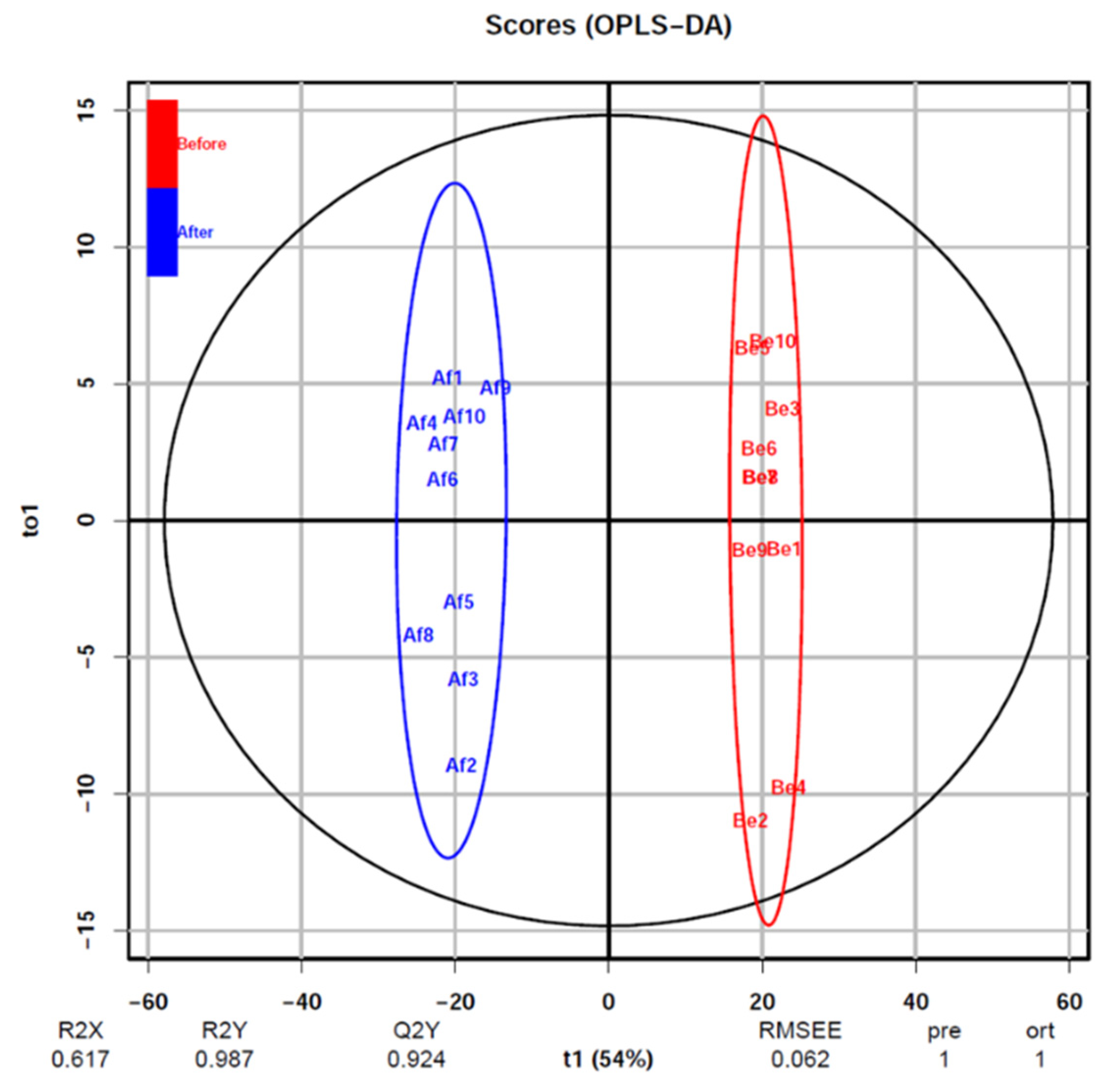
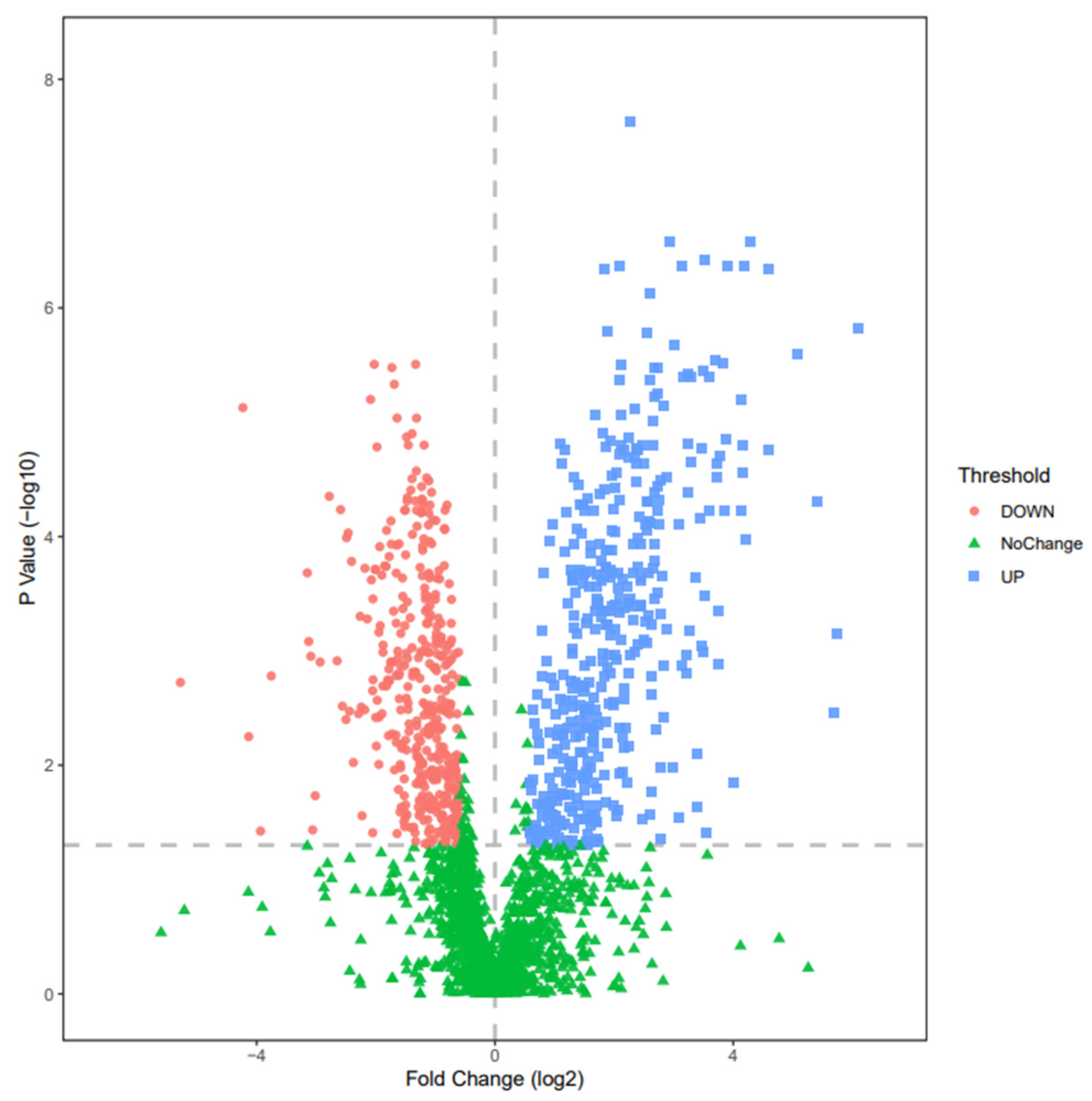
3.2. Changes of Urine Proteomics before and after the Combined Simulated Firefighting Tasks
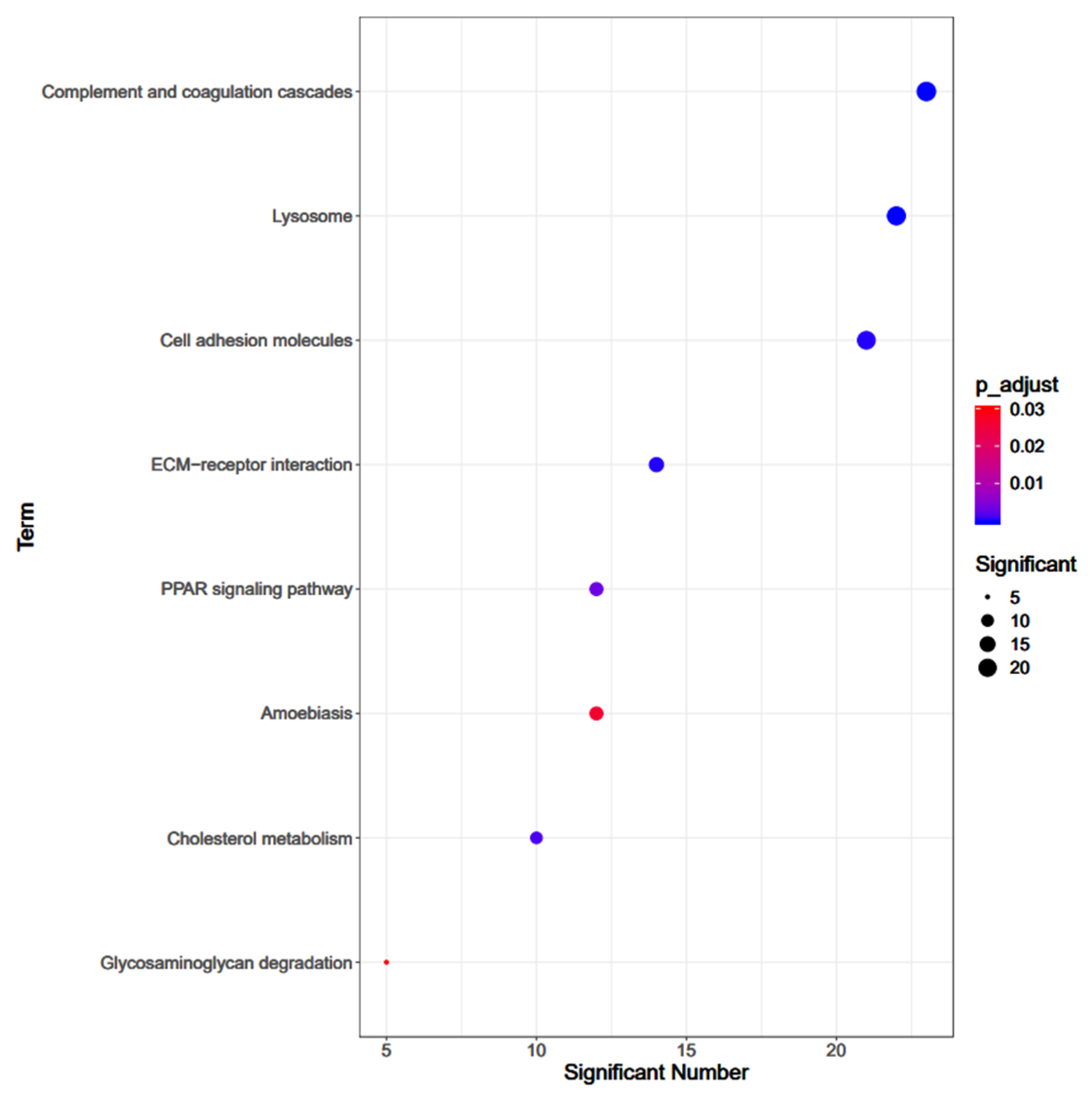
3.3. Pathway Enrichment Analysis
3.4. GO Enrichments
3.5. Correlations between the Physical Performance and Simulated Firefighting Tests
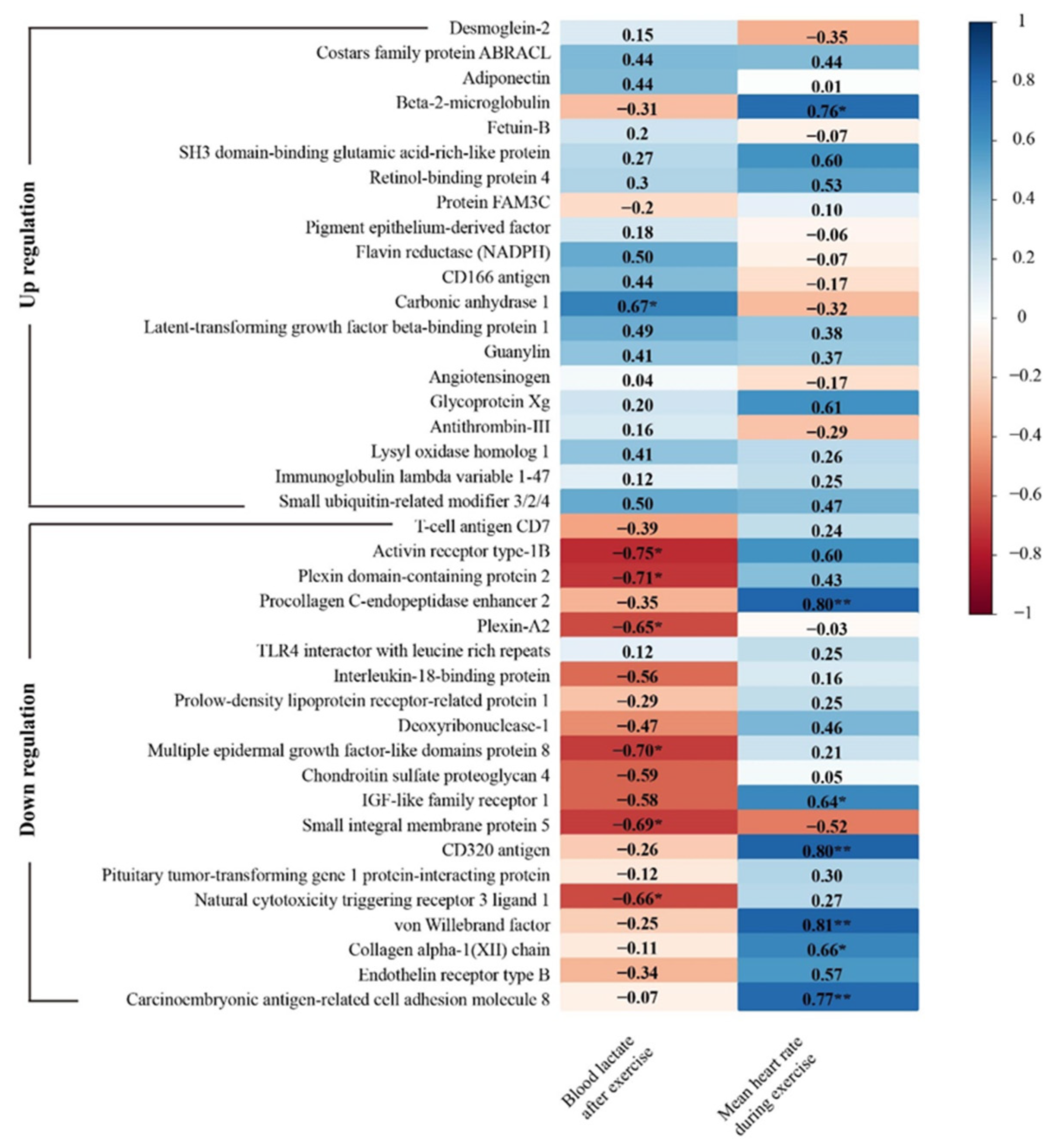
3.6. Correlations between the Urine Proteomics Changes and Simulated Firefighting Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunadharaju, K.; Smith, T.D.; DeJoy, D.M. Line–of–duty deaths among U.S. firefighters: An analysis of fatality investigations. Accid. Anal. Prev. 2011, 43, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Pollack, K.M.; Poplin, G.S.; Griffin, S.; Peate, W.; Nash, V.; Nied, E.; Gulotta, J.; Burgess, J.L. Implementing risk management to reduce injuries in the U.S. Fire Service. J. Saf. Res. 2017, 60, 21–27. [Google Scholar] [CrossRef]
- Evarts, R.C.B. United States Firefighter Injuries in 2019. Available online: https://www.nfpa.org/ (accessed on 1 June 2021).
- Hwang, J.; Xu, C.; Agnew, R.J.; Clifton, S.; Malone, T.R. Health Risks of Structural Firefighters from Exposure to Polycyclic Aromatic Hydrocarbons: A Systematic Review and Meta–Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4209. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Mani, A.; James, K.; Rao, M.B.; Bhattacharya, A. Effects of Heat Exposure from Live–Burn Fire Training on Postural Stability of Firefighters. Ergon. Int. J. 2019, 3, 213. [Google Scholar] [CrossRef]
- Smith, D.L. Firefighter fitness: Improving performance and preventing injuries and fatalities. Curr. Sports Med. Rep. 2011, 10, 167–172. [Google Scholar] [CrossRef]
- Zhuang, V.M.P.J.; Adam, B.; Kathryn, L. Total Cost of Fire in the United States. Available online: https://www.nfpa.org/–/media/Files/News–and–Research/Fire–statistics–and–reports/US–Fire–Problem/RFTotalCost.pdf (accessed on 1 June 2021).
- Zhi, L. Study on Safety Control of Fire Extinguishing Rescue Site. China Strateg. Emerg. Ind. 2021, 15, 247–248. [Google Scholar] [CrossRef]
- McAllister, M.J.; Gonzalez, A.E.; Waldman, H.S. Time Restricted Feeding Reduces Inflammation and Cortisol Response to a Firegrounds Test in Professional Firefighters. J. Occup. Environ. Med. 2021, 63, 441–447. [Google Scholar] [CrossRef]
- Storer, T.W.; Dolezal, B.A.; Abrazado, M.L.; Smith, D.L.; Batalin, M.A.; Tseng, C.H.; Cooper, C.B. Firefighter health and fitness assessment: A call to action. J. Strength Cond. Res. 2014, 28, 661–671. [Google Scholar] [CrossRef]
- Williams–Bell, F.M.; Villar, R.; Sharratt, M.T.; Hughson, R.L. Physiological demands of the firefighter Candidate Physical Ability Test. Med. Sci. Sports Exerc. 2009, 41, 653–662. [Google Scholar] [CrossRef]
- Windisch, S.; Seiberl, W.; Schwirtz, A.; Hahn, D. Relationships between strength and endurance parameters and air depletion rates in professional firefighters. Sci. Rep. 2017, 7, 44590. [Google Scholar] [CrossRef]
- Holmér, I.; Gavhed, D. Classification of metabolic and respiratory demands in fire fighting activity with extreme workloads. Appl. Ergon. 2007, 38, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Siddall, A.G.; Stevenson, R.D.M.; Turner, P.J.F.; Bilzon, J.L.J. Physical and Physiological Performance Determinants of a Firefighting Simulation Test. J. Occup. Environ. Med. 2018, 60, 637–643. [Google Scholar] [CrossRef] [PubMed]
- von Heimburg, E.D.; Rasmussen, A.K.; Medbø, J.I. Physiological responses of firefighters and performance predictors during a simulated rescue of hospital patients. Ergonomics 2006, 49, 111–126. [Google Scholar] [CrossRef]
- Lindberg, A.S.; Oksa, J.; Gavhed, D.; Malm, C. Field tests for evaluating the aerobic work capacity of firefighters. PLoS ONE 2013, 8, e68047. [Google Scholar] [CrossRef]
- Fernhall, B.; Fahs, C.A.; Horn, G.; Rowland, T.; Smith, D. Acute effects of firefighting on cardiac performance. Eur. J. Appl. Physiol. 2012, 112, 735–741. [Google Scholar] [CrossRef]
- von Heimburg, E.; Medbø, J.I.; Sandsund, M.; Reinertsen, R.E. Performance on a work–simulating firefighter test versus approved laboratory tests for firefighters and applicants. Int. J. Occup. Saf. Ergon. JOSE 2013, 19, 227–243. [Google Scholar] [CrossRef]
- Perroni, F.; Tessitore, A.; Cortis, C.; Lupo, C.; D’Artibale, E.; Cignitti, L.; Capranica, L. Energy cost and energy sources during a simulated firefighting activity. J. Strength Cond. Res. 2010, 24, 3457–3463. [Google Scholar] [CrossRef]
- Medbø, J.I.; Mamen, A.; Oseland, H.; von Heimburg, E.D. The steady–state load of five firefighting tasks. Int. J. Occup. Saf. Ergon. JOSE 2020, 26, 173–180. [Google Scholar] [CrossRef]
- Mamen, A.; Oseland, H.; Medbø, J.I. A comparison of two physical ability tests for firefighters. Ergonomics 2013, 56, 1558–1568. [Google Scholar] [CrossRef]
- Hesketh, S.J.; Stansfield, B.N.; Stead, C.A.; Burniston, J.G. The application of proteomics in muscle exercise physiology. Expert Rev. Proteom. 2020, 17, 813–825. [Google Scholar] [CrossRef]
- Oertzen–Hagemann, V.; Kirmse, M.; Eggers, B.; Pfeiffer, K.; Marcus, K.; de Marées, M.; Platen, P. Effects of 12 Weeks of Hypertrophy Resistance Exercise Training Combined with Collagen Peptide Supplementation on the Skeletal Muscle Proteome in Recreationally Active Men. Nutrients 2019, 11, 1072. [Google Scholar] [CrossRef]
- Thongboonkerd, V. Proteomics. Forum Nutr. 2007, 60, 80–90. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Zhao, D.; Zhang, L.; Chen, P.; Xu, X. Integration of metabolomics and proteomics to reveal the metabolic characteristics of high–intensity interval training. Analyst 2020, 145, 6500–6510. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lin, W.; McAinch, A.J.; Yan, X.; Weng, X. Identification of Urinary Biomarkers for Exercise–Induced Immunosuppression by iTRAQ Proteomics. BioMed Res. Int. 2020, 2020, 3030793. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Lin, S.C.; Tsai, K.Z.; Wu, T.J.; Lin, Y.P.; Lin, Y.K.; Lu, S.C.; Han, C.L.; Lin, G.M. Association of Single Measurement of dipstick proteinuria with physical performance of military males: The CHIEF study. BMC Nephrol. 2020, 21, 287. [Google Scholar] [CrossRef]
- Papassotiriou, I.; Nifli, A.P. Assessing performance in pre–season wrestling athletes using biomarkers. Biochem. Med. 2018, 28, 020706. [Google Scholar] [CrossRef]
- Available online: https://beyotime.com/Compatibility%20Chart%20For%20BCA%20Kit.pdf (accessed on 29 September 2021).
- Takao, T.; Suka, M.; Yanagisawa, H.; Kasuga, M. Combined effect of diabetic retinopathy and diabetic kidney disease on all–cause, cancer, vascular and non–cancer non–vascular mortality in patients with type 2 diabetes: A real–world longitudinal study. J. Diabetes Investig. 2020, 11, 1170–1180. [Google Scholar] [CrossRef]
- Kramer, T.A.; Sacko, R.S.; Pfeifer, C.E.; Gatens, D.R.; Goins, J.M.; Stodden, D.F. The association between the functional movement screen(tm), y–balance test, and physical performance tests in male and female high school athletes. Int. J. Sports Phys. Ther. 2019, 14, 911–919. [Google Scholar] [CrossRef]
- Sheaff, A.K.; Bennett, A.; Hanson, E.D.; Kim, Y.S.; Hsu, J.; Shim, J.K.; Edwards, S.T.; Hurley, B.F. Physiological determinants of the candidate physical ability test in firefighters. J. Strength Cond. Res. 2010, 24, 3112–3122. [Google Scholar] [CrossRef]
- Crotti, M.; Bosio, A.; Invernizzi, P.L. Validity and reliability of submaximal fitness tests based on perceptual variables. J. Sports Med. Phys. Fit. 2018, 58, 555–562. [Google Scholar] [CrossRef]
- Park, S.; Han, H.S.; Kim, G.U.; Kang, S.S.; Kim, H.J.; Lee, M.; Park, S.H.; Choi, K.H.; Kim, S.H.; Yeom, J.S. Relationships among Disability, Quality of Life, and Physical Fitness in Lumbar Spinal Stenosis: An Investigation of Elderly Korean Women. Asian Spine J. 2017, 11, 256–263. [Google Scholar] [CrossRef]
- Conkright, W.R.; Barringer, N.D.; Lescure, P.B.; Feeney, K.A.; Smith, M.A.; Nindl, B.C. Differential recovery rates of fitness following U.S. Army Ranger training. J. Sci. Med. Sport 2020, 23, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; Almeida, L.M.; IV, D.E.S.N.; NMF, D.E.S.; JA, D.E.A.; BF, D.E.S.; Bentes, C.M.; Prestes, J.; Collier, S.R.; Voltarelli, F.A. Extreme Conditioning Program Induced Acute Hypotensive Effects are Independent of the Exercise Session Intensity. Int. J. Exerc. Sci. 2017, 10, 1165–1173. [Google Scholar] [PubMed]
- Dennison, K.J.; Mullineaux, D.R.; Yates, J.W.; Abel, M.G. The effect of fatigue and training status on firefighter performance. J. Strength Cond. Res. 2012, 26, 1101–1109. [Google Scholar] [CrossRef]
- Mills, K.T.; Kobori, H.; Hamm, L.L.; Alper, A.B.; Khan, I.E.; Rahman, M.; Navar, L.G.; Liu, Y.; Browne, G.M.; Batuman, V.; et al. Increased urinary excretion of angiotensinogen is associated with risk of chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.–Eur. Ren. Assoc. 2012, 27, 3176–3181. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Wen, X.; Wu, T.; Wang, W.; Yang, M.; Wang, J.; Fang, M.; Lin, B.; Lin, H. Development of a Functional Glomerulus at the Organ Level on a Chip to Mimic Hypertensive Nephropathy. Sci. Rep. 2016, 6, 31771. [Google Scholar] [CrossRef]
- Latorre–Muro, P.; Baeza, J.; Armstrong, E.A.; Hurtado–Guerrero, R.; Corzana, F.; Wu, L.E.; Sinclair, D.A.; López–Buesa, P.; Carrodeguas, J.A.; Denu, J.M. Dynamic Acetylation of Phosphoenolpyruvate Carboxykinase Toggles Enzyme Activity between Gluconeogenic and Anaplerotic Reactions. Mol. Cell 2018, 71, 718–732.e719. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama–Kasaoka, N.; et al. The fat–derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Welters, I.D.; Bing, C.; Ding, C.; Leuwer, M.; Hall, A.M. Circulating anti–inflammatory adipokines High Molecular Weight Adiponectin and Zinc–α2–glycoprotein (ZAG) are inhibited in early sepsis, but increase with clinical recovery: A pilot study. BMC Anesthesiol. 2014, 14, 124. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, L.; Liu, S.; Li, Y.; Xia, S.; Chen, D.; Wang, M.; Wu, S.; Dai, Q.; Vu, H.; et al. γ–6–Phosphogluconolactone, a Byproduct of the Oxidative Pentose Phosphate Pathway, Contributes to AMPK Activation through Inhibition of PP2A. Mol. Cell 2019, 76, 857–871.e859. [Google Scholar] [CrossRef]
- Belkaya, S.; Michailidis, E.; Korol, C.B.; Kabbani, M.; Cobat, A.; Bastard, P.; Lee, Y.S.; Hernandez, N.; Drutman, S.; de Jong, Y.P.; et al. Inherited IL–18BP deficiency in human fulminant viral hepatitis. J. Exp. Med. 2019, 216, 1777–1790. [Google Scholar] [CrossRef]
- Kapka–Skrzypczak, L.; Popek, S.; Sawicki, K.; Wolińska, E.; Czajka, M.; Skrzypczak, M. Effect of IL–6 and IL–8 on the expression of the complement activation inhibitors MAC–inhibitory protein and decay–accelerating factor in ovarian cancer A2780 cells. Oncol. Lett. 2016, 12, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Buchser, W.J.; Smith, R.P.; Pardinas, J.R.; Haddox, C.L.; Hutson, T.; Moon, L.; Hoffman, S.R.; Bixby, J.L.; Lemmon, V.P. Peripheral nervous system genes expressed in central neurons induce growth on inhibitory substrates. PLoS ONE 2012, 7, e38101. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qiu, T.; Kathayat, R.S.; Azizi, S.A.; Thorne, A.K.; Ahn, D.; Fukata, Y.; Fukata, M.; Rice, P.A.; Dickinson, B.C. ABHD10 is an S–depalmitoylase affecting redox homeostasis through peroxiredoxin–5. Nat. Chem. Biol. 2019, 15, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, J.; Chen, X.; Liu, P.; Wang, S.; Song, F.; Zhang, Z.; Zhu, F.; Huang, X.; Liu, J.; et al. The Prenylflavonoid Xanthohumol Reduces Alzheimer–Like Changes and Modulates Multiple Pathogenic Molecular Pathways in the Neuro2a/APPswe Cell Model of AD. Front. Pharmacol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, J.; Fang, B.; Tian, X.; Feng, Y.; Cheng, Z.; Fu, Z.; Zhang, J.; Wu, J. Runners’ metabolomic changes following marathon. Nutr. Metab. 2020, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.L.; O’Connor, D.P.; Herring, M.P.; Sailors, M.H.; Jackson, A.S.; Dishman, R.K.; Bray, M.S. Exercise dose, exercise adherence, and associated health outcomes in the TIGER study. Med. Sci. Sports Exerc. 2014, 46, 69–75. [Google Scholar] [CrossRef]
- Wang, H.J.; Si, Q.J.; Shi, Y.; Guo, Y.; Li, Y.; Wang, Y.T. The prognostic values of beta–2 microglobulin for risks of cardiovascular events and mortality in the elderly patients with isolated systolic hypertension. J. Res. Med Sci. Off. J. Isfahan Univ. Med Sci. 2018, 23, 82. [Google Scholar] [CrossRef]
- Parkkila, K.; Valtonen, R.I.P.; Hiltunen, L.; Hintsala, H.E.; Jaakkola, J.J.K.; Ikäheimo, T.M. The effects of submaximal exercise and cold exposure on blood coagulation parameters in coronary artery disease patients. BMC Cardiovasc. Disord. 2021, 21, 93. [Google Scholar] [CrossRef]
- Flynn, J.M.; Meadows, E.; Fiorotto, M.; Klein, W.H. Myogenin regulates exercise capacity and skeletal muscle metabolism in the adult mouse. PLoS ONE 2010, 5, e13535. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, L.; Zhang, Z.; Ouyang, J.; Huang, H. Effects of ginsenosides–Rb1 on exercise–induced oxidative stress in forced swimming mice. Pharmacogn. Mag. 2014, 10, 458–463. [Google Scholar] [CrossRef]
- Arazawa, D.T.; Oh, H.I.; Ye, S.H.; Johnson, C.A., Jr.; Woolley, J.R.; Wagner, W.R.; Federspiel, W.J. Immobilized Carbonic Anhydrase on Hollow Fiber Membranes Accelerates CO(2) Removal from Blood. J. Membr. Sci. 2012, 404–404, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, B.W.; Kowalchuk, J.M.; Paterson, D.H.; Cunningham, D.A. O2 uptake kinetics after acetazolamide administration during moderate– and heavy–intensity exercise. J. Appl. Physiol. 1998, 85, 1384–1393. [Google Scholar] [CrossRef][Green Version]
- Yang, K.D.; Chang, W.C.; Chuang, H.; Wang, P.W.; Liu, R.T.; Yeh, S.H. Increased complement factor H with decreased factor B determined by proteomic differential displays as a biomarker of tai chi chuan exercise. Clin. Chem. 2010, 56, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Muhsen Ali, A.; Burleigh, M.; Daskalaki, E.; Zhang, T.; Easton, C.; Watson, D.G. Metabolomic Profiling of Submaximal Exercise at a Standardised Relative Intensity in Healthy Adults. Metabolites 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]


| Pro–Agility (s) | 1 RM (Bench Press) (kg) | 1 RM (Deep Squat) (kg) | The Crunch Tests (a.u) | Sit-and-Reach (cm) | The 300-Yard Shuttle Run (s) | 2400-m Run (s) |
|---|---|---|---|---|---|---|
| 4.94 ± 0.21 | 121.92 ± 15.59 | 80.21 ± 11.64 | 45.00 ± 16.78 | 43.75 ± 8.16 | 63.28 ± 3.01 | 571.87 ± 31.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Hu, Y.; Hwang, J.; Zhao, D.; Huang, L.; Qiao, L.; Wei, A.; Xu, X. Urinary Proteomics of Simulated Firefighting Tasks and Its Relation to Fitness Parameters. Int. J. Environ. Res. Public Health 2021, 18, 10618. https://doi.org/10.3390/ijerph182010618
Zhu T, Hu Y, Hwang J, Zhao D, Huang L, Qiao L, Wei A, Xu X. Urinary Proteomics of Simulated Firefighting Tasks and Its Relation to Fitness Parameters. International Journal of Environmental Research and Public Health. 2021; 18(20):10618. https://doi.org/10.3390/ijerph182010618
Chicago/Turabian StyleZhu, Ting, Yuxiang Hu, Jooyeon Hwang, Dan Zhao, Libin Huang, Liang Qiao, Ankui Wei, and Xin Xu. 2021. "Urinary Proteomics of Simulated Firefighting Tasks and Its Relation to Fitness Parameters" International Journal of Environmental Research and Public Health 18, no. 20: 10618. https://doi.org/10.3390/ijerph182010618
APA StyleZhu, T., Hu, Y., Hwang, J., Zhao, D., Huang, L., Qiao, L., Wei, A., & Xu, X. (2021). Urinary Proteomics of Simulated Firefighting Tasks and Its Relation to Fitness Parameters. International Journal of Environmental Research and Public Health, 18(20), 10618. https://doi.org/10.3390/ijerph182010618








