Postoperative Neurocognitive Disorders in Cardiac Surgery: Investigating the Role of Intraoperative Hypotension. A Systematic Review
Abstract
:1. Introduction
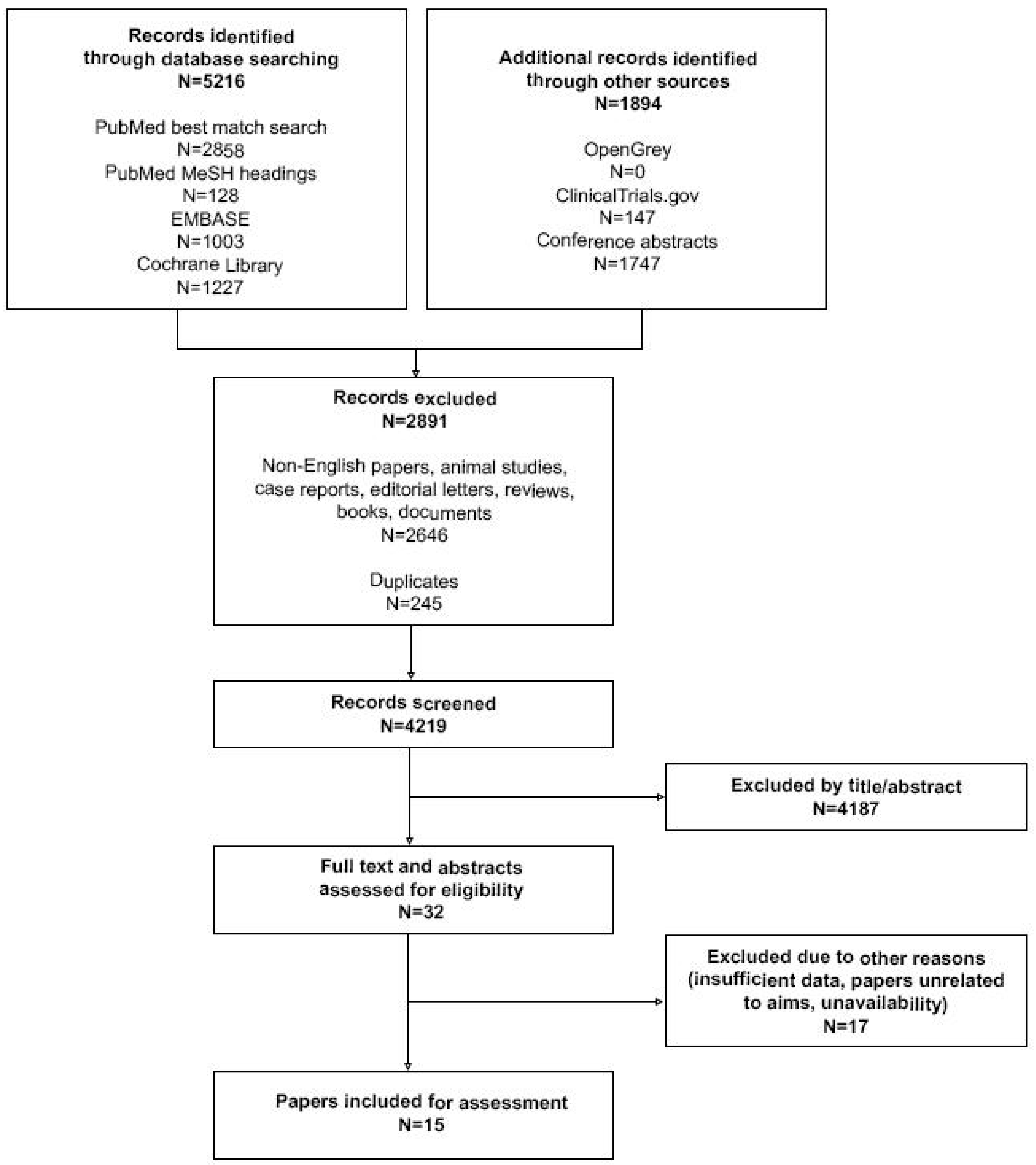
2. Materials and Methods
3. Review of Published Data
3.1. Postoperative Cognitive Decline
3.2. Postoperative Delirium
4. Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Search Strategy
References
- Evered, L.; Silbert, B.; Knopman, D.; Scott, D.A.; DeKosky, S.T.; Rasmussen, L.S.; Oh, E.; Crosby, G.; Berger, M.; Eckenhoff, R.G.; et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Br. J. Anaesth. 2018, 121, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2014. [Google Scholar]
- Inouye, S.K.; Van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying Confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Inouye, S.K.; Bernard, G.R.; Gordon, S.; Francis, J.; May, L.; Truman, B.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001, 286, 2703–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellelli, G.; Morandi, A.; Davis, D.H.; Mazzola, P.; Turco, R.; Gentile, S.; Ryan, T.; Cash, H.; Guerini, F.; Torpilliesi, T.; et al. Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age Ageing 2014, 43, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Bryson, G.L.; Wyand, A. Evidence-based clinical update: General anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can. J. Anaesth. 2006, 53, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, L.S.; Larsen, K.; Houx, P.; Skovgaard, L.T.; Hanning, C.D.; Moller, J.T.; The ISPOCD 5 Group. The assessment of postoperative cognitive function. Acta Anaesthesiol. Scand. 2001, 45, 275–289. [Google Scholar] [CrossRef]
- Sessler, D.I.; Bloomstone, J.A.; Aronson, S.; Berry, C.; Gan, T.J.; Kellum, J.A.; Plumb, J.; Mythen, M.G.; Grocott, M.P.; Edwards, M.R.; et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br. J. Anaesth. 2019, 122, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Yu, W.; Wang, T.; Zhang, L.; Heerdt, P.M.; Gelb, A.W. Blood Pressure Targets in Perioperative Care. Hypertension 2018, 72, 806–817. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, K.; Mei, W. Neurological complications after cardiac surgery: Anesthetic considerations based on outcome evidence. Curr. Opin. Anaesthesiol. 2019, 32, 563–567. [Google Scholar] [CrossRef]
- Sun, L.Y.; Chung, A.M.; Farkouh, M.E.; Van Diepen, S.; Weinberger, J.; Bourke, M.; Ruel, M. Defining an Intraoperative Hypotension Threshold in Association with Stroke in Cardiac Surgery. Anesthesiology 2018, 129, 440–447. [Google Scholar] [CrossRef]
- Hsieh, J.K.; Dalton, J.E.; Yang, D.; Farag, E.S.; Sessler, D.I.; Kurz, A.M. The Association between Mild Intraoperative Hypotension and Stroke in General Surgery Patients. Anesth. Analg. 2016, 123, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; Kappen, T.; Torn, H.; Slooter, A.; Van Klei, W. Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br. J. Anaesth. 2018, 121, 706–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzych, Ł.J.; Wybraniec, M.T.; Krupka-Matuszczyk, I.; Skrzypek, M.; Bolkowska, A.; Wilczyński, M.; Bochenek, A.A. Complex Assessment of the Incidence and Risk Factors of Delirium in a Large Cohort of Cardiac Surgery Patients: A Single-Center 6-Year Experience. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzych, Ł.J.; Pluta, M.P.; Putowski, Z.; Czok, M. Investigating Association between Intraoperative Hypotension and Postoperative Neurocognitive Disorders in Non-Cardiac Surgery: A Comprehensive Review. J. Clin. Med. 2020, 9, 3183. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hu, J.; Hua, F.; Zhang, J.; Zhang, L.; Xu, G. The correlation of intraoperative hypotension and postoperative cognitive impairment: A meta-analysis of randomized controlled trials. BMC Anesthesiol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Gold, J.P.; Charlson, M.E.; Williams-Russo, P.; Szatrowski, T.P.; Peterson, J.C.; Pirraglia, P.A.; Hartman, G.S.; Yao, F.S.F.; Hollenberg, J.P.; Barbut, D.; et al. Improvement of outcomes after coronary artery bypass: A randomized trial comparing intraoperative high versus low mean arterial pressure. J. Thorac. Cardiovasc. Surg. 1995, 110, 1302–1314. [Google Scholar] [CrossRef] [Green Version]
- Krenk, L.; Rasmussen, L.S. Postoperative delirium and postoperative cognitive dysfunction in the elderly—What are the differences? Minerva Anestesiol 2011, 77, 742–749. [Google Scholar]
- Charlson, M.E.; Peterson, J.C.; Krieger, K.H.; Hartman, G.S.; Hollenberg, J.P.; Briggs, W.M.; Segal, A.Z.; Parikh, M.; Thomas, S.J.; Donahue, R.G.; et al. Improvement of Outcomes after Coronary Artery Bypass II: A Randomized Trial Comparing Intraoperative High Versus Customized Mean Arterial Pressure. J. Card. Surg. 2007, 22, 465–472. [Google Scholar] [CrossRef]
- Larsen, M.H.; Draegert, C.; Vedel, A.G.; Holmgaard, F.; Siersma, V.; Nilsson, J.C.; Rasmussen, L.S. Long-term survival and cognitive function according to blood pressure management during cardiac surgery. A follow-up. Acta Anaesthesiol. Scand. 2020, 64, 936–944. [Google Scholar] [CrossRef]
- Newman, M.F.; Kramer, D.; Croughwell, N.D.; Sanderson, I.; Blumenthal, J.A.; White, W.D.; Smith, L.R.; Towner, E.A.; Reves, J.G. Differential age effects of mean arterial pressure and rewarming on cognitive dysfunction after cardiac surgery. Anesth. Analg. 1995, 81, 236–242. [Google Scholar]
- Friedrich, I.; Simm, A.; Kötting, J.; Thölen, F.; Fischer, B.; Silber, R.-E. Cardiac Surgery in the Elderly Patient. Dtsch. Aerzteblatt Int. 2009, 106, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Hillis, A.E.; Grega, M.A.; Borowicz, L.M.; Selnes, O.A.; Baumgartner, W.A.; McKhann, G.M. Early Postoperative Cognitive Dysfunction and Blood Pressure during Coronary Artery Bypass Graft Operation. Arch. Neurol. 2007, 64, 1111–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vedel, A.G.; Rasmussen, L.S.; Holmgaard, F.; Nilsson, J.C. Response by Vedel et al to Letters Regarding Article, “High-Target Versus Low-Target Blood Pressure Management During Cardiopulmonary Bypass to Prevent Cerebral Injury in Cardiac Surgery Patients: A Randomized Controlled Trial. ” Circulation 2018, 138, 2447–2448. [Google Scholar] [CrossRef] [PubMed]
- Siepe, M.; Pfeiffer, T.; Gieringer, A.; Zemann, S.; Benk, C.; Schlensak, C.; Beyersdorf, F. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur. J. Cardio-Thoracic Surg. 2011, 40, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Li, H.-C.; Chen, Y.-S.; Chiu, M.-J.; Fu, M.-C.; Huang, G.-H.; Chen, C.C.-H. Delirium, Subsyndromal Delirium, and Cognitive Changes in Individuals Undergoing Elective Coronary Artery Bypass Graft Surgery. J. Cardiovasc. Nurs. 2015, 30, 340–345. [Google Scholar] [CrossRef]
- Wesselink, E.; Kappen, T.H.; Van Klei, W.A.; Dieleman, J.M.; Van Dijk, D.; Slooter, A.J.C. Intraoperative hypotension and delirium after on-pump cardiac surgery. Br. J. Anaesth. 2015, 115, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Sauër, A.; Slooter, A.; Veldhuijzen, D.; Van Eijk, M.; Van Dijk, D. Intraoperative dexamethasone and delirium after cardiac surgery: A randomized clinical trial. Crit. Care 2013, 17, P396. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.G.; Patel, M.B.; Pandharipande, P.P. Pathophysiology of acute brain dysfunction: what’s the cause of all this confusion? Curr. Opin. Crit. Care 2012, 18, 518–526. [Google Scholar] [CrossRef]
- Anderson, B.J.; Chesley, C.F.; Theodore, M.; Christie, C.; Tino, R.; Wysoczanski, A.; Ramphal, K.; Oyster, M.; Kalman, L.; Porteous, M.K.; et al. Incidence, risk factors, and clinical implications of post-operative delirium in lung transplant recipients. J. Hear. Lung Transplant. 2018, 37, 755–762. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Rivelli, S.K.; Palmer, S.M.; Davis, R.D.; Mathew, J.P. Reduced Cerebral Perfusion Pressure during Lung Transplant Surgery Is Associated with Risk, Duration, and Severity of Postoperative Delirium. Ann. Am. Thorac. Soc. 2016, 13, 180–187. [Google Scholar] [PubMed] [Green Version]
- Hori, D.; Max, L.; LaFlam, A.; Brown, C.G.; Neufeld, K.J.; Adachi, H.; Sciortino, C.M.; Conte, J.V.; Cameron, D.E.; Hogue, C.W.; et al. Blood Pressure Deviations from Optimal Mean Arterial Pressure During Cardiac Surgery Measured With a Novel Monitor of Cerebral Blood Flow and Risk for Perioperative Delirium: A Pilot Study. J. Cardiothorac. Vasc. Anesth. 2016, 30, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, R.S.; Kasner, S.E. Hypertension and hypertensive encephalopathy. Interv. Neuroradiol. 2014, 119, 161–167. [Google Scholar] [CrossRef]
- Hori, D.; Brown, C.; Ono, M.; Rappold, T.; Sieber, F.; Gottschalk, A.; Neufeld, K.J.; Gottesman, R.; Adachi, H.; Hogue, C.W. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br. J. Anaesth. 2014, 113, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, K.M.; Mytar, J.O.; Kibler, K.K.; Hogue, C.W.; Lee, J.K.; Czosnyka, M.; Smielewski, P.; Easley, R.B. Noninvasive Autoregulation Monitoring with and without Intracranial Pressure in the Naïve Piglet Brain. Anesth. Analg. 2010, 111, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.H.; Neufeld, K.J.; Tian, J.; Probert, J.; LaFlam, A.; Max, L.; Hori, D.; Nomura, Y.; Mandal, K.; Brady, K.; et al. Effect of Targeting Mean Arterial Pressure During Cardiopulmonary Bypass by Monitoring Cerebral Autoregulation on Postsurgical Delirium Among Older Patients: A Nested Randomized Clinical Trial. JAMA Surg. 2019, 154, 819–826. [Google Scholar] [CrossRef]
- Nadelson, M.R.; Sanders, R.D.; Avidan, M.S. Perioperative cognitive trajectory in adults. Br. J. Anaesth. 2014, 112, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.; Nadler, J.W.; Browndyke, J.; Terrando, N.; Ponnusamy, V.; Cohen, H.J.; Whitson, H.E.; Mathew, J.P. Postoperative Cognitive Dysfunction: Minding the Gaps in Our Knowledge of a Common Postoperative Complication in the Elderly. Anesthesiol. Clin. 2015, 33, 517–550. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Futier, E.; Lefrant, J.Y.; Guinot, P.G.; Godet, T.; Lorne, E.; Cuvillon, P.; Bertran, S.; Leone, M.; Pastene, B.; Piriou, V.; et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA 2017, 318, 1346–1357. [Google Scholar] [CrossRef]
| Author | Design of the Study | Study Population | IOH Definition | POCD Screening Method | Effect |
|---|---|---|---|---|---|
| Gold et. al. [17] | Randomized controlled | 248 patients undergoing elective CABG with CPB | Two BP management strategies:MAP between 80–100 mmHg or 50–60 mmHg | Battery of 11 neuropsychological tests applied preoperatively, 7 days and 6 months post procedure | No effect at 6 months follow-up |
| Charlson et. al. [19] | Randomized controlled | 412 patients undergoing elective CABG with CPB | Two BP management strategies: MAP target determined by patient’s usual pre-bypass blood pressure or “high” MAP maintained around 80 mmHg | Battery of 11 neuropsychological tests applied preoperatively, on the 1–2 and 5–6 postoperative days and at 6 months follow-up | No effect |
| Larsen et. al. [20] | Randomized controlled | 113 patients undergoing cardiac surgery with CPB | Two BP management strategies: high target MAP = 70–80 mmHg or low target MAP = 40–50 mmHg | MMSE, ISPOCD test battery and three additional questionnaires applied before the surgery and at 3 years follow-up | No effect |
| Newman et.al. [21] | Cohort | 237 patients undergoing elective cardiac procedure with CBP | MAP < 50 mm Hg | Neuropsychologic test battery on the day prior to the surgery and the day prior to the hospital discharge | Low intraoperative MAP contributes neuropsychologic dysfunction in elderly |
| Gottesman et. al. [23] | Cohort | 15 patients with high risk for postoperative stroke undergoing elective on-pump CABG | Not defined | The neurocognitive examination consisted of MMSE, Trail Making Test A and B and the modified Rankin Scale applied 3–5 days after surgery and after 1 month | Each additional point decrease in baseline MAP during the surgery led to a 0.09-point greater decline in early postoperative MMSE score |
| Vedel et al. [24] | Randomized controlled | 197 patients undergoing cardiac surgery with CBP | Two BP management strategies: a strategy of high target for MAP (70–80 mmHg) and strategy of low target for MAP (40–50 mmHg) | ISPOCD test battery used at median day 7 and median day 90 after the procedure | No significant difference was observed at either 7 and 90 day after procedure |
| Author | Design of the Study | Study Population | IOH Definition | POD Screening Method | Effect |
|---|---|---|---|---|---|
| Siepe et al. [25] | Randomized controlled | 92 patients undergoing elective or non-elective on-pump CABG | Intervention group with MAP maintained between 60–70 mmHg | Postoperative (48 h after procedure) MMSE score 10 points lower than the preoperative assessment | Only patients with MAP kept between 60–70 mmHg exhibited POD |
| Hsiu-Ching et al. [27] | Prospective observational | 38 patients undergoing elective on-pump or off-pump CABG | MAP < 60 mmHg | Daily CAM assessment during the first week after surgery | Patients who developed POD experienced longer duration of intraoperative MAP < 60 mmHg |
| Wesselink et al. [28] | Observational study nested in another clinical trial | 734 patients undergoing on-pump cardiac surgery | Four definitions were explored: MAP < 50 mmHg, MAP < 60 mmHg, 30% and 40% decrease of baseline MAP values | Daily CAM assessments for four consecutive days after procedure | No effect regarding the hypotension thresholds |
| Anderson et al. [31] | Retrospective observational | 155 patients undergoing lung transplantation | MAP < 60 mmHg | Presence of terms: “delirious”, “delirium”, “CAM positive” or acclaimed antipsychotic treatment in medical records of hospitalization | The duration of time with an intraoperative MAP < 60 mmHg was an independent risk factor for development of POD |
| Smith et al. [32] | Prospective observational | 63 patients undergoing lung transplantation | Not defined | Daily CAM or CAM-ICU assessments for the first 7 days after surgery | Lower CPP was related with higher incidence of delirium: every 10 mmHg decrease in CPP doubled the odds of POD |
| Hori et al. [33] | Prospective observational | 99 patients undergoing on-pump cardiac surgery | Not defined | CAM or CAM-ICU assessments during the first 3 days after surgery | Blood pressure excursions above the optimal MAP correlated with presence and severity of delirium on postoperative day 2 |
| Hori et al. [35] | Prospective observational | 491 patients undergoing on-pump cardiac surgery | Not defined | Observations made by a clinical staff | Higher POD occurrence was observed in patients whose MAP exceeded upper limit of cerebrovascular autoregulation |
| Krzych et al. [14]. | Prospective observational | 5781 patients undergoing on-pump and off-pump cardiac surgery | Not defined | DSM-IV evaluations done by an attending physician and a psychiatrist | No effect |
| Vedel et al. [24] | Randomized controlled | 197 patients undergoing cardiac surgery with CPB | Two BP management strategies: a strategy of high target for MAP (70–80 mmHg) and strategy of low target for MAP (40–50 mmHg) | No screening tests provided; the data was acquired via medical records | No effect |
| Brown et al. [37] | Randomized controlled | 199 patients undergoing cardiac surgery with CPB | Two BP management strategies: standard care vs. MAP maintained above individual limit of cerebral autoregulation | CAM or CAM-ICU for the first 4 postoperative days followed by panel discussion considering DSM_V criteria | POD occurred in 38% patients from the intervention group versus 53% in control. The odds for POD were reduced by 45% in patients in the intervention group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czok, M.; Pluta, M.P.; Putowski, Z.; Krzych, Ł.J. Postoperative Neurocognitive Disorders in Cardiac Surgery: Investigating the Role of Intraoperative Hypotension. A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 786. https://doi.org/10.3390/ijerph18020786
Czok M, Pluta MP, Putowski Z, Krzych ŁJ. Postoperative Neurocognitive Disorders in Cardiac Surgery: Investigating the Role of Intraoperative Hypotension. A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(2):786. https://doi.org/10.3390/ijerph18020786
Chicago/Turabian StyleCzok, Marcelina, Michał P. Pluta, Zbigniew Putowski, and Łukasz J. Krzych. 2021. "Postoperative Neurocognitive Disorders in Cardiac Surgery: Investigating the Role of Intraoperative Hypotension. A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 2: 786. https://doi.org/10.3390/ijerph18020786
APA StyleCzok, M., Pluta, M. P., Putowski, Z., & Krzych, Ł. J. (2021). Postoperative Neurocognitive Disorders in Cardiac Surgery: Investigating the Role of Intraoperative Hypotension. A Systematic Review. International Journal of Environmental Research and Public Health, 18(2), 786. https://doi.org/10.3390/ijerph18020786






